Abstract
Purpose:
To determine if clinical evaluation of parapapillary atrophy (PPA) significantly improves the ability to distinguish open-angle glaucoma (OAG) patients from glaucoma suspects.
Methods:
Patients in this study were under evaluation for glaucoma and had open angles, at least one reliable 24-2 SITA-standard automatic perimetry, and digital stereophotographs of the optic disc. PPA was identified clinically as a parapapillary region of absent (βPPA) or hyper/hypopigmented (αPPA) retinal pigment epithelium. A single masked observer evaluated photos for: vertical cup-to-disc ratio (CDR), clock hours of total and βPPA, βPPA as percentage width of the optic disc, presence or absence of βPPA at each disc quadrant, and ordinal rating of total PPA. Generalized linear models were used to determine odds of an abnormal or borderline glaucoma hemifield test (GHT) as a function of PPA variables and covariates; model fit was assessed using the log-likelihood ratio test.
Results:
Of 410 consecutive patients, 540 eyes (of 294 patients) met inclusion criteria. Mean age was greater among patients with abnormal compared with normal GHT (P < 0.001), but sex and race/ethnicity did not differ between groups (P ≥ 0.22). Age, central corneal thickness (CCT) and CDR (P ≤ 0.006), but not intraocular pressure (IOP) (P = 0.71), were significant univariable predictors of the odds of an abnormal GHT. All PPA parameters significantly predicted GHT (P ≤ 0.03), except presence of temporal βPPA (P = 0.25). Adjustment for age, CCT, IOP, and CDR reduced the association between PPA and GHT, and model fit was not greatly improved by addition of PPA variables.
Conclusions:
Addition of most PPA parameters to a model already containing commonly assessed variables including age, CCT, IOP, and CDR does not significantly improve the ability to distinguish OAG patients from glaucoma suspects.
Introduction
Glaucomatous optic neuropathy may be evaluated by direct or indirect ophthalmoscopy of the optic nerve, optic nerve photography, or computerized imaging technologies. Clinical features of glaucomatous optic neuropathy include atrophy of the retinal nerve fiber layer, focal or diffuse narrowing of the neuroretinal rim, optic disc splinter hemorrhage (DH) and parapapillary atrophy (PPA).Citation1–Citation3
PPA is a form of outer retinal atrophy that abuts the optic disc and can be divided into alpha (α) and beta (β) zones.Citation4–Citation6 Because this atrophy most often lies adjacent to but does not completely surround the nerve, the term parapapillary may be preferable to peripapillary atrophy, though they are used interchangeably in the literature. In βPPA the sclera and large choroidal vessels are visible, as the retinal pigment epithelium (RPE) and most of the photoreceptors are absent.Citation4,Citation5 In αPPA, there is an irregular arrangement of RPE cells that can result clinically in both hypo- and hyperpigmentation. The α zone is more peripheral than the β zone when both are present.
Of note, there are no imaging devices to provide automated assessment of DH or PPA, which at the present time are assessed either by patient examination or by photographic interpretation. Interestingly, several studies addressing the topic have found that PPA and DH tend to occur together in eyes and, additionally, tend to occur in the same regions of the eye, leading to the possibility that PPA may be useful as an indicator of increased likelihood of prior, present, or future disc hemorrhage.Citation7–Citation12 Because βPPA is present in 15%–20% of normal eyes, its presence is less specific for glaucoma than DH, which occurs only in 0.6% of healthy eyes.Citation11,Citation13 Given that DH is transient, lasting weeks to months, and that PPA is stable and progressive, it may be advantageous to rely on PPA parameters for glaucoma diagnosis and monitoring.Citation8,Citation12
αPPA and βPPA have been evaluated in glaucoma using quantitative analysis of optic nerve photographs (morphometry) typically by manually outlining and measuring the area of PPA using a slide projector, imaging processing software, or with confocal scanning laser ophthalmoscopy.Citation5–Citation7,Citation14–Citation20 Both αPPA and βPPA are larger and occur more frequently in eyes with glaucoma than in normal eyes, though βPPA is more specific for glaucoma.Citation5,Citation6,Citation14,Citation15 Using these morphometric techniques, PPA has been reported to be helpful in differentiating between normal and glaucomatous eyes.Citation5,Citation6,Citation14,Citation15,Citation21–Citation24
While many morphometric investigations of PPA in glaucomatous and normal eyes have reported significant differences between these 2 groups, there is a paucity of information on how clinical evaluation of PPA may guide the clinician in the diagnosis of open-angle glaucoma (OAG). Additionally, it is difficult clinically to estimate quantitative PPA parameters, such as area of PPA, due to its heterogenous shape. Despite this, in a clinical assessment using direct ophthalmoscopy alone, information including the PPA circumferential extent and amount of neuroretinal rim narrowing increased the sensitivity and specificity for detection of glaucomatous visual field loss.Citation1 However, some previous evaluations of PPA did not consider potential confounding variables such as age or intraocular pressure (IOP) that are typically available to the clinician at the time of diagnosis.Citation1,Citation5,Citation6,Citation14 In order to determine the clinical utility of PPA evaluation in glaucoma assessment, we developed a clinical PPA grading system and conducted this investigation to determine if clinical PPA assessment could improve the prediction of glaucomatous visual field loss beyond that of standard clinical variables such as age, central corneal thickness (CCT), IOP and vertical cup-to-disc ratio (CDR).
