Abstract
This subject-masked, randomized, active and placebo-controlled study compared subjects’ perceptions of two antibiotic ophthalmic drops. One hundred and twenty-five healthy volunteers received two of the following solutions: moxifloxacin 0.5% ophthalmic solution (Vigamox®, Alcon Laboratories, Inc., Ft Worth, TX, USA), azithromycin 1% in DuraSite® (AzaSite™, Inspire Pharmaceuticals, Inc., Durham, NC, USA), or Tears Naturale II® (Alcon Laboratories, Inc., Ft. Worth, TX, USA) in contralateral eyes. Immediately following instillation and at 1, 3, 5, and 10 minutes thereafter, subjects rated comfort, acceptability, and blurring on 0–10 point analog scales stating their preference of treatment. Among subjects receiving moxifloxacin and azithromycin in contralateral eyes, 84% preferred moxifloxacin. Moxifloxacin was rated more comfortable and acceptable with less blurring than azithromycin (p < 0.0001). These differences were observed in both the adult and pediatric populations. Ocular adverse events (redness, irritation, stinging, burning, dryness, itching and chemosis) were observed in 18 (17.3%) eyes receiving azithromycin and 1 (1%) eye receiving moxifloxacin. Moxifloxacin was significantly more tolerable than azithromycin in healthy adult and pediatric eyes. Tolerability and patient acceptance affect compliance; thus these data should be of significance to the clinician.
Introduction
Bacterial conjunctivitis is a common, acute, contagious disease characterized by inflamed conjunctiva with purulent mattering of the lids. Topical antibiotics have been shown to speed clinical recovery (CitationLeibowitz et al 1981; CitationGigliotti et al 1984). Since cultures are rarely performed, the treatment is typically empiric with broad spectrum topical antibiotics (CitationAmerican Academy of Ophthalmology 2003). This practice is supported by studies previously showing clinical conjunctivitis is caused by a bacterial organism between 55% and 78% of the time (CitationBuznach et al 2005; CitationPatel et al 2007).
Moxifloxacin 0.5% ophthalmic solution (Vigamox®, Alcon Laboratories, Inc., Ft Worth, TX, USA) is a fourth generation fluoroquinolone currently indicated for 13 isolates of bacterial conjunctivitis (CitationVigamox 2003). It is a broad spectrum, concentration-dependent, bactericidal antibiotic (CitationBryskier 2005; CitationWispelway 2005). Conversely, azithromycin is derived from the parent class of macrolides known to be bacteriostatic and time-dependent (CitationBryskier and Bergogne-Bérézin 2005; CitationCarbon 1998). While it is suggested that concentration is a factor in the antibacterial effect of azithromycin, given the current high level of Gram-positive resistance patterns, this agent demonstrates a time-dependent, bacteriostatic effect. Azithromycin 1% formulated in DuraSite® (AzaSite™, Inspire Pharmaceuticals, Inc., Durham, NC, USA) is currently indicated for 5 bacterial isolates (CitationAzasite 2007). The persistent pace of evolution, however, indicates bacterial susceptibility to a systemic antibiotic may change over time (CitationChaudhry et al 1999; CitationGoldstein et al 1999; CitationAlexandrakis et al 2000; CitationMaguen and Morgan 2007). The clinical efficacy of an antibiotic can also be undermined by patient compliance.
Compliance with topical therapy is often inadequate in ophthalmology (CitationShaya 2005; CitationBradshaw 2006). For instance, in glaucoma patients the rate of non-adherence to prescribed treatment regimens ranges from 17.3% to 44% (CitationKass et al 1986; CitationKass et al 1987; CitationKonstas et al 2000; CitationGurwitz et al 1993). Comfort, convenience, and ease of administration would be expected to influence compliance with a specific eye drop dosing schedule (CitationWinfield et al 1990; CitationPatel and Spaeth 1995). One study found subjects preferred eye drops requiring fewer instillations and with less blurring and stinging (CitationJampel et al 2003). The recommended treatment regimen for azithromycin 1% in DuraSite® is 9 doses/week (CitationAzasite 2007) and the recommended treatment regimen for moxifloxacin 0.5% is 21 doses/week (CitationVigamox 2003). However, the frequency of dosing is not the only variable, nor necessarily the most important variable affecting compliance. It has been recently suggested that “pain” and “irritation” scales could have important implications for compliance with ocular antibiotics (CitationDonnenfeld et al 2004).
