Abstract
Moxifloxacin hydrochloride ophthalmic solution 0.5% (Vigamox®) is the ocular formulation/adaptation of moxifloxacin. Moxifloxacin is a broad spectrum 8-methoxyfluoroquinolone which terminates bacterial growth by binding to DNA gyrase (topoisomerase II) and topoisomerase IV, essential bacterial enzymes involved in the replication, translation, repair and recombination of deoxyribonucleic acid. Affinity for both enzymes improves potency and reduces the probability of selecting resistant bacterial subpopulations. Vigamox is a bactericidal, concentration dependent, anti-infective. It is preservative free, and well tolerated with minimal ocular side effects. It provides increased penetration into ocular tissues and fluids with improved activity against Streptococci and Staphylococci species and moderate to excellent activity against clinically relevant, gram-negative ocular pathogens.
Introduction
Moxifloxacin hydrochloride ophthalmic solution 0.5% is the ocular formulation/adaptation of moxifloxacin, an 8-methoxyfluoroquinolone, broad spectrum, anti-infective. It was introduced in 2003 as Vigamox® (Alcon Laboratories, Inc, Fort Worth, TX, USA) for the treatment of susceptible microorganisms recovered from patients with bacterial conjunctivitis. It is used more frequently off label for treatment of keratitis and as a prophylaxis agent in cataract and refractive surgeries (CitationVigamox 2004; CitationAlfonso and Crider 2005; CitationSchlech and Alfonso 2005).
It is an isotonic, preservative free, solution with a near neutral pH of 6.8. The formula of Vigamox includes 5 mg/mL (0.5%) of moxifloxacin, boric acid, and purified water. Lack of the preservative BAK (benzalkonium chloride) makes it unique among current topical antibiotics licensed for use. Vigamox is currently available in more than 40 countries. Moxifloxacin hydrochloride ophthalmic solution 0.5%, under the trade name Vegamox®, was introduced into Japan in 2006, with approval for the treatment of bacterial conjunctivitis, keratitis and surgical prophylaxis.
Chemistry
shows the basic molecule and the moxifloxacin molecule. shows the impact of core modifications.
Figure 1 Basic 4-quinolone structure (adapted from CitationDomagala 1994).
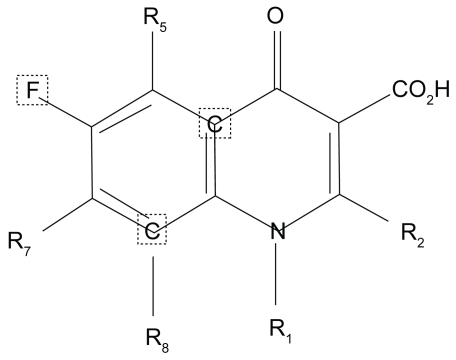
Table 1 Impact of core modification and potency to the fluoroquinolones
Moxifloxacin
Moxifloxacin is a broad spectrum, 8-methoxy fluoroquinolone with improved activity against Streptococci and Staphylococci and moderate to excellent activity against clinically relevant, gram negative ocular pathogens (CitationSmith et al 2001; CitationKeating and Scott 2004).
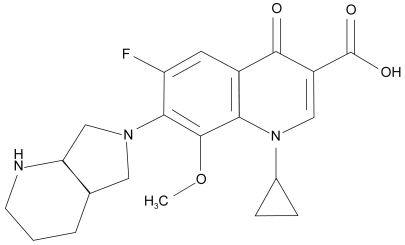
Modification of the parent molecule’s core 4-quinolone core, at positions 1, 5, 7, and 8 has engineered novel fluoroquinolones with enhanced antimicrobial activity, safety and tolerability (CitationKeating and Scott 2004). Substitutions at the N-1 nitrogen atoms are critical for the spectrum of activity and potency of the molecule. The N-1 cyclopropyl substitution in moxifloxacin confers increased activity against gram-positive and anaerobic isolates. Substitutions at the C-5 position also impact the in vitro activity against gram-positive isolates; the larger the molecule the greater the gram positive potency. Addition of a bulky C-7 (diazabicyclononyl ring) side chain and a methoxy group at the C-8 position reduces the potential for selection of resistant bacterial subpopulations and increase the binding/blocking affinity for DNA gyrase and topoisomerase IV, essential bacterial enzymes (CitationDomagala 1994; CitationBall et al 1998; CitationAppelbaum and Hunter 2000; CitationPeterson 2001; CitationZhanel et al 2002; CitationCaeiro and Iannini 2003; CitationSaravolatz and Leggett 2003)
These modifications were incorporated to meet the challenge of emerging resistance in the older fluoroquinolones among ocular and nonocular isolates (CitationBlondeau 1999; CitationChaudhry et al 1999; CitationGoldstein et al 1999; CitationAlexandrakis et al 2000; CitationZhanel and Noreddin 2001; CitationMather et al 2002; CitationHwang 2004; CitationMah 2004; CitationMarangon et al 2004; CitationVan Bambeke et al 2005).
Mechanism of action
Moxifloxacin is a bactericidal, concentration dependent, anti-infective. It interferes with bacterial survival by binding to DNA gyrase (topoisomerase II) and topoisomerase IV, essential bacterial enzymes involved in the replication, translation, repair and recombination of deoxyribonucleic acid. DNA gyrase is encoded by the genes gyra A and gyr B, while topoisomerase IV is encoded by Par C (grl A) and pare (grl B). Inhibition of either enzyme leads to bacteria death (CitationZhanel and Noreddin 2001; CitationHwang 2004; CitationMah 2004; CitationVan Bambeke et al 2005).
All fluoroquinolones bind to DNA gyrase and topoisomerase enzymes in susceptible organisms. The affinity or strength of the attachment varies; dependent on the class of fluoroquinolone and the bacteria species. Moxifloxacin binds strongly to both DNA gyrase and topoisomerase, but demonstrates preferential binding to DNA gyrase in gram-negative pathogens and Streptococcus pneumoniae. There is controversy as to the preferential target in the staphylococci. Preferential of dual targeting confirmation is dependent on methods used to evaluate the targets, and wild type strains employed to generate the mutants (CitationHooper 2001a; CitationOliphant and Green 2002; CitationBall et al 2004; CitationKeating and Scott 2004).