Patients and methods
This retrospective cross-sectional study was approved by the Institutional Review Board at Weill Cornell Medical College and New York Presbyterian Hospital. Patients under 18 years of age or with a history of corneal disease or keratorefractive surgery were excluded, as were patients with visually significant cataract (visual acuity <20/40), inflammatory eye disease, ocular trauma and non-glaucomatous optic neuropathy, or visual field loss.
Included patients were under evaluation for glaucoma and had documented open angles on examination. All patients had at least one reliable (fixation loss <33%; false-positive rate <33%; false-negative rate <33%) and repeatable 24-2 SITA-standard automatic perimetry with the Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA, USA). Patients were classified as having OAG if the glaucoma hemifield test (GHT) was borderline or outside normal limits; patients were classified as glaucoma suspects if the GHT was within normal limits. All patients had also undergone optic nerve digital stereophotography of acceptable or excellent quality with a Topcon TRC 50EX Retinal Camera (Topcon Co., Tokyo, Japan) within 18 months of inclusion. Demographic and clinical data including age, sex, self-reported race/ethnicity, CCT, and IOP on the day of examination were obtained by chart review.
One reviewer (NMR) evaluated optic nerve stereophotographs in a masked fashion using stereoscopic viewing lenses (). CDR was recorded. PPA was identified clinically as a parapapillary region of absent (βPPA) or hyper/hypopigmented (αPPA) retinal pigment epithelium. The circumferential extent of total (both α and β) PPA and βPPA were recorded in clock hours; the maximal radial βPPA extent (width) was estimated as a percentage of maximal optic disc dimensions (regardless of absolute optic disc size); the presence or absence of βPPA was assessed at each optic nerve head quadrant; and an ordinal rating (0–3) of total PPA was assigned as follows: grade 0 represented no clinically identifiable αPPA or βPPA; grade 1 represented any level of αPPA only; grade 2 represented any level of αPPA with mild and moderate βPPA; and grade 3 represented βPPA that was extensive either in circumference (12 clock hours) or volume (larger in area than the optic disc itself).
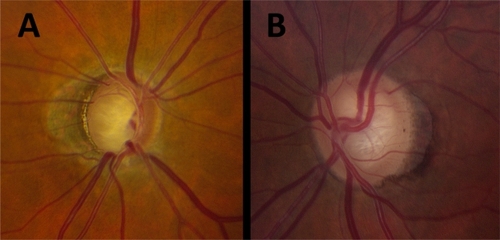
Figure 1 Clinical parapapillary atrophy (PPA) evaluation of 2 patients. A) Total PPA clock hours, 12; βPPA clock hours, 5; βPPA as percentage of disc, 60%; βPPA present, temporal; ordinal rating of total PPA, 3. B) Total PPA clock hours, 8; βPPA clock hours, 4; βPPA as percentage of disc, 20%; βPPA present, temporal; ordinal rating of total PPA, 2.
Statistical analyses were performed using Stata 11/IC (StataCorp LP, College Station, TX, USA). Differences in characteristics between patients with normal and abnormal/borderline GHT were assessed using two-sample t-tests or the chi-squared test. Differences in quantity of PPA between groups were assessed using generalized estimating equations to control for the inter-eye correlation of individuals; differences in geographic distribution of PPA were tested with the chi-squared test; and the Kruskal–Wallis test was used to test for differences in ordinal PPA rating. Generalized linear models with robust standard errors adjusted for clustering by patient were fit with a logit link function. Odds ratios (ORs) of an abnormal/borderline GHT were estimated as a function of increasing PPA and covariates. The likelihood-ratio test was used to calculate the statistical improvement in model fit when PPA parameters were added to models already containing age, CCT, IOP, and CDR. All statistical tests were 2-sided with a 0.05 level of significance.
