Abstract
Recent discoveries about the orbital tissues prompt a re-evaluation of the way that clinicians think about disorders affecting the extraocular muscles, their nerves and motoneurons in the brainstem. The revolutionary discovery that the orbital layers of the extraocular muscles insert not onto the eyeball, but into fibromuscular pulleys that guide the orbital layers, provides explanations for the kinematic properties of eye rotations and clinical findings in some patients with strabismus. The demonstration that all extraocular fibers types, except pale global fibers, lack synaptic folding provides an explanation for why saccades may remain fast in patients with limited ocular mobility due to myasthenia gravis. More than one mechanism may account for the observation that patients with disorders affecting the eye muscles or their nerves can present with the appearance of central disorders of ocular motility, such as internuclear ophthalmoplegia. New approaches to analyzing saccades in patients with disjunctive eye movements provide the means to identify disorders affecting the peripheral or central components of the ocular motor system, or both.
Introduction
Diagnosis of disorders of the peripheral part of the ocular motor system rests on time-honored tests such as those enunciated by CitationBielschowsky (1940). The advent of magnetic resonance imaging has provided the means to measure the position of extraocular muscles and other tissues as subjects direct their gaze at central and peripheral visual targets. Modern anatomical methods using histochemical stains and electron microscopy have also provided new information concerning the extraocular muscle fiber types and the way in which muscles insert into the tissues surround the globe to rotate the eye. Here we focus on three main clinical implications of these findings: (1) the role that the orbital tissues play in determining the axes of eye rotations; (2) the finding that the neuromuscular junction of extraocular muscles lacks synaptic folding, with the exception of one fiber type; and (3) the role that central adaptive mechanisms play in patients with impaired eye movements.
Orbital tissues and eye rotations
Our views about how the eye muscles turn the eye have changed dramatically over the past decade (CitationDemer 2004; CitationLeigh and Zee 2006). Certain facts remain unchanged. The four recti and the superior oblique arise from the apex of the orbit (the annulus of Zinn). The inferior oblique muscle arises from the inferior nasal aspect of the orbit. The four rectus muscles insert into the sclera anterior to the equator of the globe: the medial rectus muscle on the nasal side, the lateral rectus muscle on the temporal side, the superior rectus muscle on the superior side and the inferior rectus muscle on the inferior side. The superior and inferior oblique muscles approach the globe from its anterior and medial aspect and insert posterior to the equator of the globe. The superior oblique muscle first passes through the trochlea (a fibrous, cartilaginous, U-shaped ring that lies just inside the superior medial orbital rim) before inserting on the superior side of the globe. The inferior oblique inserts on the temporal side of the globe.
Each rectus muscle has an outer orbital layer and an inner global layer (CitationSpencer and Porter 2006), each with different fiber types, which are discussed in the second section. Although the global muscle layer inserts on the globe of the eye, the orbital layer does not; it inserts into a fascial component of Tenon’s capsule, which suspends the eye in the orbit (Miller and CitationDemer 1997; CitationDemer 2004; CitationRuskell et al 2005). Thus, the tendons of the rectus extraocular muscles, which originate from the inner global layer, pass through sleeve-like fibromuscular pulleys that lie within peripheral Tenon’s capsule. These pulleys are located approximately 10 mm behind the insertion sites of the muscles. Each rectus pulley consists of an encircling ring of collagen, located near the equator of the eyeball in Tenon’s capsule. The pulleys are attached to the wall of the orbit, adjacent extraocular muscles and Tenon’s fascia by sling-like bands containing collagen, elastin, and smooth muscle (CitationDemer et al 1997). The orbital layer of the inferior oblique muscle inserts into inferior rectus and lateral rectus pulleys. The orbital layer of the superior oblique muscle inserts into the superior rectus pulley (CitationKono et al 2005).