Studies confirmed that for the older fluoroquinolones such as ciprofloxacin, topoisomerase IV is the preferred target in gram positive bacteria (CitationBall et al 1998; CitationDalhoff and Schmitz 2003; CitationDrlica and Malik 2003; CitationZhanel et al 2006). In vitro studies supporting the dual activity of moxifloxacin have been mixed. Takei and colleagues using MIC ratios; classified moxifloxacin as a class three quinolone exhibiting dual activity against the two enzymes in Staphylococcus aureus (CitationTakei, Fukuda et al 2001). Topoisomerase IV was identified as the preferential target, with purified S. aureus DNA gyrase and topoisomerase IV enzymes by Ince and colleagues (CitationInce et al 2003). Griggs and co-workers (CitationGriggs et al 2003) selected mutants with preferred affinity for DNA gyrase.
Exposure to fluoroquinolones may select single step mutants and or bacterial populations with increased tolerance or resistance. Mechanisms of resistance to the fluoroquinolones include subpopulations (mutants) with 1) mutations in DNA gyrase and or topoisomerase genes that alter/reduce the binding affinity of the enzymes, 2) gene mutations that block drug entry, 3) presence of an efflux pump that reduces drug accumulation and 4) unique genes that confer specific resistance against S. aureus (CitationZhanel et al 2002; CitationWise 2003; CitationMah 2004; CitationJacoby 2005; CitationVan Bambeke et al 2005). Rare or emerging resistant mechanisms include 1) the presence of plasmids that protect cells from the lethal effects of the fluoroquinolones and 2) acquisition of a fluoroquinolone modifying enzyme (CitationRobicsek et al 2006; CitationRobicsek et al 2006).
Low level fluoroquinolone resistant populations usually contain a single mutation in DNA gyrase or topoisomerase IV. The preferred or primary target varies with the bacteria species and the fluoroquinolone. Key mutations usually occur in a unique region known as the quinolone resistant determining region (QRDR) of either DNA gyrase or topoisomerase IV. Secondary mutations may also occur in genes outside of these regions, in genes encoding efflux pumps, membrane permeability and cell transport. High level resistant isolates contain multiple gene mutations both in primary and in secondary targets. In areas with preexisting low levels of fluroquinolone resistance, exposure to suboptimal levels of the new fluoroquinolones will lead to rapid progression to double mutants and high level resistance (CitationHooper 2001b; CitationSmith et al 2001; CitationZhanel et al 2002; CitationHwang 2004; CitationMiller and Alfonso 2004).
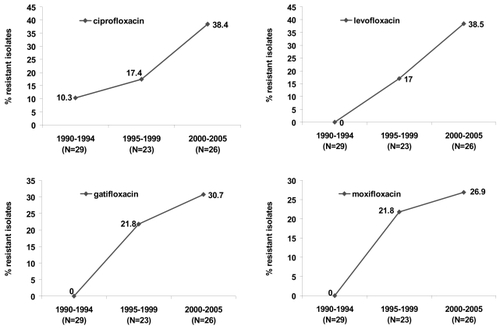
highlights the evolution of fluoroquinolones resistance among ocular isolates recovered from postoperative endophthalmitis cases from one region (CitationMiller et al 2006). At base line (1990–1994), low level resistance to ciprofloxacin (10.3%) was evident, with no documented resistance to levofloxacin, gatifloxacin, or moxifloxacin. Emergence of multistep mutants or subpopulations resistant to levofloxacin and the 8 methoxy fluoroquinolones were steeper and almost 3 times higher compared to ciprofloxacin during the initialfive years following ciprofloxacin’s introduction. Increasing resistance to ciprofloxacin and levofloxacin doubled in the last 5 years. Resistant populations increased by 8.9% for gatifloxacin and 5.1% for moxifloxacin during that same time period.
Figure 3 Evolution of fluoroquinolone resistance among coagulase negative staphylococci recovered from patients with post operative endophthalmitis (N = 78 isolates) (derived from CitationMiller and Flynn 2006).
Spectrum of activity
In vitro studies comparing minimal inhibitory concentrations (MICs) from the United States, Asia and Europe have documented the in vitro efficacy of moxifloxacin against a broad array of ocular and nonocular pathogens (CitationBlondeau 1999; CitationKrasemann, Meyer et al 2001; CitationZhanel, Ennis et al 2002; CitationCaeiro and Iannini 2003; CitationDalhoff and Schmitz 2003; CitationHwang 2004; CitationKeating and Scott 2004; CitationMah 2004). Emerging trends indicate enhanced activity and excellent coverage for S. pneumoniae, Haemophilus influenzae, and methicillin sus-ceptibile staphylococci compared to older fluoroquinolones. There was near equivocal coverage for Enterobacteriaceae, Pseudomonas aeruginosa, and other nonfermenters, but suboptimal coverage for methicillin resistant staphylococci and enterococci, .
Table 2 Comparative in vitro susceptibility of ciprofloxacin, gatifloxacin, and moxifloxacin against select nonocular pathogens
Greater than 10% resistance for moxifloxacin and or gatifloxacin was documented for several ocular pathogens, including S. pneumoniae (11.9%), methicillin resistant S. aureus (95% USA, 23.3% Taiwan, methicillin resistant Staphylococcus epidermidis (69% USA, 40% Taiwan) and P. aeruginosa (18.3% Taiwan).
Currently, there are few large, credible studies evaluating in vitro activity of moxifloxacin against new and older fluoroquinolones for common ocular pathogens. Many are hampered by low numbers of isolates (less than 30 per species), incomplete panel of challenge, comparative antibiotics or spectrum of pathogens and use conflicting or outdated interpretation standards.
compares minimal inhibitory concentrations needed to inhibit 90% of pathogens (MIC90s) for select ocular pathogens from North and South America for ciprofloxacin, gatifloxacin, and moxifloxacin. Results are impacted by methodology, isolate mix, testing period and interpretation standard applied. Minimal inhibitory concentrations against relevant ocular pathogens indicated that the 8-methoxy fluoroquinolones have improved efficacy against common ocular pathogens compared to ciprofloxacin. MIC90s were 2–4 times lower for moxifloxacin versus ciprofloxacin and lower than or equivocal to gatifloxacin among important ocular pathogens. Percent susceptible ranged from 24% (methicillin-resistant staphylococci) to 100% (S. pneumoniae) (CitationMather et al 2002; CitationKowalski et al 2003; CitationKowalski et al 2005; CitationStroman et al 2005; CitationOliveira et al 2007). Isolates resistant to ciprofloxacin were in general also resistant to moxifloxacin and gatifloxacin.