This study assessed other factors which may affect patient compliance including drop preference, comfort, acceptability, blurring, residue, and sensation. The purpose of this study was to compare subjects’ perceptions of moxifloxacin and azithromycin in healthy adult and pediatric subjects.
Subjects and methods
Subjects 9 years of age or older with normal ocular health were enrolled in this single center, single-visit, subject-masked, randomized, active, and placebo-controlled study. To determine eligibility for the study, medical and ophthalmic histories were taken, best corrected ETDRS visual acuity (BCVA) was measured, and a slit lamp biomicroscopy exam was performed. All subjects were healthy volunteers with ETDRS visual acuity ≤0.3 LogMAR, who had not used contact lenses or ophthalmic medications recently (within 5 days). Subjects had no recent history of disease (ocular or systemic) or ocular surgery. Written informed consent was obtained for each qualified subject. Institutional review board approval was obtained from Coast IRB.
A trained technician instilled one drop of each study medication in the eyes of 125 healthy volunteers. Adult subjects were randomized in a 3:1:1 ratio and pediatric subjects were randomized in a 5:1:1 ratio, according to the following treatment scheme:
One drop of moxifloxacin HCL 0.5% ophthalmic solution (moxifloxacin) in one eye and one drop of azithromycin 1% in DuraSite® ophthalmic solution (azithromycin) in the fellow eye or
One drop of moxifloxacin in one eye and 1 drop of Tears Naturale II® (placebo) (Alcon Laboratories, Inc, Ft Worth, TX, USA) in the fellow eye or
One drop of azithromycin in one eye, and 1 drop of placebo (Tears Naturale II®) in the fellow eye.
After drop comfort, acceptability, blurring, residue, and preference assessments had been completed at all 5 time points (0, 1, 3, 5, and 10 minutes post-instillation), BCVA and slit lamp biomicroscopy exams were performed. As a safety measure, BCVA and slit lamp results before and after drop instillation were compared. Adverse events, both elicited and observed, were monitored. The study was conducted in compliance with Good Clinical Practices and the International Conference on Harmonization guidelines.
Chi-square analysis was used to compare the treatment preference between groups. Average comfort, acceptability and blurriness scores were compared using paired t-test in subjects receiving moxifloxacin and contralateral azithromycin, moxifloxacin and contralateral placebo, and azithromycin and contralateral placebo. In addition, a pooled analysis of all subjects receiving moxifloxacin and azithromycin (ie, scores in all subjects receiving either antibiotic even when administered with contralateral placebo) was also performed and scores compared using paired t-test.
Results
Demographics
One hundred and twenty-five subjects were enrolled and randomly assigned to a treatment group; 84 subjects (34 adults and 50 children) received moxifloxacin and contralateral azithromycin, 21 subjects (11 adults and 10 children) received moxifloxacin and contralateral placebo, and 20 subjects (10 adults and 10 children) received azithromycin and contra-lateral placebo. Sixty-one percent of subjects were female and 97% were Caucasian. The treatments groups were balanced for gender and race distribution.
Overall preference
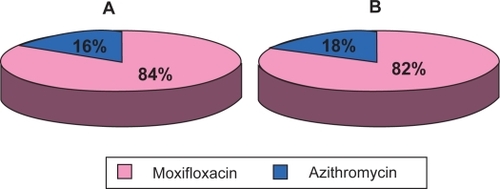
When asked if they preferred the drop in their left eye or the drop in their right eye, more subjects chose the eye instilled with moxifloxacin (p < 0.0001). In both pediatric and adult subjects receiving moxifloxacin and azithromycin simultaneously there was a significant preference for moxifloxacin over azithromycin at every time point (p ≤ 0.0001 and p ≤ 0.0002, respectively). An average of 84% of all subjects and 82% of pediatric subjects preferred moxifloxacin over azithromycin (). Subjects who received azithromycin and contralateral placebo (n = 20) significantly preferred placebo at most (4/5) time points (p < 0.01). For subjects receiving moxifloxacin and contralateral placebo (n = 21) there was no difference between treatments in terms of preference at any time point.