What is the functional significance of this anatomy? One important function of the fibromuscular pulleys is to limit sideslip movement of the rectus muscles during eye rotations. Thus, the pulleys effectively change the point of origin of the rectus muscles, just as the trochlea changes the functional point of origin of the superior oblique muscle. Another probable role of the fibromuscular pulleys is to constraint the axes of rotation of the eyes during visually guided movements to Listing’s plane, which is perpendicular to the fixation line in primary position (CitationHaslwanter 1995). Direct electrical stimulation of the abducens nerve in monkey causes horizontal movements if the eye starts in primary position, but also induces torsional rotations if the eye starts from a position in upgaze or downgaze (CitationKlier et al 2006). Thus, the fibromuscular pulleys may simplify for the brain the challenging job of governing 3-D eye rotations, although there is evidence that neural factors also play a role (CitationVan Opstal et al 1996).
The clinical impact of the fibromuscular pulleys is still being determined, but has already provided new approaches to evaluate eye movement disorders (CitationDemer 2004). Some forms of congenital strabismus have been ascribed to congenital misplacement of pulleys (CitationClark et al 1998). Disorders affecting the connective tissues, such as Marfan’s syndrome, may cause increase mobility of pulleys with consequent strabismus (CitationOh et al 2002).
In patients with abducens nerve palsy, small vertical or torsional deviations may be apparent in addition to the predominant esotropia (CitationWong et al 2002); such deviations may be explained by role of the pulleys, such that a weak lateral rectus muscle also affects the positions of pulleys which, in turn, influence the pulling directions of muscles with predominantly vertical actions. Finally, measurement of the axis about which the eyes rotate provides potentially important information to researchers. For example, saccades and smooth pursuit eye movements obey Listing’s law, such that the axis of rotation lies in Listing’s plane, which is approximately frontal (coronal). However, vestibular eye movements responding to head rotations do not obey Listing’s law (CitationTian et al 2005); consider, for example, torsional eye rotations induced by ear-to-shoulder head roll. Conversely, vestibular eye movements responding to head translations (linear motion) do obey Listing’s law, which is evidence for their later evolution, along with frontal binocular vision and smooth pursuit (CitationZee et al 2002).
Properties of extraocular muscles
Extraocular muscles show substantial differences, anatomically, physiologically, and immunologically, from striate limb muscle (CitationSpencer and Porter 2006). Extraocular muscle fibers are smaller, more variable in size, and more richly innervated than limb muscle fibers. Motor unit size is small – about 10–20 muscle fibers per motoneuron. Like limb muscle, the extraocular muscle contains twitch fibers that have a single endplate per fiber and can generate action potentials. In addition, there are non-twitch fibers that do not generate action potentials and show graded contractions to trains of electrical pulse stimuli (CitationShall and Goldberg 1992; CitationMorgan and Proske 1984). At their insertion into the muscle tendon, fibers are covered by axonal terminals (pallisade endings), which are probably proprioceptive in function (CitationRuskell 1999). Another difference from limb muscles is that extraocular muscle expresses virtually all known striated muscle isoforms of myosin heavy chain, including skeletal, cardiac and embryonic isoforms, in the proximal and distal portions of muscle fibers in the orbital layers (CitationPorter et al 1995; CitationYu Wai Man et al 2005; CitationSpencer and Porter 2006). Expression of myosin may vary along the length of single muscle fibers, with “fast” forms being more prominent in the central region of most fibers, thus accounting for the ability of both orbital and global fibers to contract quickly (CitationBriggs and Schachat 2002). The basement membranes of the extraocular muscle also differs from skeletal muscle (CitationKjellgren et al 2004), being low in enzymes and regulators related to glycogen metabolism, a finding consistent with low glycogen content of extraocular muscle and pointing to important differences in energy metabolism (CitationPorter 2002). Extraocular muscles show other differences from skeletal muscles, including transcriptional regulation, sarcomeric organization, excitation-contraction coupling, intermediary metabolism and the immune response (CitationSpencer and Porter 2006).
