Abstract
While investigative ophthalmologists access peer-reviewed journals as part of their daily routine, and while they regularly visit scientific congresses, they rarely peruse patent documents as an information source. Among the reasons for this negligence are the incompatibility of patent search algorithms with those known from journal databases, a legalistic and frequently redundant language, and misconceptions about the nature of the patenting system. Here we present key data and analyses from the ophthalmology module of a patent database system that we are developing to address some of these problems. We show that international patent applications consistently reflect developer interest in the ocular drug and diagnostics field; that they are technically focused lead indicators of developments that frequently feature in peer-reviewed patenting only much later; and that patenting targets are well aligned with the unmet therapeutic needs of populations in industrialized countries. Most applications (74%–78% in years since 2006) are supported with experimental data, and most (on average, 80%–90%) faced at least one objection to patentability during their initial stage of examination. In contrast to the peer-reviewed scenery that is highly diverse, the corresponding patenting arena shows a pronounced focus on the United States.
Introduction
Ophthalmologists, like most other medical specialists, obtain scientific information mainly from two sources: from the peer-review literature, and from scientific congresses that cover their specialty. This provides an impressive amount of information: MEDLINE covers more than 50 peer-reviewed periodicals which exclusively focus on ocular medicine, and there are about 100 additional journals which have a broader scope or a different focus but which regularly publish papers on eye medicine. The number of substantial international congresses that compete for the time and attention of research ophthalmologists amounts to at least a dozen in any given year. However, there is another extremely important source of scientific and technical information – patent documents – which is utilized to a much lesser degree if at all, even though its basic data sources are universally accessible without charge.
There are several reasons why most researchers hardly ever peruse intellectual property documents to an extent comparable to their use of the peer-reviewed literature. First, the primary purpose of a patent is to unequivocally establish the applicant’s priority rights to the claimed invention. Scientists and clinicians are not accustomed to the legalistic language of intellectual property documents, which makes the extraction of information difficult for them. Second, many aspects of the criteria for novelty, nonobviousness and utility as used by the patent system differ substantially from what the scientific community believes these terms stand for. Third, and most importantly, public patent databases cannot be meaningfully searched using intuitive standardized and structured keywords in the way MEDLINE can be searched using MESH terms. They have their own hierarchically nested document classification system that is incomprehensible to anybody who is not a patent specialist. As a result, the knowledge contained in the “patent information space” is largely inaccessible to the average researcher or clinician, even though the raw data is available online.
This is an extremely wasteful situation because patent documents provide a perspective of applied science and technology that is substantially different from what is offered by peer-reviewed papers, which are primarily written to communicate new insights and to advance basic understanding.
This general dilemma fully translates to research, development, and practice of clinical ophthalmology, where the specialist journals rarely cite patents as scientific references. There is no reasonably quick and easy way to search for patent documents of substantial ophthalmological content that would return a set of data that is highly focused and complete. If ocular research periodicals specifically address patenting at all, it is to discuss controversial uses of the system,Citation1,Citation2 or to investigate the interaction of sponsorship with research.Citation3,Citation4 Systematic reviews of ocular patenting activity are usually published in journals that are focused either on patentingCitation5–Citation9 or on general drug discovery and development.Citation10,Citation11
We are currently developing a database system that is designed to make patent information more accessible to scientists and clinicians in select specialties; one of its modules focuses on ophthalmology. Here we present an analysis of the key features of international ocular patenting that highlights its potential relevance for the development of therapeutic and diagnostic products.
Methods
Using internally optimized and validated search algorithms supplemented with manual searches, we have exhaustively identified patents centered on ocular subjects from the Patent Convention Treaty (PCT) public database PatentScope(R).Citation12 A document was included in our collection if it had ocular drugs, drug delivery, tissue culture, gene therapy, or diagnostics (including biomarkers) as at least one of its major subjects, or if it had these ocular uses as a secondary subject but reported experimental data that directly supported such applications. The time frame for publication dates was the period between January 1986 and June 2008. Document key data were captured in a local SQL database. Document content was indexed according to the Medical Subject Headings (MeSH)-controlled vocabularyCitation13 which MEDLINE employs for indexing articles, and textual content was processed for metadata.
While the inclusion of substantial experimental data in patent applications is not mandatory (such data can be submitted during the examination process up to one year after filing the patent), their presence in the published version is obviously crucial for the document’s utility as a source of scientific information. As one of its sets of metadata, our database flags each document for the presence or absence of such substantial experimental data that support one or more of the claims.