Figure 1 Preference among subjects receiving moxifloxacin or azithromycin. (A) Preference for all subjects (n = 84) who received both antibiotics. (B) Preference for pediatric subjects (n = 50) who received both antibiotics. Pie charts based on an average of subjects at each visit who preferred either agent. Chi-square test comparison at each visit was statistically significant, p ≤ 0.0001.
Comfort, acceptability, and blurriness
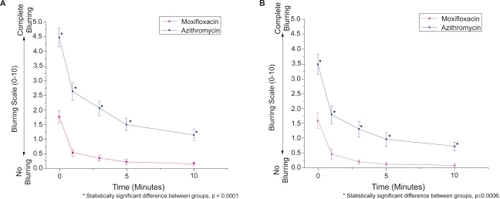
In subjects receiving moxifloxacin and azithromycin simultaneously, moxifloxacin was significantly more comfortable (p < 0.0001), acceptable (p < 0.0001), and resulted in less blurring (p < 0.0001) than azithromycin at every time point (–). These statistical differences were seen in both the pediatric and adult populations (p < 0.02). Pooled analysis comparing antibiotics (ie, moxifloxacin and azithromycin eyes from subjects receiving contralateral placebo and subjects receiving both antibiotics contralaterally) also demonstrated favorable comfort, acceptability, and blurriness scores for moxifloxacin (p < 0.0001 for each parameter) over azithromycin.
Figure 2 Average comfort score at each time point among subjects receiving moxifloxacin or azithromycin. (A) Average comfort scores for all subjects (n = 84) who received both antibiotics. (B) Average comfort scores for pediatric subjects (n = 50) who received both antibiotics. Errors bars indicate standard error of the mean.
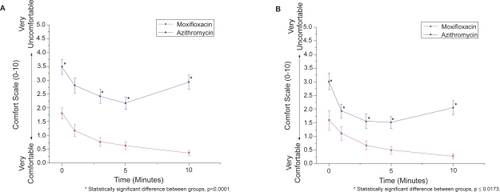
Figure 3 Average acceptability score vs. time among subjects receiving moxifloxacin or azithromycin. (A) Average acceptability scores for all subjects (n = 84) who received both antibiotics. (B) Average acceptability scores for pediatric subjects (n = 50) who received both antibiotics. Errors bars indicate standard error of the mean.
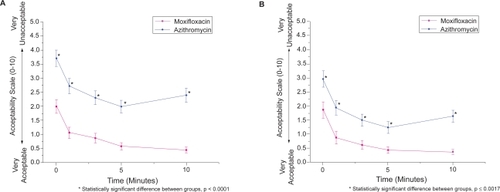
Figure 4 Average blurring score vs. time among subjects receiving moxifloxacin or azithromycin. (A) Average blurring scores for all subjects (n = 84) who received both antibiotics. (B) Average blurring scores for pediatric subjects (n = 50) who received both antibiotics. Errors bars indicate standard error of the mean.
Comparison of the comfort, acceptability, and blurriness scores for subjects receiving moxifloxacin with contralateral placebo demonstrated no difference in comfort scores between moxifloxacin and placebo at 4 of the 5 time points. Furthermore, there were no differences in acceptability or blurriness scores between moxifloxacin and placebo at any time point. In subjects receiving azithromycin with contralateral placebo, azithromycin was significantly less comfortable (p < 0.003) and acceptable (p < 0.03) compared to placebo at every time point. Azithromycin also resulted in blurred vision significantly more (p < 0.02) than placebo at every time point.