Disorders affecting extraocular muscles
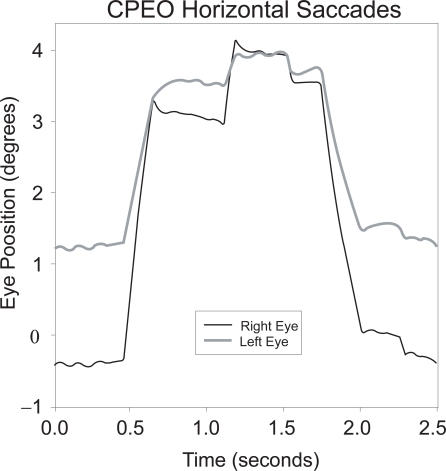
It is well known that one cause of chronic progressive ophthalmoplegia, the Kearns-Sayre–Daroff syndrome (CitationKearns and Sayre 1958; CitationDaroff et al 1966), is due, in most cases, to deletions or duplications of mitochondrial DNA (CitationBrockington et al 1995; CitationMoraes 1996; CitationShoffner 1996; CitationFromenty et al 1997; CitationWilichowski et al 1997; CitationMcFarland et al 2002; CitationTaylor and Turnbull 2005). Affected patients may have strabismus, and eye movements may be disjunctive () but few present with the complaint of double vision (CitationRichardson et al 2005). Mitochondrial DNA mutations appear to be more common in tissues with higher oxidative metabolism, which is true of the extraocular muscles (CitationYu Wai Man et al 2005). This multisystem disorder is characterized by progressive ophthalmoparesis beginning in childhood or adolescence, atypical pigmentary degeneration of the retina, and heart block – the cardiac complications of Kearns-Sayre syndrome may be life-threatening. Ultrastuctural analysis of extraocular muscle from one patient with a mitochondrial DNA deletion and complete bilateral ptosis with non-restrictive exotropia showed a selective vacuolization of some muscle fibers, with abnormal mitochondria (CitationCarta et al 2000). An uneven distribution of deletions of mitochondrial DNA in different tissues probably accounts for the different phenotypic expressions. Both limb and extraocular muscle sections show ragged-red fibers with trichrome stains, due to increased numbers of abnormal sarcolemmal mitochondria. Therapy with vitamins and agents such as Coenzyme Q10 aims to improve respiratory chain activity (CitationBresolin et al 1988), but their efficacy is still unproven (CitationMcFarland et al 2002).
Figure 1 Horizontal saccades in CPEO due to mitochondrial myopathy; these horizontal movements are slow and disjunctive (note different size of movements of the right and left eyes). Positive values indicate rightward movements. Eye movements were recorded using the magnetic search coil technique (CitationLeigh and Zee 2006).
The neuromuscular junctions of the extraocular muscles
Fibers with single and multiple nerve end plates have different acetylcholine receptor isoforms. Thus, on the one hand, adult skeletal muscle and the levator of the eyelid possess only the adult isoforms of the acetycholine receptor. On the other hand, in extraocular muscle, the adult epsilon isoform is expressed in singly innervated fibers, whereas the fetal gamma subunit is expressed in multiply innervated fibers (CitationFraterman et al 2006). Another important difference between extraocular and skeletal muscle is that synaptic folding is sparse in most types of extraocular fibers; thus, the “safety factor” is likely to be low, making them susceptible to neuromuscular fatigue in myasthenia gravis. Only the pale global fibers, which are responsible for the large acceleration of saccades, have well developed synaptic folding (CitationSpencer and Porter 2006). Electromyographic studies of the extraocular muscles indicate that global fibers discharge mainly for saccades, but are not as active as orbital layers during eccentric gaze holding (CitationScott and Collins 1973).
Myasthenia gravis revisited
Although several independent factors may contribute to the predominant involvement of the extraocular muscles in myasthenia gravis, the differences in synaptic folding make several predictions concerning the involvement of eye movements in myasthenia: (1) eye muscles will be more commonly affected that skeletal muscles in myasthenia and (2) saccades may remain fast despite limited range of movement (leading to “quiver movements”) because of synaptic folding in pale global fibers, which probably generate the high acceleration of these movements (CitationKaminski et al 1990, Citation2002; CitationPorter and Baker 1996). A second reason for sparing of pale global fibers is that they are only called upon to contract during saccades. These ideas were originally put forward by Cogan and colleagues (CitationCogan et al 1976; CitationYee et al 1976), before the fine structure of the neuromuscular junctions of the extraocular muscles had been studied.