Results
Patient characteristics, including PPA parameters, are presented in . Patients with OAG were significantly older than glaucoma suspects (61.9 versus 55.3 years; P < 0.001), and there was no difference in sex or ethnic/racial distribution between groups (P ≥ 0.22). Patients with OAG had significantly greater PPA than glaucoma suspects for all PPA variables measured (P ≤ 0.02) except for presence of temporal PPA (P = 0.25).
Table 1 Patient and parapapillary atrophy characteristics
Univariable logistic models were fit to predict the change in odds of OAG as a function of increasing age, CCT, IOP, and CDR (). Increasing age (OR = 1.4 per 10 years, 95% confidence interval (CI) 1.2, 1.6, P < 0.001) and CDR (OR = 1.3 per 0.1 units, 95% CI 1.2, 1.4, P < 0.001) but not IOP (P = 0.71) were significantly associated with greater odds of OAG. Likewise, increasing CCT was significantly associated with decreased odds of an OAG (OR = 0.9 per 10 μm, 95% CI 0.9, 1.0, P < 0.001).
Table 2 Simple logistic models: odds of open-angle glaucoma as a function of common clinical variables
Univariable logistic models were also fit to determine the association of PPA variables with OAG (). Again, an increase in each of the PPA parameters (P ≤ 0.03), except for presence of temporal βPPA (P = 0.30), was found to be significantly associated with an increase in the odds of OAG.
Table 3 Simple and multiple logistic models: odds of open-angle glaucoma as a function of parapapillary atrophy (PPA) variables
Each PPA variable was then added to a logistic model already containing age, CCT, IOP, and CDR, and the odds of OAG as a function of increasing PPA was determined when adjusting for covariates (). With adjustment for age, CCT, IOP, and CDR, the magnitude of association between nasal βPPA and OAG increased compared with the crude association and trended toward statistical significance (OR = 1.6, 95% CI 1.0, 2.7, P = 0.06). However, after adjustment for clinical covariates no other PPA variables were statistically significant predictors of the odds of OAG (P ≥ 0.10).
Finally, the likelihood-ratio test was used to calculate the change in statistical fit of the model containing age, CCT, IOP, CDR, and a single PPA variable compared with the same model without a PPA variable (). Inclusion of the nasal βPPA variable significantly improved model fit (X2[1] = 4.5, P = 0.03). Other PPA variables did not have a statistically significant impact on the fit of the logistic model (P ≥ 0.06). While the results of the likelihood-ratio test for total PPA clock hours (X2[1] = 3.6, P = 0.06) and ordinal rating of PPA (X2[3] = 6.8, P = 0.08) trended toward statistical significance, these variables were not significant independent predictors of OAG in their respective multivariable models. The logistic model containing age, CCT, IOP, and CDR correctly predicted the OAG status of 63.2% of eyes. Inclusion of total PPA clock hours in this model improved prediction of OAG status more than other PPA variables, and the percentage of eyes correctly classified increased by 1.2% to a total of 64.4%.
Discussion
Past investigations have demonstrated a strong association between PPA and glaucoma.Citation5–Citation7,Citation14–Citation17,Citation25–Citation28 The purpose of the current investigation was to determine if the clinician could utilize this association in the evaluation of glaucoma patients using visual inspection only. In this investigation, using our clinical grading scale, we replicated trends in the spatial and quantitative distribution of PPA that have been reported among patients with and without glaucoma.Citation6,Citation14,Citation15,Citation29 While PPA variables were, on their own, significantly predictive of the odds of OAG, this association was greatly attenuated by adjustment for 4 variables that comprise part of a typical glaucoma evaluation: age, CCT, IOP, and CDR. Furthermore, when values for these covariates were already known, modeling of the odds of OAG was not greatly improved by the consideration of PPA variables. This suggests that in clinically evaluating and diagnosing glaucoma there may be little incremental value to assessing PPA.