Table 3 Comparative MIC90s for moxifloxacin, gatifloxacin, and ciprofloxacin against select ocular pathogens
Resistance patterns to the fluoroquinolones vary by region, country, and ocular site. Studies comparing endemic background ciprofloxacin resistance demonstrated wide variation across regions in Europe, and North and South America (). Emerging resistant rates to moxifloxacin and gatifloxacin correlated with background ciprofloxacin resistant rates. Regions with the highest endemic resistance also reported the highest resistant rates to moxifloxacin and gatifloxacin (CitationMather et al 2002; CitationKowalski et al 2003; CitationKowalski et al 2005; CitationOliveira et al 2007) (CitationMiller 2006, unpublished).
Table 4 Regional and geographic variation in endemic fluoroquinolone resistance (%) among ocular pathogens
Other microbial pathogens considered susceptible to moxifloxacin include Chlamydia pneumoniae, Chlamydia trachomatis, Legionella pneumoniae and the some Mycobacteria species (CitationVigamox 2004).
Treatment of nontuberculosis with the 8-methoxyfluoroquinolones has been mixed. In a review of the early literature, Abshire and colleagues (Alcon Laboratories) offered data to support the use of moxifloxacin in the prevention and treatment on mycobacterial keratitis (CitationAbshire et al 2004). Several case and series report successful treatment outcomes with the 8 methoxyfluoroquinolones as adjunctive therapy (CitationLee et al 2005; CitationChang and Welty 2006 p 272; CitationJohn and Velotta 2005 p 461). Others have reported therapeutic failures with these drugs (CitationHofling-Lima et al 2005; CitationMoshirfar et al 2007).
The majority of the mycobacterial keratitis cases have been reported following outbreaks (CitationFreitas et al 2003; CitationKarp et al 2003; CitationWinthrop et al 2003; CitationJohn and Velotta 2005). Increased awareness, control measures and modified surgical techniques have reduced the frequencies of mycobacterial infections in the United States. The current recommendation for treatment of mycobacterial keratitis is alternative treatment with amikacin and one of the 8-methoxyfluoroquinolones (CitationDonnenfeld et al 2005).
Case reports and series from other ocular sites have been rare (CitationGupta et al 2003; CitationWilhelmus 2003; CitationNielsen et al 2004; CitationSpencer et al 2005; CitationMatieli et al 2006). Treatment strategies include amikacin and clarithromycin (CitationWilhelmus 2003).
The antifungal activities of the 8-methoxyfluoroquinolones have been investigated. There is both in vitro and in vivo evidence for some efficacy of the 8 methoyl fluoroquinolones to reduce fungal loads in the lab and for patients with contact lens associated fungal keratitis. Additional studies need to be done (CitationOzdek et al 2006; CitationMunir et al 2007)
Pharmacokinetics
Direct application of topical antimicrobial to conjunctival and corneal tissues can initially provide very high local and aqueous chamber concentrations. Final or sustained concentrations are altered by rapid tear film dissipation, underlying tissue health and dosing frequency (CitationRobertson et al 2005; CitationStroman et al 2005).
Penetration of moxifoxacin has been studied in ocular tissues and fluids in both humans and animals. Human studies have revealed wide variation in drug concentrations. Recorded concentrations are impacted by route of administration, dosing frequency, site of infection, presence or absence of epithelial defect and underlying disease. The recommended dosing frequency for the treatment of bacterial conjunctivitis is one drop 3 times a day for 5 days. (CitationVigamox 2004; CitationAlfonso and Crider 2005; CitationRobertson et al 2005; CitationStroman et al 2005)
In general, moxifloxacin’s high concentration formulation, enhanced bioavailability, and solubility have allowed for levels 2- to 4-fold higher ocular tissues levels than gatifloxacin (Zymar®, Allergan, Irvine CA), ciprofloxacin (Ciloxacin®, Alcon, Ft. Worth, TX), levofloxacin (Quixin®, Vistakon Pharmaceuticals, USA), or ofloxacin (Ocuflox®, Allergan, Irvine, CA) (CitationCekic et al 1999a; CitationCekic et al 1999b; CitationGarcia-Saenz et al 2001; CitationSmith et al 2001; CitationDonnenfeld et al 2004; CitationHwang 2004; CitationMah 2004; CitationKoch et al 2005; CitationRobertson et al 2005; CitationChang Lin and Welty 2006; CitationO’Brien 2006)
summarizes studies evaluating the penetration of moxifloxacin, gatifloxacin and ciprofloxacin into the aqueous and vitreous chambers by topical and oral routes of administration. Topical dosing protocols that mimic pre and post dosing frequencies for cataract and refractive surgeries of 4 times a day, pulsing dosing or a combination of the two, report concentration levels in the aqueous chamber that ranged from 0.38 ± 0.32 μg/mL to 2.28 ± 1.23 μg/mL.
Table 5 Penetration studies of moxifloxacin and comparators into aqueous and vitreous humor
Resultant concentration in the vitreous ranged from 0.011 ± 0.008 μg/mL to 0.11 ± 0.05 μg/mL. Vitreal concentrations were 20- to 40-fold lower than those obtained in the aqueous. (CitationDonnenfeld et al 2004; CitationHariprasad et al 2005; CitationHariprasad et al 2005; CitationKatz et al 2005; CitationKim et al 2005a; CitationKim et al 2005b; CitationSolomon et al 2005; CitationCostello et al 2006; CitationMcCulley et al 2006; CitationOng-Tone 2007). Concentration levels in the aqueous and the vitreous were usually 2-fold higher for moxifloxacin compared to gatifoxacin or ciloxacin.
Oral administration of 400–800 mg of moxifloxacin ranged from 0.21 ± 0.21 μg/mL to 2.33 μg/mL ± 0.85 and produced concentrations that were comparable to topical administration in the aqueous chamber. Drug levels were negligible in the vitreous (CitationGarcia-Saenz et al 2001; CitationKampougeris et al 2005; CitationHariprasad et al 2006; CitationVedantham et al 2006; CitationFuller et al 2007; CitationWalter et al 2007).
