Abstract
Aim:
To evaluate proton-beam radiotherapy (PBRT) in the management of uveal melanoma in Scotland.
Methods:
A retrospective review was undertaken on all patients receiving PBRT for uveal melanoma (1994–2005). Data obtained included: gender, past ocular/medical history, age, presenting complaint(s), diagnosis, laterality, tumor location/ultrasound characteristics, visual acuity (VA) and intraocular pressure. At post-treatment reviews (3, 6, 12, and 24 months), the following data was obtained: VA, intraocular pressure, tumor appearance and ultrasound characteristics. Mean follow up was 38.8 months.
Results:
Seventy-six patients were included. Mean age was 64 years; male to female ratio was 1.1:1. Ninety-seven percent demonstrated initial treatment response; 87% had successful control of tumor growth. Mean pre-treatment tumor height was 6.2 mm v.s. 4.8 mm post-irradiation (p < 0.001). Pre-irradiation VA was <3/60 in 18.5% compared with 74% post-irradiation (p < 0.0001). There was a statistically significant association between adverse events (enucleation, metastasis) and greater maximal basal tumor diameter. Eighteen eyes were enucleated. The median survival time was estimated to be 54 months.
Conclusion:
In our experience, PBRT is a precise, reliable and effective treatment in the management of large, and previously treated uveal melanomas. It prevents enucleation in the majority at short term follow-up.
Introduction
Uveal melanoma is the most common primary adult ocular malignancyCitation1–Citation5 with an incidence of 0.6/100,000.Citation1 It is the only potentially fatal intraocular tumor in the adult.Citation2
The standard treatment of choroidal melanoma used to be primary enucleation.Citation4–Citation6 ZimmermanCitation7 in the 1970’s suggested this may not be the best management. Globe preserving management is now favored. Such techniques include: laser photocoagulation,Citation1,Citation8,Citation9 transpupillary thermotherapy,Citation1,Citation8,Citation10 local resection, radiotherapy (charged particle or brachytherapy) and, most recently, the gamma knife.Citation11, Citation12 Enucleation is reserved for an end stage disease.
Proton-beam radiotherapy (PBRT) was first utilized in the management of uveal melanoma in 1975.Citation13 It is now predominantly used for choroidal and ciliary body melanomas. Ocular complications may arise,Citation2,Citation5,Citation14 such as radiation retinopathy, neuropathy and cataract.Citation2,Citation14 They are largely dependent on tumor size and location.Citation2 This retrospective study was performed to evaluate the use of PBRT in the management of relatively large uveal melanoma and to review our treatment experience.
Methods
A retrospective case note review was performed on 76 patients who received PBRT for uveal melanoma. The diagnosis was established utilizing clinical examination features, photographs, and ultrasound findings. Histopathological confirmation was performed on all enucleation specimens. All patients were jointly managed at the Tennent Institute of Ophthalmology, Glasgow, Scotland (referral centre) and the Clatterbridge Centre for Oncology, Wirral, England (treatment centre).
Initially, some lesions underwent photocoagulation, local resection (medium sized equatorial lesions), or brachytherapy (maximal vertical tumor height [MVTH] of <5 mm) when they proved to be amenable to such modality of treatment. Multiple treatment failure was managed with enucleation. Patients were offered PBRT if their lesions were unsuitable for alternative treatments, if they demonstrated prior treatment failure or had iris/ciliary body tumors.
All patients were assessed by the same consultant ophthalmologist (EGK) and had ultrasound examinations performed by three consultant radiologists. All patients had irradiation planning and treatment delivered by the same multidisciplinary team.
PBRT protocol
Stage one involved surgical placement of tantalum clips to demarcate the tumor periphery. Stage two incorporated the simulation and planning of patient specific treatment. Cranial X-rays, fundus photographs, and ocular ultrasound data were utilized to create computer-generated patient eye/tumor models. Clip positions were used at simulation to plan individualized irradiation fields. Stage three involved delivery of PBRT in four consecutive, daily fractions (30 seconds each) to a total dose of 53.1 protons Gray (58 Gy Co-60 equivalents).