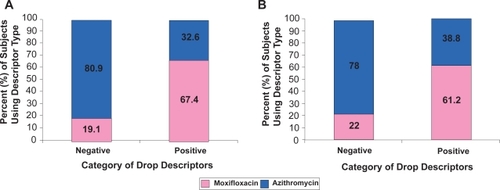
Drop descriptors
Subjects were asked to describe how each drop felt with three words. Descriptors were categorized as positive and negative. If the subject thought the drop was “comfortable”, “cool”, “refreshing”, “smooth”, or “soothing”, that was considered a positive indicator of tolerability. If the subject thought the drop was “achy”, “burning”, “gritty”, “irritating”, “sticky”, “filmy”, “thick”, or “stinging”, that was considered a negative indicator of tolerability. The percentages included in represent the relative frequency a certain type of descriptor was used within each of the antibiotic treatment groups. A greater percentage of negative descriptors were reported with azithromycin compared to moxifloxacin.
Figure 5 Percent of categorized descriptors attributable to each antibiotic drop. Total frequency of negative and positive descriptors used among (A) all subjects (n = 84) who received both antibiotics and (B) pediatric subjects (n = 50) who received both antibiotics. Positive descriptors included “comfortable, cool, refreshing, smooth or soothing” and negative descriptors included “achy, burning, gritty, irritating, sticky, filmy, thick, or stinging”.
Residue
Subjects were given a mirror and asked if they noticed any residue around their eyes. Residue was categorized as either “absent” or “present.” Residue was more frequently seen around eyes which received azithromycin (27.4%) compared to eyes which received moxifloxacin (4%) (p < 0.0001). The difference in residue incidence among pediatric subjects receiving both antibiotics was significant (p < 0.04) at all time points (residue was present in 28.4% of eyes receiving azithromycin and 4% of eyes receiving moxifloxacin). Among adult subjects, the difference was significant (p < 0.03) at most (3/5) time points.
Safety
Mild post-instillation changes were observed during slit lamp examinations. Twenty-seven ocular adverse events were observed in 19 subjects during the study, 6 among adult subjects and 13 among pediatric subjects. One subject receiving moxifloxacin experienced 2 ocular adverse events and 18 subjects receiving azithromycin experienced 25 adverse events (). Some of the adverse events were similar to the negative descriptors observed during the study (ie, burning, stinging, irritation). One non-ocular adverse event was observed in a subject who received both antibiotics (post-nasal drip).
Table 1 Reported ocular adverse events (AEs)
Discussion
In this study results for our primary endpoints were statistically in favor of moxifloxacin without a single dissenting data point. In every population significantly more subjects preferred moxifloxacin over azithromycin. Moxifloxacin was more comfortable and acceptable to subjects and resulted in less blurred vision than azithromycin. These differences were observed in both the adult and pediatric populations. Unlike azithromycin which was generally less tolerable than placebo, moxifloxacin was similar to placebo in tolerability.
Formulation differences between moxifloxacin and azithromycin may explain the differences in tolerability measures. Moxifloxacin is self-preserved at an approximate pH of 6.8, while azithromycin is preserved with 0.003% benzalkonium chloride (BAK) at a pH of approximately 6.3. Solutions which are more acidic than average tear film (pH 7.35) may induce ocular discomfort (Bartlett and Jaanus 1995). For the azithromycin formulation, BAK may affect drop tolerability (CitationFurrer et al 2002). In addition, the DuraSite® component in the azithromycin formulation may increase the length of time BAK is in contact with the corneal surface. DuraSite® may also be responsible for the negative acceptability and blurring attributes found in this study. Both antibiotics have approximately the same osmolality (290 mOsm/kg), which is within the limits of normal tear osmolality (ie, between 280 and 330 mOsm/kg) (CitationFarris et al 1981; CitationCraig and Tomlinson 1995).