In the current study, patients with OAG had greater PPA that glaucoma suspects for every measure of PPA that was considered (though the difference was not statistically significant for βPPA of the temporal quadrant). Past studies have shown that βPPA tends to be larger in areas where the neuroretinal rim is focally narrow.Citation14 Also, as measured with planimetry, the spatial distribution of PPA differs in glaucoma, with PPA being present nasally in 5%–9% of normal patients and 21%–38% of glaucoma patients.Citation6,Citation14,Citation15 A similar trend was confirmed between patients with OAG and glaucoma suspects in the current study. However, the existence of nasal βPPA among 34.7% of OAG patients and only 18.3% of suspects indicates that nasal βPPA is not a sensitive predictor of disease. Past investigations have also demonstrated that PPA is most commonly located temporally, followed by inferotemporally and then superotemporally.Citation29 In the present study, βPPA was most commonly located temporally among both OAG patients and glaucoma suspects.
In a study by Jonas and colleagues, the investigators sought to determine which features of the optic disc best distinguished normal and glaucomatous patients.Citation20 The authors found that size of α and β zone PPA, assessed using planimetric techniques, were among the least useful optic disc characteristics for differentiating normal subjects from subjects with either preperimetric or perimetric glaucoma. The variables that consistently offered the greatest diagnostic power in the study by Jonas et al were: CDR, neuroretinal rim area, rim-to-disc area and cup-to-disc area.Citation20 While these findings largely agree with results from the current study, the current study did not morphometrically investigate optic disc parameters as was done by Jonas et al. Our investigation more specifically addressed the incremental importance of PPA variables, since in nearly all instances the clinician will have knowledge of a patient’s age, CCT, IOP, and some measurement of CDR. Like Jonas and colleagues, we determined that PPA variables are not the most important clinical variables for distinguishing glaucomatous and non-glaucomatous patients.
The present study also has the advantage of utilizing a multivariable technique that allows for outcomes to be modeled as a function of multiple variables, as often occurs in the clinical setting. In this study, we considered a model that contained four standard clinical glaucoma variables (age, CCT, IOP, and CDR) with and without the addition of PPA variables. The addition of most PPA variables to this model resulted in little incremental improvement in the modeling of our data. This suggests that the more parsimonious model – the model without PPA – more appropriately represents these data.
A growing literature suggests that PPA may be more useful for evaluating progression than for detecting glaucoma.Citation16–Citation18,Citation25 Studies have shown that PPA increases in size as glaucoma progresses and that it is not specific to the mechanism of glaucoma.Citation16–Citation18,Citation22,Citation25 In one report of eyes with progressive cupping, the area of PPA enlarged in 64%, as opposed to 17% of eyes with glaucoma that did not have progressive cupping.Citation16 Teng and colleagues showed that over a period of 3 years, glaucomatous eyes with β zone PPA were more likely to show evidence of visual field progression and that a greater area of βPPA was predictive of more rapid progression. As in the present study, Teng et al found that CCT was significantly associated with PPA. However, the association between visual field progression and presence of βPPA persisted in a multivariable model controlling for baseline demographic and ocular characeristics.Citation25 Likewise, Jonas et al found that the area of βPPA was significantly larger among patients with progressive disease and that progression was associated with age, and the area of βPPA and the neuroretinal rim.Citation17,Citation18 However, in a study of ocular hypertensives Quigley and colleagues did not find a significant difference in the prevalence of PPA between patients with and without progressive glaucoma.Citation30
The current investigation was retrospective in nature so its results cannot be extrapolated to determine risk or future odds of glaucoma. It is possible that patients with normal visual fields (glaucoma suspects) considered in the study might have had pre-perimetric glaucoma, and some of the glaucoma suspects considered might have been referred for evaluation in part based upon the presence of PPA. However, both of the above limitations are commonly encountered in clinical glaucoma management and may actually add to the relevance of this work. This study is less quantitative than past studies that have used planimetric measurement of PPA. However, the clinical grading system that was developed for this study was designed to more closely resemble the sort of evaluation routinely performed in a clinical glaucoma examination. Notwithstanding, by assessing the additional benefit conferred by inclusion of PPA evaluation as part of a standard glaucoma exam, this investigation contributes to the understanding of the association between PPA and OAG.
Disclosure
The authors report no conflicts of interest in this work, which was partially supported by a grant from the American Glaucoma Society.