The following data were obtained for each patient: gender, past ocular/medical history, age at presentation, presenting complaint(s), ocular diagnosis, laterality, anatomical tumor location, tumor ultrasound characteristics [(MVTH) and maximal basal tumor diameter (MBTD)], best corrected visual acuity (BCVA), intraocular pressure (IOP) and PBRT data (total dose, duration, number of fractions, maximum exposure dose to various ocular structures). At post-treatment review (3, 6, 12, and 24 months), the following data was obtained: BCVA, IOP, tumor appearance (clinical examination, fundus photography) and ultrasound characteristics. The development of metastatic disease and patient mortality was documented. Tumor growth was monitored utilizing serial ultrasound examinations, clinical examination, and fundus photography. Treatment endpoints included evidence of tumor growth, enucleation, and patient death. The follow-up period was calculated from first treatment date until documentation of tumor growth, enucleation, or last clinic visit.
Statistical analysis was performed using p-value for assessment of statistical significance of tumor dimensions and changes in BCVA. Kaplan-Meier survival analysis was applied for assessment of the cumulative adverse event rate over time. The effects of baseline characteristics on subsequent adverse event rates were estimated using Cox proportion hazards models.
Results
Demographics
Seventy-six patients received PBRT for uveal melanoma between January 1994 and June 2005 (Table ). Seventy had a clinical diagnosis of choroidal melanoma and six had presumed iris/ciliary body melanoma. Nine patients had previously received treatment: Ruthenium-106 plaque (n = 4), local tumor resection (n = 2), and laser photocoagulation (n = 3). Patients were followed up for a mean of 38.8 months (range 3–122).
Table 1 Baseline characteristics
Tumor growth
Seventy-four patients (97%) demonstrated an initial response to treatment (stabilization of tumor growth, with or without regression). There was evidence of continued tumor growth <2 months post treatment in both remaining patients, one with extension along the optic nerve. Sixty-six (87%) patients had successful control of tumor growth throughout the entire follow-up period. Ten (13%) demonstrated evidence of long-term treatment failure (no response to treatment [n = 2], delayed tumor progression [n = 4], development of metastatic disease [n = 4], of which one patient died during follow-up). Three (out of 10) demonstrated both tumor progression and metastatic disease.
Pre- and post-irradiation characteristics
In patients for whom both ultrasound measurements were available, there was a statistically significant reduction in MVTH post treatment (N = 57; mean change, −1.65 mm; p < 0.001) (Table ). Fifty-one percent demonstrated a 1.5 mm decrease in height by one and a half years post-treatment. A reduced MBTD was also observed post-treatment, without reaching statistical significance (N = 70; mean change, −0.52 mm; p = 0.072).
Table 2 Pre- and post-irradiation clinical characteristics
Visual acuity
Fourteen patients (18.5%) had a BCVA of <3/60 prior to radiotherapy compared with 56 (74%) afterwards (p < 0.0001) (Table ). Seven patients showed post-treatment improvement in BCVA. Tumors in these cases were located inferotemporally (n = 5), superonasally (n = 1), and at the posterior pole in the remaining patient. In this improved BCVA subgroup, the mean (SD) pre treatment MBTD was 14 mm (2.4 mm) which reduced to 12.4 mm (1.2 mm) by the last visit. The mean (SD) MVTH was 6.0 mm (2.0 mm) and reduced to 4.4 mm (1.8 mm) by the last visit. Reduction in vision is mainly due to complications of treatment (42 patients, 55.2%).
Complications
The most common complication following PBRT was associated retinopathy. Rubeosis iridis was the second most common followed by cataract (Table ).
Table 3 Ocular complications of proton beam irradiation (multiple in some patients)
Enucleation and metastasis
Eighteen patients (24%, 11 were male) underwent enucleation during the follow-up period, of which 13 had associated retinal detachment. Indications for enucleation were: local tumor recurrence (n = 5), refractory glaucoma (n = 12), and optic nerve involvement (n = 1). Two patients had received prior treatment (laser and local resection). Enucleation was performed on average 22.3 months post-treatment.