Recent studies of saccades in ocular myasthenia support these postulates (CitationKhanna et al 2007). Thus, although the relationship between the peak velocity and amplitude of saccades (the main-sequence relationship) is more variable in ocular myasthenia than that in normal subjects (CitationBarton and Sharpe 1995), perhaps no other condition that myasthenia causes restricted movement of the eyes with fast saccades; this paradox may be due to selective sparing of pale, global fibers. Further, although conjugacy of eye movements is quite variable (presumably reflecting fluctuations in tenuous neuromuscular transmission), the initial component of saccades is consistently similar in each eye. This latter feature has been demonstrated using phase-plane analysis, in which time differences between the abducting and adducting eyes are removed (). Thus, it seems that the global pale fibers of yoke-muscle pairs contract similarly at the onset of saccades (achieving large peak velocities) even though subsequent eye drifts may be very disjunctive.
Figure 2 Comparison of true internuclear ophthalmoplegia due to multiple sclerosis (MS) (A and B) with pseudo-internuclear ophthalmoplegia due to ocular myasthenia gravis (MG) (C and D). Note that both patients show slower adducting movements (evident on the velocity channels of the time plots). However, on the corresponding binocular phase planes, the initial movements of abducting and adducting eyes are conjugate for the myasthenic (D – arrow) but differ from the onset of the movement for the patient with true INO (C – arrow). Positive values indicate rightward movements. Eye movements were recorded using the magnetic search coil technique (CitationLeigh and Zee 2006).
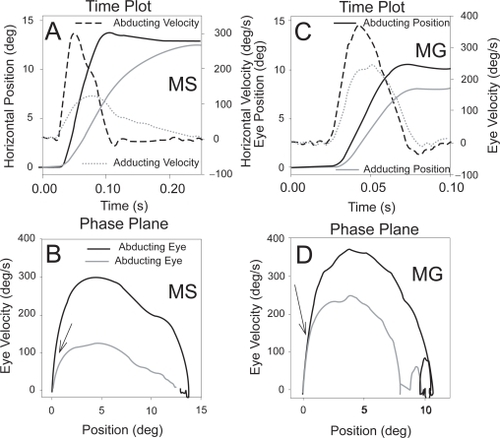
It is also possible that other mechanisms may affect the extraocular muscles to produce clinical presentations such as INO. Thus, disturbance of ion channels may be responsible for some forms of congenital myasthenic syndromes (CitationColomer et al 2006), and the calcium channelopathy, episodic ataxia type 2, may present the appearance of INO (CitationRucker et al 2005).
The role that central adaptive mechanisms play in patients with impaired eye movements
As noted in the prior section, patients with neuromuscular disorders (especially ocular myasthenia) as well as neuropathies affecting the ocular motor nerves (especially Miller Fisher syndrome) may present with eye movements that suggest central rather than peripheral disease. Thus, internuclear ophthalmoplegia, gaze palsies, one-and-half syndrome, and dorsal midbrain syndrome may all be mimicked by these peripheral disorders. In some cases of Miller Fisher syndrome, it is possible that there is some involvement of brainstem or cerebellum, since an overlap with Bickerstaff’s encephalitis has been demonstrated (CitationWillison and Yuki 2002). However, central involvement is not the case in myasthenia gravis. What other explanation could account for disorders of the ocular motor periphery mimicking central disorders? Insights are provided from the results of the edrophonium (Tensilon) test, which sometimes causes saccadic hypermetria (overshoots) or even square-wave oscillations may occur. This occurs because the brain has made adaptive increases in the level of innervation (in response to weakness) and, with reversal of the neuromuscular block by edrophonium, saccadic eye movements are now too large, leading to oscillations around the target. This is a classic example of how central adaptation can contribute to the overall picture in peripheral disorders causing diplopia. Similarly, in generalized neuropathies that affect eye movements, such as Miller Fisher syndrome, the disorder of ocular motility often mimics central disorders and, at least in part, central adaptive processes contribute to this behavior (CitationLeigh and Zee 2006). Thus, in diseases affecting the extraocular muscles, their nerves and motoneurons, both peripheral and central factors may combine to produce unusual disturbances of ocular motility.