PCT patent documents are either published together with an international search report (ISR), or this report is published separately within the subsequent months (unless the application is retracted or fails to meet the formal criteria). ISRs are standardized patent office replies where the examiner cites the references on which the initial rejection of individual claims for alleged lack of novelty or inventiveness are based. This amounts to the approximate equivalent of the initial stage of an open peer review. Critical rejections are of two types: type X, which is based on a single earlier document, and type Y which is based on the content of two or more documents considered together. The combined number of X and Y rejections can be considered a surrogate measure of the novelty and inventiveness of the claimed invention as judged by the examiner. For each document for which an ISR was available we have captured the number and type distribution of such rejections.
Results
Time profile of applications, 1986–2008
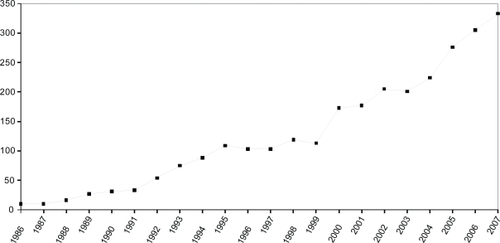
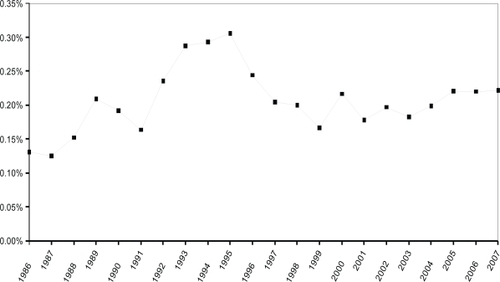
A total of 2,967 PCT ophthalmology documents were identified. The annual count of published applications increased dramatically and almost consistently during the 22.5 year period investigated, from 10 documents each in 1986 and 1987 to 333 in 2007. Based on the data from the first half of the year we expect almost 400 patents to be published in 2008 (Figure ). However, most of this growth reflects the steep increase in the overall number of PCT documents published by the World Intellectual Property Organization. The fractional share of “predominantly ocular” documents meeting our inclusion criteria – a surrogate measure for the relative interest in ophthalmology patenting – exhibited far less variation (between 0.13% and 0.31% of all published PCT documents; Figure ). An initial increase until 1995 was followed by a pronounced drop that was reversed in 2000. Subsequently there has been a slow but steady increase in relative interest, and the figure has remained constant at 0.22% for the most recent 2005–2007 period. A deeper exploratory analysis with temporal linkage to the peer-review literature (data not shown) suggests that the relative peak period which is evident for the 1993–1994 period might have been a precursor to the surge in research attention to ophthalmology triggered by the discovery of the role of vascular endothelial growth factor (VEGF) in retinal disease.
Language and origin of applications, 1999 to mid-2008
Of the 2199 applications published between January 1999 and June 2008, 1821 (82.8%) were in English, and therefore immediately accessible to virtually the entire community of research ophthalmologists. The second most common language was Japanese, at 12.6%. The other languages allowed by the PCT system were rarely used; German, French, and Spanish accounted only for 1.9, 1.2 and 1.0%, respectively and there were only five applications made in Russian and three in Chinese.
Analyzing the priority countries (ie, the countries were the initial patent filing was submitted) for all documents published since 1986, we found the following distribution: 69.8% came from the United States; 17.0% originated from individual European countries or from the European Patent Organization; 12.3% came from Japan; and only 0.9% came from elsewhere. In recent years the balance has shifted towards a further strengthening of the United States’ position (data not shown) because major European patentees in the ophthalmology drugs and diagnostics scenery have merged with US companies. (For example, Pharmacia – which used to file first in Sweden – has been absorbed into Pfizer.)
Presence of supporting data and pattern of initial claim rejections
The results of this dual analysis are presented in Table . Since 1999 the percentage of PCT ophthalmology documents that present substantial experimental data in support of their claims has varied between 63% and 84% but has remained almost constant (in the range of 74%–78%) for the applications published since January 2006. The number of published applications without “X” or “Y” rejections (ie, those which received an tentative “clean bill of patentability” during initial examination) remained relatively constant (10%–19%) from 1999 to 2003, and then began to fluctuate. The low figure for 2007 is not due to a higher number of yet unpublished search reports but reflects a real decrease in expressed objections.