Seven patients (four were male) developed clinically evident distant metastases during follow-up. One died from their disease during this period and one had histopathology confirmed disease (liver biopsy). Three presented with clinical evidence of distant metastases 11, 15, and 16 months post enucleation, respectively; two of these three patients showed initial tumor treatment response, the third did not. The remaining two patients developed clinically evident metastatic disease 25 and 31 months post-PBRT, respectively.
The mean age at diagnosis of metastasis was 60 years. The mean (SD) MBTD for this subgroup was 12.2 mm (2.8 mm) and MVTH was 6.2 mm (1.9 mm). All had hepatic involvement; two also demonstrated breast and spleen metastases. Six had accompanying secondary retinal detachment prior to PBRT.
The duration from treatment date to development of first adverse event (enucleation or late metastatic disease) or the end of follow-up was calculated for every patient; Figure shows the Kaplan-Meier estimate of the cumulative adverse event rate over time. The estimated survival at the maximal follow-up time was in excess of 50%, so that the median time until the first adverse event could not be estimated from these data. However, the lower 95% confidence boundary for the median survival time was estimated to be 54 months.
Figure 1 Kaplan-Meier estimated cumulative adverse event rate (enucleation or distant metastasis) compared with follow-up time after proton beam irradiation.
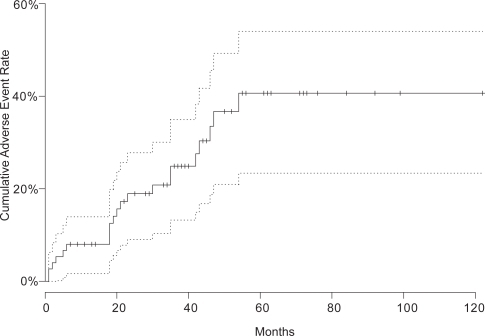
The effects of baseline characteristics on subsequent adverse event rates were estimated using Cox proportion hazards models. The results are presented in Table . The only association observed was an increased risk of adverse events in those with greater MBTD, with an estimated 14.1% increased hazard (95% CI 0.3−29.8, p = 0.045) for every 1 mm increase in basal diameter. This finding must be viewed with some caution, since several variables were tested in this way, and the observed association is of borderline statistical significance.
Table 4 Estimated effects of baseline characteristics on adverse event rates following treatment with proton beam irradiation–hazard ratios with 95% confidence intervals and p-values
Resolution of pre-treatment secondary retinal detachment
Secondary retinal detachment was present in 50 patients (66%) prior to irradiation treatment. All resolved during follow up, 46% within the first 3 months. Resolution of the detachment was noted prior to tumor regression.
Discussion
Proton beam irradiation treatment for uveal melanoma offers several advantages over other methods.Citation6,Citation15 It is a precise and reliable technique which achieves excellent tumor-normal tissue dose ratio.Citation6,Citation8,Citation14–Citation16 The density of ionization of protons increases markedly near the end of their path (Bragg peak). This characteristic enables accurate treatment, especially important for lesions close to vital ocular structures.Citation6,Citation14,Citation16 No handling of radioactive material is required by ophthalmologists dealing with PBRT, in contrast to brachytherapy where handling is required.Citation8 External beam radiotherapy with photons will always result in less favorable dose distribution than PBRT.Citation17 It appears that the use of gamma-knives leads to higher enucleation rate.Citation18
Demographics
The average patient age in this study population was slightly higher than other reports.Citation6,Citation10,Citation19–Citation21 Age however, was not found to be associated with treatment failure in our study (P = 0.47) and by others.Citation8
Women with uveal melanoma have previously been found to have more favorable outcomes than men.Citation8 This was not duplicated in our study, possibly due to relatively limited follow up time.