Pharmacodynamics
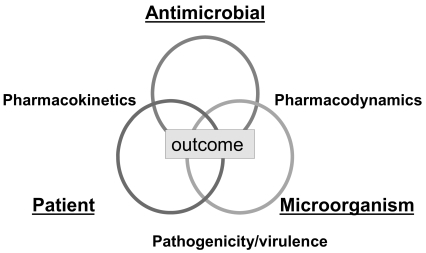
The therapeutic success or potency of an antibacterial agent is a complex interrelationship between drug and its ability to reach the target site (pharmacokinetics), the microbial pathogen and susceptibility to the selective drug (pharmacodynamics), and the underlying immune status of the patient ().
Pharmacokinetics is the dispersion and metabolism of the drug in the body. It is defined by the absorption, distribution, dosage and protein binding characteristics of the drug, which may vary among individual drugs in a class. Pharmacodynamics defines the impact of the antimicrobial agent on the infecting microorganism. It is characterized by the bacterial species, mechanism of microbial resistance, growth phase, infecting inoculum, degree of kill, time kill and MIC distribution. The third partner in this complex relationship is what both the drug and the pathogen do to the patient. This interplay is described by the patient’s age, genetic background, underlying disease and prior antimicrobial exposure.
The ratio of peak concentration (Cmax) to the MIC and the area under the concentration curve (AUC) are the pharmacodynamic indices that correlate most favorable with clinical outcomes for concentration-dependent anti-infectives. Maintaining adequate concentration of an antibiotic above a certain level known as the mutant prevention concentration (MPC) can also reduce the probability of selecting resistant subpopulations and increasing a favorable clinical outcome.
Antibiotic penetration into ocular tissues and fluids must not only reach but exceed the minimal inhibitory concentration (MIC) sufficiently to meet the targeted pharmacodynamic indices (Cmax: MIC or MPC) by a factor of 10. A Cmax:MIC ratio or MPC values greater than 10 have been documented to eradicate pathogens and suppress emergence of resistance in patients treated with fluoroquinolones (CitationAllen et al 2004; CitationMetzler et al 2004; CitationSmith et al 2004; CitationHermsen et al 2005).
Wilhelmus (CitationWilhelmus 2003; CitationWilhelmus et al 2003) confirmed the application and utility of pharmacodynamic indices to predict clinical outcome in patients with bacterial keratitis. The pharmacodynamic indices (PDI): Cmax:MIC and AUC:MIC were use to correlate clinical outcome for 391 patients with bacterial keratitis. Clinical improvement was associated with a Cmax:MIC ratio greater than 8 and an AUC:MIC ratio greater than 152. Corneal pathogens included S. aureus (21%), P. aeruginosa (12%), S. pneumoniae (7%), Streptococcus viridans (5%), other gram-positive isolates (44%), and other gram-negative isolates (11%).
displays calculated pharmacodynamic indices (PDI) for moxifloxacin and gatifloxacin using reported aqueous concentrations and MIC50s/MIC90s values for coagulase negative staphylococci recovered from endophthalmitis (CitationMather et al 2002; CitationMiller et al 2006)
Table 6 Comparative MIC50s, MIC90s, and calculated pharmacodynamic indices for moxifloxacin and gatifloxacin for coagulase-negative staphylococci versus reported aqueous concentrations
Targeted PDIs ranged from less than or equal to 0.038 μg/mL to 0.228 μg/mL for moxifloxacin and less than or equal to 0.048–0.094 μg/mL for gatifloxacin. Obtainable moxifloxacin concentrations exceeded the MIC and met the PDI factor of 10 for 89% (8/9) and 78% (7/9) of the isolates when using the MIC50. None of the moxifloxacin concentrations were sufficient to provide coverage for fluoroquinolone resistant coagulase negative staphylococci for reported MIC90s.
Gatifloxacin concentrations met the PDI factor of 10 for 28% (2/7) of the isolates at the MIC50 value of 0.09 μg/mL. None of the obtainable Zymar concentrations met the PDI factor of 10 for fluoroquinolone resistant isolates at values reported by CitationMiller et al (2006).
In a more recent report from the same Institution, Harper and colleagues (CitationHarper et al 2007) confirmed the low peak concentration:mic ratios for both gatifloxacin and moxifloxacin using reported intraocular levels against 59 coagulase negative staphylococci isolates collected between 1993 and 2006. Moxifloxacin ratios (Cmax:MIC90) were higher than gatifloxacin (0.05 μg/mL vs 0.02 μg/mL) but lower than vancomycin (0.45 μg/mL) for reported mean (1.66 μg/mL) aqueous concentrations. A significant difference in the PDI parameter was observed for moxifloxacin when the MIC50 rather than the MIC90 was used. The ratio for moxifloxacin using the MIC50 was 2.2 μg/mL vs 0.83 μg/mL for gatifloxacin and 0.67 μg/mL for vancomycin.
Clinical efficacy
No clinical trials have been conducted evaluating moxifloxacin vs. nonfluroquinolone antibiotics for the treatment of keratitis and or endophthalmitis. In two pre-marketing, randomized, double-masked, multi-centered, controlled clinical trials to assess, safety and efficacy of moxifloxacin for the treatment of bacterial conjunctivitis, clinical cures were documented in 66%–69% of patients by day 4. Micro-biological eradication occurred in 84%–94% of the patients. Patients were dosed 3 times a day for 4 days. Age groups ranged from 2 to 92. No adverse events were reported in this group (CitationAlfonso and Crider 2005).
Deramo and colleagues reported no significant difference in the rate of endophthalmitis using 4 times a day dosing of moxifloxacin or gatifloxacin pre- and post-operative compared to established endophthalmitis infection rates. In a retrospective, multicentered review of 20,013 patients from 9 cataract centers across 7 states, the overall rate of endophthalmitis following cataract surgery was 0.07%. The rate of postoperative endophthalmitis in the gatifloxacin-treated group (81%, 16,209) was 0.06% (9 cases) and the rate for the moxifloxacin-treated group (19%, 3804) was 0.1% (5 cases). The difference was not significant (p = 0.11, nor was this rate lower than the earlier study by Miller et al using clear corneal phacoemulsification (CitationMiller et al 2005; CitationDeramo et al 2006).
Safety and biocompatibility
Reported adverse reactions with 0.5% moxifloxacin hydrochloride ophthalmic solution administration have included: conjunctivitis, keratitis, decreased visual acuity, ocular hyperemia, dry eye, itching, subconjunctival hemorrhage, and tearing (Alcon Laboratories package insert) (CitationHariprasad et al 2005). Other infrequent ocular adverse events reported for the fluoroquinolones as a class include chemosis, eyelid edema, and punctuate epithelial keratitis (CitationMah 2004).