At 10 minutes post-instillation, comfort and acceptability scores for azithromycin were higher than previous time points, indicating less comfort and acceptability over time ( and ). It is possible that the relative discomfort seen at the 10 minute time point is related to viscosity differences between azithromycin in DuraSite® and the other study drops. Studies of artificial tears indicate increased viscosity in eye drops increases the incidence of blurred vision, stickiness, and eyelash residue (CitationLaMott et al 2002; CitationNoecker 2006). In this study, we found azithromycin was frequently described with viscosity-related words like “sticky”, “thick”, and “filmy” (mentioned 33.1%, 30.2%, and 26.7% of the time, respectively, compared to 1.7%, 1.2%, and 4.8% for moxifloxacin). In addition, we found subjects receiving azithromycin more frequently noticed residue around their eyes compared to the other study drops.
Complaints from subjects after the last (10 minute) time point were considered adverse events. Mild ocular complaints between time points (ie, ≤10 minutes post-instillation) were considered drop descriptors, not adverse events. Some of the negative words used frequently to describe azithromycin drops within 10 minutes of instillation were similar to adverse events observed in eyes treated with azithromycin after 10 minutes post-instillation. This suggests some adverse events were not aberrations but instead persistent symptoms of ocular sensations. Overall, the incidence of ocular adverse events was higher in azithromycin-treated eyes (17.3%) than moxifloxacin-treated eyes (1.0%).
Missing doses of prescribed eye drops can undermine the clinical efficacy of ophthalmic anti-infective treatment (CitationKonstas et al 2000). Many variables affect compliance, for example: gender, number of prescribed medications, and treatment cost (CitationGurwitz et al 1993; CitationKonstas et al 2000; Patel and Spaeth 2005). Additionally, some subjects may be unintentionally noncompliant because they do not instill drops correctly (CitationWinfield et al 1990; CitationKonstas et al 2000). Compliance can also be affected by the tolerability of an agent with less tolerable agents increasing patient noncompliance. In the present study, azithromycin was significantly less comfortable or acceptable than moxifloxacin in the eyes of healthy volunteers. In diseased eyes (inflamed eyes with vascular dilation and mucopurulent discharge) it is also expected that the tolerability of both antibiotics would be even worse.
Compliance is also affected by dosing of a medication. The recommended treatment course for azithromycin is only 9 drops/week and the recommended treatment course of moxifloxacin is 21 drops/week. Agents requiring fewer doses may be expected to result in greater compliance; however, less frequent dosing should be considered in light of the drug’s tolerability. If an agent is not well tolerated, the less frequent dosing regimen may not be advantageous. In addition, ocular tolerability may indirectly affect the efficacy of an antibiotic drop for current and future patients. The package insert for azithromycin 1% warns “skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance” (CitationAzasite 2007). This concern regarding skipping a dose becomes more important for azithromycin where each drop represents 11% of the full treatment. Conversely, the threat of resistance would be expected to be less for moxifloxacin where one missed dose only represents 4.7% of full treatment.
In conclusion, 84% of subjects receiving moxifloxacin and azithromycin prefer moxifloxacin drops. Tolerability endpoints provide a number of possible explanations for this preference. The moxifloxacin drop is consistently more comfortable and more acceptable than the azithromycin drop and produces less blurring than the azithromycin drop. Azithromycin is also more frequently characterized with negative descriptors within 10 minutes of instillation and induces ocular adverse events after 10 minutes post-instillation more frequently (17.3% for azithromycin-treated eyes, compared to 1% of moxifloxacin-treated eyes). Since tolerability is expected to affect compliance, especially in children, these data are clinically important and suggest that moxifloxacin should be the preferred therapy for the treatment of bacterial conjunctivitis.
Tradenames are property of respective owners.
Acknowledgements
This study was supported by Alcon Laboratories, Inc. Drs Granet and Lichtenstein serve as consultants for Alcon.
References
- AlexandrakisGAlfonsoEMillerD2000Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolonesOphthalmol1071497502
- The American Academy of Ophthalmology2003Preferred practice pattern for conjunctivitis [online]. Accessed 10 October, 2007. URL: http://www.aao.org/education/guidelines/ppp/conjunctivitis_new.cfm
- Azasite [package insert]2007 Inspire Pharmaceuticals, Durham, NC.