Growth
Local control of disease is defined as clinical evidence of cessation of tumor growth or evidence of tumor regression.Citation4 In this cohort, 87% demonstrated this following PBRT. Ultrasound examination revealed a statistically significant decrease (p < 0.001) in MVTH following treatment in the last visit. Other studies have shown less dramatic changes in tumor dimensions post-treatment.Citation22 The degree of regression response did not appear to be related to initial tumor size in this study. This finding is similar to previous reports.Citation19 Most of the treated tumors showed complete or partial regression after the first 6 months of treatment, with a usual range between 1 and 24 months.Citation19
Proton beam radiotherapy is an ocular-conserving option that may be considered for the treatment of extra-large uveal melanoma in carefully selected patients.Citation17,Citation21
Visual acuity
Visual acuity deteriorated post-treatment in most patients. Almost two thirds had a pre-treatment BCVA of ≥6/18 compared with almost three quarters having BCVA of <3/60 post-treatment, (P < 0.0001, McNemar’s test). This was most likely due to direct radiation damage of vital visual structures. This level of acuity still enables spatial awareness perception, otherwise not present in enucleation cases. Other studies have shown higher levels of retained BCVA post-PBRT.Citation4,Citation10,Citation23 It is difficult to compare study outcomes due to variable pre-irradiation VA levels, anatomical sites of the tumors, irradiation doses, and variable complications of treatment.
Male gender has been associated with poorer final BCVA.Citation5 This result was not reproduced in our study.
Complications
Thirty nine percent of our study population developed radiation retinopathy, higher than previously reported.Citation15,Citation21,Citation24 Proton beam-induced retinal ischemia predisposes to rubeosis iridis. Twenty one percent of our population developed this complication, Conway and colleagues showed higher rates.Citation21 Rubeosis iridis resulting in secondary refractory glaucoma was the most common indication for enucleation in our study, this finding is similar to other reports in the literature.Citation14 Tumor-induced angiogenic factors, inflammation secondary to necrosis of the melanoma, retinal detachment, and ischemic retina and iris from irradiation are possible stimuli.Citation25 The presence of subretinal fluid was not a significant risk factor in this study for the development of neovascular glaucoma. Similar findings have been reported by Kim and colleagues.Citation26 More recently Gragoudas and Lane found that larger tumor volume is the most significant factor associated with iris neovascularisation.Citation27 A similar finding was reported by Foss.Citation14 The early detection of neovascular glaucoma is important in the management of patients with uveal melanoma.Citation28 Rubeosis and neovascular glaucoma can be reduced when the anterior segment is spared.Citation25 With a very sharp Bragg peak of proton beam, however the irradiation of the anterior segment could be reduced; it resulted in a lower number of anterior segment complications requiring enucleation.Citation29
Resolution of pre-treatment secondary retinal detachment
Secondary retinal detachment resolved clinically in all cases prior to the evidence of tumor regression post-treatment. This finding has been confirmed by a previous study.Citation19 Secondary retinal detachments have been documented to develop after PBRT. None of our patients developed this. These have also been shown to resolve completely over time.Citation21
Enucleation
Secondary glaucoma and tumor growth were found to be the leading causes for enucleation following PBRT, both in this study and others.Citation30 The post-irradiation enucleation rate in our study (18/76, 24%) was higher than that previously reported by some groupsCitation4,Citation8,Citation14,Citation23,Citation30,Citation31 and lower than that reported by others (Table ).Citation21,Citation24 A higher probability of enucleation (46%) has been shown for very large tumors.Citation21,Citation29 Enucleation was performed on average 22.3 months post-treatment, which was slightly longer than demonstrated by Kodjikian and colleaguesCitation8 and shorter than reported by Finger.Citation4 The presence of retinal detachment prior to PBRT has been shown to be a significant risk factor for subsequent enucleation.Citation14 Our data was consistent with this finding although we had insufficient power to demonstrate this conclusively, with a hazard ratio of 2.01 (95%CI 0.74–5.52).