In vitro and animals studies have demonstrated a concentration dependent toxicity in studies of corneal epithelial cell migration and or proliferation, key components in corneal wound healing (CitationMallari et al 2001; CitationDonnenfeld et al 2004; CitationKovoor et al 2004; CitationBurka et al 2005; CitationDurrie and Trattler 2005; CitationMcGee et al 2005; CitationRobertson et al 2005; CitationSolomon et al 2005; CitationDonaldson et al 2006; CitationKaufman et al 2006; CitationLy et al 2006; CitationMatsumoto et al 2006; CitationMcDermott and Wheater 2006; CitationStern et al 2006; CitationWalter and Tyler 2006).
Matsumoto and colleagues reported low and equivocal cell migration inhibition scores for moxifloxacin and gatifloxacin versus ciprofloxacin at low concentrations (<0 .4 mmol/L), but greater toxicity for moxifloxacin and ciprofloxacin versus gatifloxacin at higher concentrations (≥0.64 mmol/L) (CitationMatsumoto et al 2006).
McDermott and Wheater correlated dilutions and effects on migration, adhesion, collagen type four expression and presence of fibronectin of the two commercially available 8-methoxy fluoroquinolones (moxifloxacin and gatifloxacin) on human corneal and conjunctival epithelial cell lines (CitationMcDermott and Wheater 2006). Increased toxicity was again correlated with higher drug concentrations. Gatifloxacin was reported to be less toxic than moxifloxacin at all concentrations.
Results of other in vitro studies evaluating, the toxicity of the 8-methoxyfluoroquinolones in human and animal corneal tissues have been mixed. Stern and colleagues used several animal models to compare the cellular effects of gatifloxacin and moxifloxacin on the rate and quality of corneal wound healing. In general, they reported greater corneal epithelial degradation, greater inhibition of collagen IV synthesis, and increased loss of normal structure in the basal lamina (Decemet’s membrane) in moxifloxacin treated eyes (CitationStern et al 2006).
In two studies from the University of Texas Southwestern Medical Center, investigators reported moxifloxacin to be less toxic to the corneal epithelium than all currently available ophthalmic fluoroquinolones (CitationKovoor et al 2004). Confocal assessment documented maintenance of corneal epithelial integrity and tight junction organization after short term intense dosing with moxifloxacin versus gatifloxacin. Under similar conditions, moxifloxacin induced cell loss and breakdown of tight junctions (CitationLy et al 2006).
Outcomes of human studies comparing the biocompatibility of moxifloxacin with gatifloxacin and or older fluoroquinolones were also mixed. In 14 healthy volunteers, where 0.5% moxifloxacin and 0.3% gatifloxacin drops were randomly administered to the right or left eye at 1 minute intervals for 5 minutes, higher levels of conjunctival injection, discomfort and corneal cell drop out per high power field were reported for the moxifloxacin eyes than for the gatifloxacin eyes. No significant change in pupil size or visual acuity was recorded for the two drugs (CitationKaufman et al 2006).
Walter reported two cases of severe corneal toxicity after moxifloxacin therapy. Both patients were treated for persisted sterile corneal ulcers that worsened with intense topical dosing with moxifloxacin, but resolved after change in therapy to corticosteroids and gatifloxacin (CitationWalter and Tyler 2006).
Donaldson and coworker reported no differences in visual acuity, tear breakup time or ocular surface integrity in the moxifloxacin treated vs. non treated eyes of healthy subjects dosed 4 times daily for 3 days. Authors concluded that moxifloxacin was safe during the 3 day treatment period that mimicked a prophylactic dosing regimen for patients scheduled for cataract surgery (CitationDonaldson et al 2006).
Durrie and Trattler compared the safety and tolerability of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% ophthalmic solution for treatment and prophylaxis in patients undergoing laser-assisted in situ keratomileusis (LASIK) and laser-assisted subepithelial keratomileusis (LASEK). No differences between the two antibiotics was documented for visual acuity pupil size, SSPK, edema, haze, day and night-time glare halos, clarity of day or night vision, or dry eye symptoms up to 1 week in LASIK patients. Moxifloxacin and gatifloxacin were equivalent in terms of ease of use, speed of recovery, overall vision, and overall comfort for this group of patients. No differences in corneal healing were observed after LASEK surgery (CitationDurrie and Trattler 2005).
Burka et al evaluated the effect of the 8 methoxyfluoroquinolones on epithelial healing following photorefractive keratectomy (PRK). At one month follow up, the moxifloxacin treated eyes had smaller defects and healed faster than patients treated with gatifloxacin (CitationBurka et al 2005). No significant differences in visual outcomes were found in the six month follow up for these patients (CitationBurka et al 2007).
Solomon et al compared penetration and safety of ciprofloxacin, moxifloxacin and gatifloxacin in patients scheduled for cataract surgery. No clinical evidence of epithelial or intraocular toxicity was noted for any of the three drugs (CitationSolomon et al 2005).
In general animal, in vitro, and clinical studies indicate the ocular and systemic safety and tolerability of moxifloxacin for the treatment of ocular infections in children (3 days to 17 years) and adults (up to age 93). Reported adverse events including conjunctivitis, keratitis and endophthalmitis have been low (CitationMcGee et al 2005; CitationKleinmann et al 2006).
Emerging resistance issues
Greater than 94% of the isolates in the Endophthalmitis Vitrectomy Study were gram positive bacteria (CitationEndophthalmitis Vitrectomy Study Group 1995; CitationHaimann et al 1996; CitationHan et al 1996) . There are increasing reports of gram-positive pathogens recovered from post refractive surgery infections. One of the anticipated advantages of the new 8-methoxyfluoroquinolones was the increased activity (lower MICs) against resistant gram positive cocci. What has emerged among ocular and nonocular comparative studies is that the gap in improved coverage for resistant gram positive ocular pathogens is less than optimal (CitationKowalski et al 2003; CitationMather et al 2004; CitationMiller et al 2006; CitationMoshirfar et al 2006; CitationOliveira et al 2007).