- BradshawJ2006Ophthalmology: spreading the word on compliancePaediatr Nurs182117193914
- BartlettJDJaanusSD2005Clinical ocular pharmacologyBoston, MAButterworth-Heinemann411
- BryskierA2005FluoroquinolonesBryskierAAntimicrobial agents: antibacterials and antifungalsWashington, DCASM Press668788
- BryskierABergogne-BérézinE2005MacrolidesBryskierAAntimicrobial agents: antibacterials and antifungalsWashington, DCASM Press475526
- BuznachNDaganRGreenbergD2005Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance eraPediatr Infect Dis J24823816148850
- CarbonC1998Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogensClin Infect Dis2728329675445
- ChaudhryNAFlynnHWMurrayTG1999Emerging ciprofloxacin-resistant Pseudomonas aeruginosaAm J Ophthalmol1285091010577596
- CraigJPTomlinsonA1995Effect of age on tear osmolalityOptom Vis Sci7271378570160
- DonnenfeldEPerryHDChruscickiDA2004A comparison of the fourth-generation fluoroquinolones gatifloxacin 0.3% and moxifloxacin 0.5% in terms of ocular tolerabilityCurr Med Res Opin201753815537475
- FarrisRLStuchellRNMandelID1981Basal and reflex human tear analysis. I. Physical measurements: osmolarity, basal volumes, and reflex flow rateOphthalmology8885277322504
- FurrerPMayerJMGurnyR2002Ocular tolerance of preservatives and alternativesEur J Pharm Biopharm532638011976014
- GurwitzJHGlynnRJMonaneM1993Treatment for glaucoma: adherence by the elderlyAm J Public Health8371168484454
- GigliottiFHendleyJOMorganJ1984Efficacy of topical antibiotic therapy in acute conjunctivitis in childrenJ Pediatr10462366323667
- GoldsteinMHKowalskiRPGordonYJ1999Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year reviewOphthalmology1061313810406613
- JampelHDSchwartzGFRobinAL2003Patient preferences for eye drop characteristics: a willingness-to-pay analysisArch Ophthalmol121540612695251
- KassMAMeltzerDWGordonM1986Compliance with topical pilocarpine treatmentAm J Ophthalmol101515233706455
- KassMAGordonMMorleyRE1987Compliance with topical timolol treatmentAm J Ophthalmol103188933812621
- KonstasAGMaskalerisGGratsonidisS2000Compliance and viewpoint of glaucoma patients in GreeceEye14752611116698
- LeibowitzHMHyndiukRASmolinGR1981Tobramycin in external eye disease: a double-masked study vs. gentamicinCurr Eye Res1259667030632
- MaguenEMorganMA2007A case of vancomycin-resistant enterococcus conjunctivitis and its clinically successful topical treatmentCornea26223417251818
- LaMotteJORidderWHKuanT2002The effect of artificial tears with different CMC formulations on contrast sensitivityInvest Ophthalmol Vis Sci ARVO poster 3151.
- NoeckerRJ2006Comparison of initial treatment response to two enhanced-viscosity artificial tearsEye Contact Lens321485216702870
- PatelSCSpaethGL1995Compliance in patients prescribed eyedrops for glaucomaOphthalmic Surg2623367651690
- PatelPBDiazMCBennettJE2007Clinical features of bacterial conjunctivitis in childrenAcad Emerg Med141517119185
- ShayaFT2005Compliance with medicineOphthalmol Clin North Am18611716314223
- Vigamox [package insert]2003Fort Worth, TXAlcon Laboratories, Inc
- WinfieldAJJessimanDWilliamsA1990A study of the causes of non-compliance by patients prescribed eyedropsBr J Ophthalmol74477802390523
- WispelwayB2005Clinical implications of pharmacokinetics and pharmacodynamics of fluoroquinolonesClin Infect Dis41S1273515942879