Conclusion
Advances in understanding the structure and function of the extraocular periphery have provided a number of insights and resolved some stubborn paradoxes. One attraction of studying eye movements is that the relationship between the discharge of motoneurons and rotations of the eye seemed much simpler than the control of limb movements. The latter is surely still true, but now the means by which the extraocular muscles rotate the eye involves an apparently more complex mechanism – fibromuscular pulleys; however, pulleys may actually simplify the neural commands required to rotate the eye and also account for a range of disorders of ocular motility that were not previously understood. Even if the brain does delegate aspects of 3-D rotations of the globe to the orbital tissues, there is abundant evidence that central factors play an important role in adaptive processes, which themselves may cause patients to present with the appearance of central disorders.
Funding
Supported by NIH grant EY06717, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and the Evenor Armington Fund.
References
- BartonJJSSharpeJA1995“Saccadic jitter” is a quantitative ocular sign in myasthenia gravisInvest Ophthalmol Vis Sci361566727601637
- BielschowskyA1940Lectures on motor anomaliesHanover, New HampshireDartmouth College Publications
- BresolinNBetLBindaA1988Clinical and biochemical correlations in mitochrondrial myopathies treated with coenzyme Q10Neurology3889293368070
- BriggsMMSchachatF2002The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscleJ Exp Biol20531334212235193
- BrockingtonMAlsanjariNSweeneyMG1995Kearns-Sayre syndrome associated with mitochondrial DNA deletion or duplication: a molecular genetic and pathological studyJ Neurol Sci13178877561952
- CartaAD’AddaTCarraraF2000Ultrastructural analysis of extraocular muscle in chronic progressive external ophthalmoplegiaArch Ophthalmol11811415
- ClarkRAMillerJMRosenbaumAL1998Heterotopic muscle pulleys or oblique muscle dysfunctionJ AAPOS2172510532362
- CoganDGYeeRDGittingerJ1976Rapid eye movements in myasthenia gravis. I. Clinical observationsArch Ophthalmol9410835938289
- ColomerJMullerJSVernetA2006Long-term improvement of slow-channel congenital myasthenic syndrome with fluoxetineNeuromuscul Disord163293316621558
- DaroffRBSolitaireGBPincusJH1966Spongioform encephalopathy with chronic progressive external ophthalmoplegia. Central ophthalmoplegia mimicking ocular myopathyNeurology1616195948507
- DemerJL2004Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lectureInvest Ophthalmol Vis Sci457293814985282
- DemerJLPoukensVMillerJM1997Innervation of extraocular pulley smooth muscle in monkeys and humansInvest Ophthalmol Vis Sci381774859286266
- FratermanSKhuranaTSRubinsteinNA2006Identification of acetylcholine receptor subunits differentially expressed in singly and multiply innervated fibers of extraocular musclesInvest Ophthalmol Vis Sci4738283416936094
- FromentyBCarrozzoRShanskeS1997High proportions of mtDNA duplications in patients with Kearns-Sayre syndrome occur in the heartAm J Med Genet71443529286453
- HaslwanterT1995Mathematics of three-dimensional eye rotationsVision Res351727397660581
- KaminskiHJMaasESpiegelP1990Why are the eye muscles frequently involved in myasthenia gravisNeurology40166391700335
- KaminskiHJRichmondsCRKusnerLL2002Differential susceptibility of the ocular motor system to diseaseAnn N Y Acad Sci956425411960792
- KearnsTPSayreGP1958Retinitis pigmentosa, external ophthalmoplegia and complete heart blockArch Ophthalmol602809
- KhannaSLiaoKKaminskiHJ2007Myasthenia revisited: New insights from pseudo-internuclear ophthalmoplegiaJ NeurologyIn press.