Pong et al evaluated the in vitro efficacy of moxifloxacin against clinical isolates with varying degrees of resistance to ciprofloxacin. There was a high correlation between increasing ciprofloxacin resistant levels and resistant MICs for both ofloxacin and moxifloxacin for gram positive isolates. The comparative MICs, however, were lower for moxifloxacin than for ofloxacin. In general moxifloxacin MICs were 8- to 32-fold lower for gram-positive isolates and up to four fold lower for susceptible gram negative isolates than for the older fluoroquinolones (CitationPong et al 1999).
The improved activity of moxifloxacin and gatifloxacin against methicillin resistant S. aureus (MRSA) does not translated to in vivo efficacy. No route of drug administration (oral, intravitreal, subconjunctival or topical) has provided concentrations that adequately cover the majority of MRSA with moderate or high level ciprofloxacin resistance.
Kotulus and colleagues documented clinical failure in a subset of 9 patients with MRSA infections treated with moxifloxacin or gatifloxacin. A third of the patients improved with continued treatment with the 8-methoxyfluoroquinolones; however, two thirds needed additional therapeutic intervention and only improved when switched to vancomycin and or other combination therapy. Patients who failed therapy were treated for an average of 4.5 days, while the third with favorable outcomes were treated more long term (18.1 days) (CitationKotlus et al 2006). Others have also reported treatment failures for patients with MRSA. (CitationSolomon et al 2003; CitationMoshirfar et al 2006; CitationSolomon et al 2007; CitationWoodward and Randleman 2007).
Coagulase-negative staphylococci remain the most frequent pathogen recovered from post cataract endophthalmitis. The consensus is that the origin of pathogens recovered from post cataract infections are seeded from the patient’s conjunctiva. Small populations of organisms resistant to the 8 methoxyfluoroquinolones may be presence as part of the resident conjunctiva flora. These may have been “selected” following exposure to older fluoroquinolone. The high concentration and broad spectrum of the fluoroquinolone may disrupt normal conjunctival flora and allow for colonization of more resistant bacterial and or more nonbacterial pathogens.
Mino de Kaspar and colleagues demonstrated a low rate of resistance (2%) among coagulase negative staphylococci in their study evaluating the normal conjunctiva flora of patients scheduled for anterior segment surgery (CitationMino de Kaspar et al 2005).
Miller et al documented a high level fluoroquinolone cross resistance among coagulase negative endophthalmitis isolates. Increasing resistance to ciprofloxacin was paralleled by increasing resistance to both moxifloxacin and gatifloxacin. Moxifloxacin provides coverage for 10/38, 26% and gatifloxacin 13/38, 66% for the ciprofloxacin-resistant isolates (CitationMiller et al 2006).
Role of moxifloxacin in the management of ocular bacterial infections
No anti-infective provides ideal coverage for all pathogens for all infected sites. Selection of an effective anti-infective for ophthalmology is dependent on clinical efficacy, background resistance, site of infection, and toxicity.
Moxifloxacin hydrochloride ophthalmic solution 0.5% is a unique, preservative free, anti-infective which offers elevated tissue concentrations, broad spectrum of activity and a moderate to high rate of clinical success against common ocular pathogens. Declining efficacy against methicillin susceptible and resistant staphylococci and pseudomonas species is a concern. Judicious use is warranted to maintain utility and reduce selection of resistant populations.
References
- AbshireRCockrumP2004Topical antibacterial therapy for mycobacterial keratitis: potential for surgical prophylaxis and treatmentClin Ther26191615038942
- AlexandrakisGAlfonsoEC2000Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolonesOphthalmology107149750210919897
- AlfonsoECriderJ2005Ophthalmic infections and their anti-infective challengesSurv Ophthalmol50Suppl 1S1616257307
- AllenGPKaatzGW2004In vitro activities of mutant prevention concentration-targeted concentrations of fluoroquinolones against Staphylococcus aureus in a pharmacodynamic modelInt J Antimicrob Agents241506015288314
- AppelbaumPCHunterPA2000The fluoroquinolone antibacterials: past, present and future perspectivesInt J Antimicrob Agents1651511185413
- BallPFernaldA1998Therapeutic advances of new fluoroquinolonesExpert Opin Investig Drugs776183
- BallPStahlmannR2004Safety profile of oral and intravenous moxifloxacin: cumulative data from clinical trials and postmarketing studiesClin Ther269405015336463
- BlondeauJM1999Expanded activity and utility of the new fluoroquinolones: a reviewClin Ther21340 discussion 1–210090423
- BlondeauJM1999A review of the comparative in-vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolonesJ Antimicrob Chemother43Suppl B11110382869
- BurkaJMBowerKS2005The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomyAm J Ophthalmol14083715953577
- BurkaJMBowerKS2007The effect of moxifloxacin and gatifloxacin on long-term visual outcomes following photorefractive keratectomyJ Refract Surg23414717455838
- CaeiroJPIanniniPB2003Moxifloxacin (Avelox): a novel fluoroquinolone with a broad spectrum of activityExpert Rev Anti Infect Ther13637015482134
- CekicOBatmanC1999aPenetration of ofloxacin and ciprofloxacin in aqueous humor after topical administrationOphthalmic Surg Lasers304658
- CekicOBatmanC1999bHuman aqueous and vitreous humour levels of ciprofloxacin following oral and topical administrationEye135558
- Chang LinJEWeltyD2006Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humansSurv Ophthalmol51530 author reply 530–116950254
- ChaudhryNAFlynnHWJr1999Emerging ciprofloxacin-resistant Pseudomonas aeruginosaAm J Ophthalmol1285091010577596
- CostelloPBakriSJ2006Vitreous penetration of topical moxifloxacin and gatifloxacin in humansRetina26191516467677
- DalhoffASchmitzFJ2003In vitro antibacterial activity and pharmacodynamics of new quinolonesEur J Clin Microbiol Infect Dis222032112687416
- DeramoVALaiJC2006Acute endophthalmitis in eyes treated prophylactically with gatifloxacin and moxifloxacinAm J Ophthalmol142721516989762