Table 1 Selected metadata for ocular drug, tissue culture and diagnostic patents, 1999–2008
Targets of recent ocular patenting
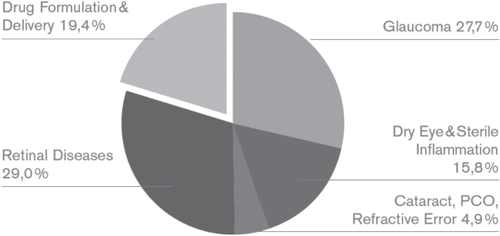
An analysis of the patenting targets for the most recent two-year publishing period with complete information (October 2006 to September 2007) revealed the pattern presented in Figure . Among the patent documents that had a single or predominant application focus, retinal diseases and glaucoma headed the list of target conditions, followed by dry eye syndrome and the group comprised of lens and refractive disorders. About one fifth of all applications dealt with ocular drug delivery technologies.
Case study in ocular patenting: iontophoretic drug delivery
Since our database features a MeSH term index in addition to patent codes, it allows identical parallel searches using MEDLINE conventions. To illustrate how ophthalmological patenting can supplement or anticipate information published in academic journals, we have conducted a search for all international patent documents published between 1986 and June 2008 for which ocular iontophoretic drug delivery is the primary subject of the invention.
The retrieved set of 18 PCT documents revealed no dedicated document for ocular iontophoresis prior to August 1999. (Although several earlier applications mention that the subject drugs of the respective invention can be administered to the eye by iontophoresis, they do not point out workable examples.) This is quite surprising because United States national patents with a main focus on this subject go back to at least 1950, and the pertinent peer-reviewed literature can be traced back even to the 1910s. However, by the 1990s the actual use of this technology had diminished considerably and few clinical ophthalmologists were familiar with it.
In 1999 the business development arm of the Hebrew University of Jerusalem published the first international patent application which claimed a iontophoretic drug delivery device employing a hydrogel probe.Citation14 It took five more years for this system to appear in the peer-reviewed literature.Citation15 But developers had already taken note and from 2002 onward a steady stream of patent applications were published. The first ones came from the iontophoresis specialist company Iomed, Inc., (Salt Lake City, UT, USA), covering what soon became known as the OcuPhor™ device, and applications of this device to deliver vascular endothelial growth factor (VEGF) inhibitor aptamers,Citation16 interferons,Citation17 methotrexate,Citation18 and steroidsCitation19 to the eye. A clinical study published in 2003 reported the first clinical data obtained with this device.Citation20 While ocular iontophoresis of the corticosteroid, dexamethasone had been reported in the peer-reviewed literature as early as 1989,Citation21 methotrexate delivery featured there only in 2007.Citation22 For interferons no ocular iontophoretic delivery has been reported in the nonpatent literature even until today. However, applications involving oligonucleotide aptamers including those blocking VEGF actions appeared almost at the same time as the respective Iomed patent application, in a paper by the French research organization INSERMCitation23 which had been preceded by a PCT patent document jointly filed by Optis France (Paris) and INSERM.Citation24 Optis (which later became Eyegate Pharma) followed up with several additional applications outlining the design of its various devices.Citation25–Citation30 While Iomed’s ocular iontophoresis developments came to an end in 2007 when it was acquired by a medical device company, another industrial player from Salt Lake City, Aciont, Inc., had emerged with its first patent application including data and claims for ocular iontophoresis in 2004.Citation31 Later applications addressed devices for the delivery of steroid-sparing immunosuppressive agents (including monoclonal antibodies)Citation32 and two new types of devices.Citation33,Citation34 The inventor team has published peer-reviewed papers on the general subject,Citation35,Citation36 but no clinical studies have been reported from Aciont so far.
There are only two other PCT patent assignees in this field, both of whom produced single documents. One came from Ceramatec, Inc.Citation37 (another Salt Lake City company), and one from the University of South Florida.Citation38 In both cases the inventors have published on iontophoretic drug delivery, but only in fields other than ophthalmology.
Discussion
For reasons of research capacity, our database of international patent applications in ophthalmology does not yet cover medical devices that are not related to drug delivery (such as intraocular lenses or glaucoma shunts unless they elute drugs, or surgery and diagnostic equipment such as lasers or cameras). However, in its present state – with coverage of drugs, gene therapy, tissue- and cell-based technologies, and diagnostics from 1986 to present – it has already revealed indications of the value which a user-friendly database on ocular patenting that is tightly selected, comprehensive, and annotated for content would have as a scientific information resource for the clinical ophthalmology field.
Using the MEDLINE-compatible index feature we were able to show, in a case study of patent applications centered on iontophoretic drug delivery to the eye, that these documents can contain technical information of high potential relevance to clinical development. We have also shown that much of this information emerges in peer-review papers only later, or in many cases not at all. Even where information is apparently duplicated it is presented in a different context and from a different perspective, making it a valuable asset for researchers and developers.