- KjellgrenDThornellLEVirtanenI2004Laminin isoforms in human extraocular musclesInvest Ophthalmol Vis Sci454233915557425
- KlierEMMengHAngelakiDE2006Abducens nerve/nucleus stimulation produces knematically correct three-dimensional eye movementJ Neurosci262732716525052
- KonoRPoukensVDemerJL2005Superior oblique muscle layers in monkeys and humansInvest Ophthalmol Vis Sci46In Press
- LeighRJZeeDS2006The neurology of eye movements4th ed[book/DVD]New YorkOxford University Press
- McFarlandRTaylorRTurnbullD2002The neurology of mitochondrial DNA diseaseLancet134351
- MillerJMDemerJL1997New orbital constraints on eye rotationsFetterMHaslwanterTMisslischHTweedDThree-dimensional kinematics of eye, head, and limb movementsThe NetherlandsHarwood Academic Publishing
- MoraesC1996Mitochondrial disordersCurrent opinion in neurology936789011305
- MorganDLProskeU1984Vertebrate slow muscle: Its structure, pattern of innervation, and mechanical propertiesPhysiol Rev64103696320233
- OhSYClarkRAVelezF2002Incomitant strabismus associated with instability of rectus pulleysInvest Ophthalmol Vis Sci4321697812091413
- PorterJD2002Extraocular muscle: Cellular adaptations for a diverse functional repertoireAnn NY Acad Sci95671611960789
- PorterJDBakerRS1996Muscles of a different ‘color’: The unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic diseaseNeurology463078559415
- PorterJDBakerRSRagusaRJ1995Extraocular muscles: basic and clinical aspects of structure and functionSurv Ophthalmol39451847660301
- RichardsonCSmithTSchaeferA2005Ocular motility findings in chronic progressive external ophthalmoplegiaEye192586315272295
- RuckerJCJenJStahlJS2005Internuclear ophthalmoparesis in episodic ataxia type 2Ann N Y Acad Sci1039571415827025
- RuskellGL1999Extraocular muscle proprioceptors and proprioceptionProg Retin Eye Res182699110192514
- RuskellGLHaugenI-BKBruenechJR2005Double insertions of extraocular rectus muscles in humans and the pulley theoryJ Anatomy206295306
- ScottABCollinsCC1973Division of labor in human extraocular muscleArch Ophthalmol90319224746649
- ShallMSGoldbergSJ1992Extraocular motor units: type classification and motoneuron stimulation frequency-muscle unit force relationshipsBrain Res5872913001525662
- ShoffnerJM1996Maternal inheritance and the evaluation of oxidative phosphorylation diseasesLancet348128388909383
- SpencerRFPorterJD2006Biological organization of the extraocular musclesProg Brain Res1513379
- TaylorRWTurnbullDM2005Mitochondrial DNA mutations in human diseaseNat Rev Genetics638940215861210
- TianJZeeDSWalkerMF2005Eye-position dependence of torsional velocity during step-ramp pursuit and transient yaw rotation in humansExp Brain Res16
- Van OpstalAJHeppKSuzukiY1996Role of monkey nucleus reticularis tegmenti pontis in the stabilization of Listing’s planeJ Neuroscience16728496
- WilichowskiEGrutersAKruseK1997Hypoparathyroidism and deafness associated with pleioplasmic large scale reqrrangements of the mitochondrial DNA: a clinical and molecular genetic study of four children with Kearns-Sayre syndromePediatric Res41193200
- WillisonHJYukiN2002Peripheral neuropathies and anti-glycolipid antibodiesBrain125259162512429589
- WongAMTweedDSharpeJA2002Vertical misalignment in unilateral sixth nerve palsyOphthalmology10913152512093657
- YeeRDCoganDGZeeDS1976Rapid eye movements in myasthenia gravis. II. Electro-oculographic analysisArch Ophthalmol94146572962656
- Yu Wai ManCYChinneryPFGriffithsPG2005Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disordersNeuromuscul Disord15172315639116
- ZeeDSWalkerMFRamatS2002The cerebellar contribution to eye movements based upon lesions: binocular three-axis control and the translational vestibulo-ocular reflexAnn N Y Acad Sci9561788911960803