- DomagalaJM1994Structure-activity and structure-side-effect relationships for the quinolone antibacterialsJ Antimicrob Chemother336857068056688
- DonaldsonKEMarangonFB2006The effect of moxifloxacin on the normal human corneaCurr Med Res Opin2220738017022866
- DonnenfeldEPerryHD2004A comparison of the fourth-generation fluoroquinolones gatifloxacin 0.3% and moxifloxacin 0.5% in terms of ocular tolerabilityCurr Med Res Opin201753815537475
- DonnenfeldEDKimT2005ASCRS White Paper: Management of infectious keratitis following laser in situ keratomileusisJ Cataract Refract Surg3120081116338575
- DrlicaKMalikM2003Fluoroquinolones: action and resistanceCurr Top Med Chem32498212570763
- DurrieDSTrattlerW2005A comparison of therapeutic regimens containing moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% ophthalmic solution for surgical prophylaxis in patients undergoing LASIK or LASEKJ Ocul Pharmacol Ther212364115969641
- Endophthalmitis Vitrectomy Study Group1995Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study GroupArch Ophthalmol1131479967487614
- FreitasDAlvarengaL2003An outbreak of Mycobacterium chelonae infection after LASIKOphthalmology1102768512578767
- FullerJJLottMN2007Vitreal penetration of oral and topical moxifloxacin in humansAm J Ophthalmol1433384017258525
- Garcia-SaenzMCArias-PuenteA2001Human aqueous humor levels of oral ciprofloxacin, levofloxacin, and moxifloxacinJ Cataract Refract Surg2719697411738912
- GoldsteinMHKowalskiRP1999Emerging fluoroquinolone resistance in bacterial keratitis:a 5-year reviewOphthalmology1061313810406613
- GriggsDJMaronaH2003Selection of moxifloxacin-resistant Staphylococcus aureus compared with five other fluoroquinolonesJ Antimicrob Chemother511403712716775
- GuptaVGuptaA2003Presumed tubercular serpiginouslike choroiditis: clinical presentations and managementOphthalmology1101744913129872
- HaimannMHWeissH1996The Endophthalmitis Vitrectomy StudyArch Ophthalmol1141025 author reply 1026–7
- HanDPWisniewskiSR1996Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy StudyAm J Ophthalmol1221178659579
- HariprasadSMBlinderKJ2005Penetration pharmacokinetics of topically administered 0.5% moxifloxacin ophthalmic solution in human aqueous and vitreousArch Ophthalmol123394415642810
- HariprasadSMShahGK2005Determination of aqueous and vitreous concentration of moxifloxacin 0.5% after delivery via a dissolvable corneal collagen shield deviceJ Cataract Refract Surg312142616412929
- HariprasadSMShahGK2006Vitreous and aqueous penetration of orally administered moxifloxacin in humansArch Ophthalmol1241788216476886
- HarperTMillerD2007In vitro efficacy and pharmacodynamic indices for antibiotics against coagulase-negative Staphylococcus endophthalmitis isolatesOphthalmology114871517383732
- HermsenEDHovdeLB2005Mutant prevention concentrations of ABT-492, levofloxacin, moxifloxacin, and gatifloxacin against three common respiratory pathogensAntimicrob Agents Chemother491633515793158
- Hofling-LimaALde FreitasD2005In vitro activity of fluoroquinolones against Mycobacterium abscessus and Mycobacterium chelonae causing infectious keratitis after LASIK in BrazilCornea24730416015094
- HooperDC2001aEmerging mechanisms of fluoroquinolone resistanceEmerg Infect Dis73374111294736
- HooperDC2001bMechanisms of action of antimicrobials: focus on fluoroquinolonesClin Infect Dis32Suppl 1S9S1511249823
- HwangDG2004Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fluoroquinolonesSurv Ophthalmol49Suppl 2S798315028483
- InceDZhangX2003Activity of and resistance to moxifloxacin in Staphylococcus aureusAntimicrob Agents Chemother471410512654680
- JacobyGA2005Mechanisms of resistance to quinolonesClin Infect Dis41Suppl 2S120615942878
- JohnTVelottaE2005Nontuberculous (atypical) mycobacterial keratitis after LASIK: current status and clinical implicationsCornea242455515778593
- KampougerisGAntoniadouA2005Penetration of moxifloxacin into the human aqueous humour after oral administrationBr J Ophthalmol896283115834098
- KarpCLTuliSS2003Infectious keratitis after LASIKOphthalmology1105031012623812
- KatzHRMasketS2005Absorption of topical moxifloxacin ophthalmic solution into human aqueous humorCornea24955816227840
- KaufmanSCRusinekC2006Comparison of the biocompatibility of gatifloxacin 0.3% and moxifloxacin 0.5%Cornea259 Suppl 2S314
- KeatingGMScottLJ2004Moxifloxacin: a review of its use in the management of bacterial infectionsDrugs6423477715456331
- KimDHStarkWJ2005aOcular penetration of moxifloxacin 0.5% and gatifloxacin 0.3% ophthalmic solutions into the aqueous humor following topical administration prior to routine cataract surgeryCurr Med Res Opin21934
- KimDHStarkWJ2005bAqueous penetration and biological activity of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% solution in cataract surgery patientsOphthalmology11219926
- KleinmannGLarsonS2006Intraocular concentrations of gatifloxacin and moxifloxacin in the anterior chamber via diffusion through the cornea using collagen shieldsCornea252091316371785
- KochHRKulusSC2005Corneal penetration of fluoroquinolones: aqueous humor concentrations after topical application of levofloxacin 0.5% and ofloxacin 0.3% eyedropsJ Cataract Refract Surg3113778516105610
- KotlusBSWymbsRA2006In vitro activity of fluoroquinolones, vancomycin, and gentamicin against methicillin-resistant Staphylococcus aureus ocular isolatesAm J Ophthalmol142726917056356
- KovoorTAKimAS2004Evaluation of the corneal effects of topical ophthalmic fluoroquinolones using in vivo confocal microscopyEye Contact Lens3090415260356
- KowalskiRPDhaliwalDK2003Gatifloxacin and moxifloxacin:an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolatesAm J Ophthalmol136500512967804
- KowalskiRPYatesKA2005An ophthalmologist’s guide to understanding antibiotic susceptibility and minimum inhibitory concentration dataOphthalmology112198716183128
- KrasemannCMeyerJ2001Evaluation of the clinical microbiology profile of moxifloxacinClin Infect Dis32Suppl 1S516311249830
- LeeSBOliverKM2005Fourth-generation fluoroquinolones in the treatment of mycobacterial infectious keratitis after laser-assisted in situ keratomileusis surgeryCan J Ophthalmol40750316391641
- LyLTCavanaghHD2006Confocal assessment of the effects of fourth-generation fluoroquinolones on the corneaEye Contact Lens32161516845259