Approximately three quarters of all international applications from our database include experimental data. At least for the ocular drug and diagnostic field, the frequently expressed opinion that patents tend to make exhaustive claims without offering hard data does not seem broadly valid. Even in those cases where such data support only some of the claims, or where they would not be considered sufficiently elaborate or precise by the peer reviewers for an academic paper, they are of considerable value in the technical context of the application. Frequently they are among the first hard data that become available in reproducible form. In another analysis we show that patent examiners tend to be quite rigid and thorough in their initial rejections, with only a small fraction of ocular applications not drawing formal objections at the start of the review process. We have not investigated to which degree the applicants have overcome such opposition in the subsequent national phases of patenting.
As has recently been shown for drug development efforts targeting Alzheimer’s disease,Citation39 patenting activity is a leading indicator of developer interest in any given competitive segment of the medical sciences. We have provided a preliminary analysis for the ocular drugs and diagnostics segment that suggests that this attention, while largely consistent over recent times, might also reflect developer responses to new insights that are believed to have the potential to result in marketable products.
Our analysis of patenting targets from a recent 2-year period reveals that the distribution of disease targets closely resembles the relevance of the respective disorders for eyesight problems in the industrialized nations. It is no surprise to see that typical eye diseases of developing countries are hardly addressed in patenting since their effective treatment would in most cases not require patentable innovation.
Our ocular patent database still has considerable limitations. International patent applications that are published in languages other than English are not yet properly represented in our content index. We are currently working on identifying and including the English versions of such documents, which become accessible if and when the applicant files the corresponding US and/or European patent applications. Finally, because our database is centered on international PCT applications it does not yet contain documents for which no PCT filings were made. A pilot investigation that we have undertaken for the year 2005 indicates that the exclusion of US-only patents might have caused 10%–15% of the total ocular patents to be missed in the analysis. This factor is likely to play a significantly larger role in the years prior to about 1995, ie, in the time before the ophthalmology developer community utilized the PCT filing system as intensely as it does today. Future expansions of our database will also include applications made only in the United States and Europe.
In summary, we are in the process of building a database covering substantially all inventive activity in ophthalmology that has led to patent applications in the major intellectual property systems. This will provide a unique resource for developers in the field, amounting to the opening of a “third space of information” (peer-review literature and scientific congresses being the first two dimensions). Further plans include features such as interfaces with public databases and integrated visualization of query results.
Disclosure
The authors report no conflicts of interest in this work.
References
- PackerSEthics and medical patentsArch Ophthalmol1999117824610369598
- KottowMHPatenting medical proceduresArch Ophthalmol2000118114010922218
- EllweinLBKrollPNarinFLinkage between research sponsorship and patented eye-care technologyInvest Ophthalmol Vis Sci19963724955038933766
- NovackGDThe role of pharmaceutical companies in sponsored researchOphthalmology20071141037817544773
- ClarkABingamanDKapinMOcular angiostatic agentsExp Opin Ther Patents20001042748
- De WitDLightmanSEmerging approaches to the treatment of uveitis: patents of 2000–2004Exp Opin Ther Patents20051586174
- JanoriaKGHariharanSDasariCRRecent patents and advances in ophthalmic drug deliveryRecent Patents on Drug Delivery and Formulations2007116170
- SallamAJajakumarSLightmanSIntraocular delivery of anti-infective drugs-bacterial, viral, fungal and parasiticRecent Patents Anti-Infect Drug Disc200835363
- ConwayBRRecent patents on ocular drug delivery systemsRecent Patents on Drug Delivery and Formulations2008218
- GurtlerFGurnyRPatent literature review of ophthalmic insertsDrug Dev Industrial Pharmacy199521118
- MuckeHAMNew ocular therapeutics: a view from the patenting perspectiveIDrugs200710374117187313
- PATENTSCOPE(R) Search Service[www.wipo.int/pctdb/]GenevaWorld Intellectual Property Organization
- Medical Subject Headings[http://www.nlm.nih.gov/mesh/]BethesdaNational Library of Medicine – National Institutes of Health
- DombAFrucht-PeryJShapiroMinventorsHadasit Medical Research Services and Development Co. Ltd. and Yissum Research Development Co of the Hebrew University of Jerusalem, assigneesA device for iontophoretic administration of drugsPCT patent application WO 99/40967. 1999 Aug 19.