Table 5 Local control, metastasis, complications, and visual acuity after proton radiotherapy for uveal melanoma
Some would argue that enucleation following PBRT is inevitable and that primary enucleation should be advocated instead. However, only a minority of our study population required enucleation. Survival outcome therefore should not be a reason for suggesting enucleation. An increased fractionation scheme or treatment with combination of radiation and angiogenic agents could decrease functional loss and enucleation.Citation25
Zehetmayer launched a study using a stereotactic linear accelerator to avoid higher rate of enucleation, which is more convenient for delivery of fractionated treatment.Citation18
Metastasis
Seven patients developed metastases during follow up. Higher and lower rates have been reported (Table ).Citation8,Citation21,Citation23,Citation24,Citation29,Citation31 Maximum tumor vertical height and accompanying retinal detachment have both been established as recognized risk factors for the development of metastatic disease in uveal melanoma.Citation14 Our findings support this data.
Despite tumor regression, most melanoma-related metastatic disease manifests within the first five years post-treatment; Citation15,Citation32,Citation33 this finding is supported by our finding.
Six patients (7.9%) showed evidence of local recurrence following PBRT. This rate is in keeping with other literature reports.Citation19 Recurrence in our patients was first documented 0.5 to 63 months post-treatment. There are reports demonstrating a lower incidence with earlier recurrence time.Citation8,Citation23,Citation24 All six recurrent tumors in this study were large at diagnosis. All but one was located equatorially. The remaining was posteriorly located. These findings are echoed in the literature.Citation8
It is difficult to compare reported results referring to various radiation modalities because both dose and fractionation can be very different.Citation31 Table showed the least enucleation and metastasis rate in tumors (extra large or located too close to the optic nerve or fovea) treated with noninvasive linear accelerator-based stereotactic irradiation.Citation31 However, the prescribed radiation dose is higher than in the current study.
Other fractionation schedules might help to reduce the number of side effects without compromising local control.Citation31 It has now become possible to treat small to medium size ocular melanomas using stereotactic radiotherapy with acceptable dose distributions and target localizations. Stereotactic radiotherapy should provide a good alternative to proton therapy.Citation34
Conclusions
Proton beam irradiation treatment was used in this study primarily for the management of large choroidal melanomas and those having failed previous alternative treatment(s). Almost all (97%) patients demonstrated a response to treatment and 87% showed successful control of tumor growth throughout the entire follow-up period with an associated statistically significant reduction in MVTH post-treatment.
Best corrected visual acuity was markedly reduced (statistically significant) in the majority post treatment although notably those with inferotemporal, superonasal, and posterior pole tumors demonstrated an improvement in BCVA after treatment. Many patients required enucleation (24%), mainly due to refractory glaucoma. Less than 10% of patients developed clinically evident distant metastases during follow up, all involving the liver. This study demonstrated a statistically significant association between increased risk of adverse event (enucleation, metastasis) and greater maximal basal tumor diameter.
In our experience, PBRT is a precise, effective, and reliable technique which achieves excellent tumor–normal tissue dose ratio. It is an effective mode (although very expensive)Citation35,Citation36 of treatment in the management of large and previously treated choroidal melanomas and is associated with low rates of enucleation over the short term.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChiquetCGrangeJDAyzacLEffects of proton beam irradiation on uveal melanomas: a comparative study of Ki-67 expression in irradiated versus non-irradiated melanomasBr J Ophthalmol2000849810210611107
- GragoudasESLaneAMReganSA randomized controlled trial of varying radiation doses in the treatment of choroidal melanomaArch Ophthalmol2000118773810865313