References
- HarperRReevesBThe sensitivity and specificity of direct ophthalmoscopic optic disc assessment in screening for glaucoma: a multivariate analysisGraefes Arch Clin Exp Ophthalmol20002381294995511196356
- TheodossiadesJMurdochIWhat optic disc parameters are most accurately assessed using the direct ophthalmoscope?Eye (Lond)200115Pt 328328711450721
- JonasJBBuddeWMPanda-JonasSOphthalmoscopic evaluation of the parapapillary region of the optic nerve headKlin Oczna2004106Suppl 1–227928915510524
- KubotaTJonasJBNaumannGODirect clinico-histological correlation of parapapillary chorioretinal atrophyBr J Ophthalmol19937721031068435408
- JonasJBNaumannGOParapapillary chorioretinal atrophy in normal and glaucoma eyes. II. CorrelationsInvest Ophthalmol Vis Sci19893059199262722448
- JonasJBNguyenXNGusekGCNaumannGOParapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric dataInvest Ophthalmol Vis Sci19893059089182722447
- HayakawaTSugiyamaKTomitaGCorrelation of the peripapillary atrophy area with optic disc cupping and disc hemorrhageJ Glaucoma1998753063119786558
- RadcliffeNMLiebmannJMRozenbaumIAnatomic relationships between disc hemorrhage and parapapillary atrophyAm J Ophthalmol2008146573574018723142
- AhnJKKangJHParkKHCorrelation between a disc hemorrhage and peripapillary atrophy in glaucoma patients with a unilateral disc hemorrhageJ Glaucoma200413191414704537
- JonasJBMartusPBuddeWMInter-eye differences in chronic open-angle glaucoma patients with unilateral disc hemorrhagesOphthalmology2002109112078208312414418
- SugiyamaKTomitaGKawaseKDisc hemorrhage and peripapillary atrophy in apparently healthy subjectsActa Ophthalmol Scand199977213914210321526
- LawSKChoeRCaprioliJOptic disk characteristics before the occurrence of disk hemorrhage in glaucoma patientsAm J Ophthalmol2001132341141311530060
- BudenzDLAndersonDRFeuerWJDetection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment StudyOphthalmology2006113122137214316996592
- UhmKBLeeDYKimJTHongCPeripapillary atrophy in normal and primary open-angle glaucomaKorean J Ophthalmol199812137509753950
- XuLWangYYangHJonasJBDifferences in parapapillary atrophy between glaucomatous and normal eyes: the Beijing Eye StudyAm J Ophthalmol2007144454154617651676
- UchidaHIncreasing peripapillary atrophy is associated with progressive glaucomaOphthalmology19981058154115459709771
- JonasJBPredictive factors of the optic nerve head for development or progression of glaucomatous visual field lossInvest Ophthalmol Vis Sci20044582613261815277484
- JonasJBMartusPBuddeWMJünemannAHaylerJSmall neuroretinal rim and large parapapillary atrophy as predictive factors for progression of glaucomatous optic neuropathyOphthalmology200210981561156712153811
- KwonYHKimYIPereiraMLMRate of optic disc cup progression in treated primary open-angle glaucomaJ Glaucoma200312540941614520149
- JonasJBBerguaASchmitz-ValckenbergPPapastathopoulosKIBuddeWMRanking of optic disc variables for detection of glaucomatous optic nerve damageInvest Ophthalmol Vis Sci20004171764177310845597
- WilenskyJTKolkerAEPeripapillary changes in glaucomaAm J Ophthalmol1976813341345943951
- RockwoodEJAndersonDRAcquired peripapillary changes and progression in glaucomaGraefes Arch Clin Exp Ophthalmol198822665105153209077
- JonasJBClinical implications of peripapillary atrophy in glaucomaCurr Opin Ophthalmol2005162848815744137
- ParkKHParkSJLeeYJKimJYCaprioliJAbility of peripapillary atrophy parameters to differentiate normal-tension glaucoma from glaucomalike diskJ Glaucoma20011029510111316103
- TengCCde MoraesCGPrataTSTelloCRitchRLiebmannJMBeta-Zone parapapillary atrophy and the velocity of glaucoma progressionOphthalmology2010117590991520132988
- KawanoJTomidokoroAMayamaCCorrelation between hemifield visual field damage and corresponding parapapillary atrophy in normal-tension glaucomaAm J Ophthalmol20061421404516815249
- TezelGKolkerAEKassMAParapapillary chorioretinal atrophy in patients with ocular hypertension. I. An evaluation as a predictive factor for the development of glaucomatous damageArch Ophthalmol199711512150315089400782
- TezelGKolkerAEWaxMBParapapillary chorioretinal atrophy in patients with ocular hypertension. II. An evaluation of progressive changesArch Ophthalmol199711512150915149400783
- HeltzerJMProgression of peripapillary atrophyOphthalmology1999106585710328376
- QuigleyHAKatzJDerickRJGilbertDSommerAAn evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damageOphthalmology199299119281741133