- Eljarrat-BinstockERaiskupFFrucht-PeryJHydrogel probe for iontophoresis drug delivery to the eyeJ Biomater Sci Polym Ed20041539741315212325
- LloydLBParkinsonTMSzlekMinventorsIomed, Inc., assigneeOcular iontophoretic device and method for inhibiting vascular endothelial growth factor (VEGF) using the samePCT patent application WO 02/058786. 2002 Aug 1.
- ParkinsonTMSzlekMLloydLBinventorsIomed, Inc., assigneeOcular iontophoretic device and method of usePCT patent application WO 02/058789. 2002 Aug 1.
- WarrenSHamiltonSinventorsIomed, Inc., assigneeMethods for treating neoplastic, angiogenic, fibroplastic, and/or immunosuppressive ocular irregularities via administration of methotrexate based medicaments, and ocular iontophoretic devices for delivering methotrexate based medicamentsPCT patent application WO 03/007961. 2003 Jan 30.
- ParkinsonTMSzlekMLloydLBinventorsIomed, Inc., assigneeOcular iontophoretic device and method for using the samePCT patent application WO 03/008036. 2003 Jan 30.
- ParkinsonTMFergusonEFebbraroSTolerance of ocular iontophoresis in healthy volunteersJ Ocul Pharmacol Ther2003191455112804059
- LamTTEdwardDPZhuXATransscleral iontophoresis of dexamethasoneArch Ophthalmol198910736871
- Eljarrat-BinstockEDombAJOrucovFMethotrexate delivery to the eye using transscleral hydrogel iontophoresisCurr Eye Res2007327–86394617852187
- BerdugoMValamaneshFAndrieuCDelivery of antisense oligonucleotide to the cornea by iontophoresisAntisense Nucleic Acid Drug Dev2003131071412804037
- De BizemontTSennlaubFBehar-CohenFinventorsOptis France S.A.Institut National de la Sante et de la Recherche Medicale (INSERM), assigneesGene therapy with chimeric oligonucleotides delivered by a method comprising a step of iontophoresisPCT patent application WO 02/083184. 2002 Oct 21
- BeharFRoyPinventorsOptis France S.A. assigneeDevice for delivering medicines by transpalpebral electrophoresisPCT patent application WO 03/030989. 2003 Apr 17.
- BeharFRoyPinventorsOptis France S.A. assigneeDevice for medicine delivery by intraocular iontophoresis or electroporationPCT patent application WO 03/043689. 2003 May 30.
- RoyPKleinsingerAinventorsOptis France S.A. assigneeOcular device for current controlled variable delivery of active principlesPCT patent application WO 2004/105864. 2004 Dec 9.
- RoyPinventorOptis France S.A. assigneeIrritation-reducing ocular iontophoretic devicePCT patent application WO 2005/115281. 2005 Dec 8.
- RoyPinventorEyegate Pharma S.A. assigneeOcular iontophoresis device for delivering siRNA and aptamersPCT patent application WO 2006/072887. 2006 Jul 13
- RoyPinventorEyegate Pharma S.A. assigneeOcular iontophoresis devicePCT patent application WO 2007/099406. 2007 Sep 7.
- HiguchiWIMillerDJLiKSinventorsAciont, Inc., assigneeMethods and systems for controlling and/or increasing iontophoretic fluxPCT patent application WO 2004/077012. 2004 Sep 10.
- HiguchiJKochambilliRTuitupouAinventorsAciont, Inc. assigneeOcular administration of immunosuppressive agentsPCT patent application WO 2007/038687. 2007 Apr 5.
- HiguchiJWLiKSinventorsAciont, Inc., assigneeIntraocular iontophoretic device and associated methodsPCT patent application WO 2007/050645. 2007 May 3
- LiKSHiguchiJWinventorsAciont, Inc., assigneeMethod and device for minimally invasive site specific ocular drug deliveryPCT patent application WO 2008/013913. 2008 Jan 31.
- LiSKZhangYZhuHInfluence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transportJ Pharm Sci2005948476015736190
- MolokhiaSAJeongEKHiguchiWIExamination of penetration routes and distribution of ionic permeants during and after transscleral iontophoresis with magnetic resonance imagingInt J Pharm20073351–2465317236728
- JoshiAVinventorCeramatec Inc., assigneeNovel iontophoretic drug delivery systemsPCT patent application WO 03/039622. 2003 May 15.
- HellerRJaroszeskiMJGilbertRAinventorsUniversity of South Florida, assigneeElectroporation device and method for delivery to ocular tissuePCT patent application WO 2005/123183. 2005 Dec 29.
- LecanuLPapadopoulosVCutting-edge patents in Alzheimer’s disease drug discovery: anticipation of potential future treatmentsRecent Patents CNS Drug Discov2007211322