- WrightPKDamatoBEAuditing outcomes after treatment of Scottish patients with uveal melanoma in LiverpoolJ R Coll Surg Edinb199944260410453150
- MindelJFingerPRadiation therapy for choroidal melanoma. Therapeutic reviewSurv Ophthamol19974221532
- MeliaBMAbramsonDHAlbertDMCollaborative Ocular Melanoma Study Group. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16Ophthalmology20011083486611158813
- GragoudasESGoiteinMVerheyLProton beam irradiation-An alternative to enucleation for intraocular melanomasOphthalmology198087571816251410
- ZimmermanLEMcLeanIWFosterWDDoes enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cellsBr J Ophthalmol1978624205352389
- KodjikianLRoyPRouberolFSurvival after proton-beam irradiation of uveal melanomasAm J Ophthalmol200413710021015183783
- FineSHow should we manage a patient with uveal melanoma?Arch Ophthaloml198510391011
- SpireMDevouassouxMSKodjikianLPrimary transpupillary thermotherapy for 18 small posterior pole uveal melanomasAm J Ophthalmol2006141840916678505
- MuellerAJTaliesSSchallerUCStereotactic radiosurgery of large uveal melanomas with the gamma-knifeOphthalmology20001071381710889116
- HaasAPinterOPapaefthymiouGIncidence of radiation retinopathy after high-dosage single-fraction gamma knife radiosurgery for choroidal melanomaOphthalmology20021099091311986096
- GragoudasESGoiteinMKoehlerAMProton irradiation of small choroidal malignant melanomasAm J Ophthalmol19778366573405869
- FossAJEWhelehanIHungerfordJLPredictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanomaBr J Ophthalmol199781748549422926
- KincaidMCForbergRTorczynskiEComplications after proton beam therapy for uveal malignant melanoma. A clinical and histopathologic study of five casesOphthalmology198895982912845323
- YoungLHYGragoudasESMacular uveal melanoma treated with proton beam irradiationRetina1994144368016461
- TokuuyeKAkineYSumiMFractionated stereotactic radiotherapy for choroidal melanomaRadiother Oncol19974387919165142
- ZehetmayerMKitzKMenapaceRLocal tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanomaRadiother Oncol2000551354410799725
- WilkesSRGragoudasESRegression patterns of uveal melanomas after proton beam irradiationOphthalmology19828984046289219
- EmaraKWeisbrodDJSahgalAStereotactic radiotherapy in the treatment of juxtapapillary choroidal melanoma: preliminary resultsInt J Radiat Oncol Biol Phys2004599410015093904
- ConwayRMPoothullilAMDaftariIKEstimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapyArch Ophthalmol20061248384316769837
- GragoudasESGoiteinMKoehlerAProton beam irradiation of choroidal melanomasArch Ophthalmol19789615839199132
- GragoudasESSeddonJMEganKLong-term results of proton beam irradiated uveal melanomasOphthalmology198794349533035451
- BrovkinaAFZarubeiGDCiliochoroidal melanomas treated with a narrow medical proton beamArch Ophthalmol198610440243006648
- GragoudasESProton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld LectureInvest Ophthalmol Vis Sci20064746667317065472
- KimMKCharDHCastroJLNeovascular glaucoma after helium ion irradiation for uveal melanomaOphthalmology198693189922419816
- GragoudasESLaneAMUveal melanoma: proton beam irradiationOphthalmol Clin North Am2005181111815763196
- LeeJLoganiSLakoshaHPreretinal neovasculaisation associated with choroidal melanomaBr J Ophthalmol20018513091211673295
- EggerEZografosLSchalenbourgAEye retention after proton beam radiotherapy for uveal melanomaInt J Radiat Oncol Biol Phys2003558678012605964
- EganKMGragoudasESSeddonJMThe risk of enucleation after proton beam irradiation of uveal melanomaOphthalmology1989961377822550868
- DieckmannKGeorgDZehetmayerMLINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experienceRadiother Oncol20036719920612812851
- RobertsonDMA rationale for comparing radiation to enucleation in the management of choroidal melanomaAm J Ophthalmol1989108448512801866
- LiWGragoudasESEganKMTumor basal area and metastatic death after proton beam irradiation for choroidal melanomaArch Ophthalmol2003121687212523887
- JaywantSMOseiEKLadakSStereotactic radiotherapy in the treatment of ocular melanoma: A noninvasive eye fixation aid and tracking systemJ Appl Clin Med Phys200341566112777151
- ConstableIJProton irradiation therapy for ocular melanomaTrans Opthalmol Soc U K197797430
- GragoudasESGoiteinMVerheyLProton beam irradiations of uveal melanomasArch Ophthalmol1982100928346284097