- MahFS2004Fourth-generation fluoroquinolones: new topical agents in the war on ocular bacterial infectionsCurr Opin Ophthalmol153162015232471
- MallariPLMcCartyDJ2001Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitisAm J Ophthalmol131131311162991
- MarangonFBMillerD2004Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitisAm J Ophthalmol137453815013867
- MatherRKarenchakLM2002Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibioticsAm J Ophthalmol133463611931779
- MatherRStewartJM2004The effect of cataract surgery on ocular levels of topical moxifloxacinAm J Ophthalmol138554915488780
- MatieliLCDe FreitasD2006Mycobacterium abscessus endophthalmitis: treatment dilemma and review of the literatureRetina26826916963860
- MatsumotoSWayW2006Comparative toxicity of fluoroquinolone antibiotics on corneal cells in vitroCornea259 Suppl 2S17
- McCulleyJPCaudleD2006Fourth-generation fluoroquinolone penetration into the aqueous humor in humansOphthalmology113955916603244
- McDermottMWheaterM2006In vitro comparison of the effects of clinically available ophthalmic solutions of gatifloxacin 0.3% and moxifloxacin 0.5% on human corneal and conjunctival epithelial cell adhesion and migration and on collagen type IV protein expressionCornea259 Suppl 2S25S30
- McGeeDHHoltWF2005Safety of moxifloxacin as shown in animal and in vitro studiesSurv Ophthalmol50Suppl 1S465416257310
- MetzlerKHansenGM2004Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureusInt J Antimicrob Agents24161715288315
- MillerDAlfonsoEC2004Comparative in vitro activity of levofloxacin, ofloxacin, and ciprofloxacin against ocular streptococcal isolatesCornea232899315084863
- MillerDFlynnPM2006In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolatesArch Ophthalmol1244798316606872
- MillerJJScottIU2005Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatmentAm J Ophthalmol139983715953426
- Mino de KasparHKossMJ2005Antibiotic susceptibility of preoperative normal conjunctival bacteriaAm J Ophthalmol139730315808182
- MoshirfarMMeyerJJ2007Fourth-generation fluoroquinolone-resistant mycobacterial keratitis after laser in situ keratomileusisJ Cataract Refract Surg3319788117964409
- MoshirfarMMirzaianG2006Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgeryJ Cataract Refract Surg32515816631067
- MunirWMRosenfeldSI2007Clinical response of contact lens-associated fungal keratitis to topical fluoroquinolone therapyCornea26621417525664
- NielsenJSBlattS2004Clinicopathologic case report: scleral buckle associated nontuberculous mycobacterial scleritisSemin Ophthalmol193–4101415590546
- O’BrienTP2006Evidence-based review of moxifloxacinInt Ophthalmol Clin46617217060792
- OliphantCMGreenGM2002Quinolones: a comprehensive reviewAm Fam Physician654556411858629
- OliveiraADD’AzevedoPA2007In vitro activity of fluoroquinolones against ocular bacterial isolates in Sao Paulo, BrazilCornea26194817251812
- Ong-ToneL2007Aqueous humor penetration of gatifloxacin and moxifloxacin eyedrops given by different methods before cataract surgeryJ Cataract Refract Surg33596217189794
- OzdekSCMillerD2006In vitro antifungal activity of the fourth generation fluoroquinolones against Candida isolates from human ocular infectionsOcul Immunol Inflamm143475117162605
- PetersonLR2001Quinolone molecular structure-activity relationships: what we have learned about improving antimicrobial activityClin Infect Dis33Suppl 3S180611524717
- PongAThomsonKS1999Activity of moxifloxacin against pathogens with decreased susceptibility to ciprofloxacinJ Antimicrob Chemother44621710552978
- RobertsonSMCurtisMA2005Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humansSurv Ophthalmol50Suppl 1S324516257309
- RobicsekAJacobyGA2006The worldwide emergence of plasmid-mediated quinolone resistanceLancet Infect Dis66294017008172
- RobicsekAStrahilevitzJ2006Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferaseNat Med1283816369542
- SaravolatzLDLeggettJ2003Gatifloxacin, gemifloxacin, and moxifloxacin:the role of 3 newer fluoroquinolonesClin Infect Dis371210514557966
- SchlechBAAlfonsoE2005Overview of the potency of moxifloxacin ophthalmic solution 0.5% (VIGAMOX)Surv Ophthalmol50Suppl 1S71516257313
- SharmaSKDY1999Trends in antibiotic resistance of corneal pathogens: Part I. An Analysis of commonly used antibioticsIndian J Ophthalmol4795100
- SmithAPennefatherPM2001Fluoroquinolones: place in ocular therapyDrugs617476111398907
- SmithHJWaltersM2004Mutant prevention concentrations for single-step fluoroquinolone-resistant mutants of wild-type, efflux-positive, or ParC or GyrA mutation-containing Streptococcus pneumoniae isolatesAntimicrob Agents Chemother483954815388458
- SolomonRDonnenfeldED2003Bilateral methicillin-resistant staphylococcus aureus keratitis in a medical resident following an uneventful bilateral photorefractive keratectomyEye Contact Lens29187912861116
- SolomonRDonnenfeldED2007Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgeryAm J Ophthalmol1436293417320811
- SolomonRDonnenfeldED2005Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humorOphthalmology112466915745775
- SpencerTSTeskeMP2005Postcataract endophthalmitis caused by Mycobacterium goodiiJ Cataract Refract Surg311252316039508
- SternMEGaoJ2006Effects of fourth-generation fluoroquinolones on the ocular surface, epithelium, and wound healingCornea259 Suppl 2S12S24
- StromanDWDajcsJJ2005In vitro and in vivo potency of moxifloxacin and moxifloxacin ophthalmic solution 0.5%, a new topical fluoroquinoloneSurv Ophthalmol50Suppl 1S163116257308
- TakeiMFukudaH2001Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibitionAntimicrob Agents Chemother453544711709337
- Van BambekeFMichotJM2005Quinolones in 2005: an updateClin Microbiol Infect112568015760423
- VedanthamVLalithaP2006Vitreous and aqueous penetration of orally administered moxifloxacin in humansEye201273816200061
- Vigamox. 2004. [package insert]. Fort Worth, TX, Alcon Laboratories.
- WalterKTylerME2006Severe corneal toxicity after topical fluoroquinolone therapy: report of two casesCornea25855717068466
- WalterSKuchenbeckerJ2007Concentration of moxifloxacin in serum and human aqueous humor following a single 400 mg oral doseJ Cataract Refract Surg335535
- WilhelmusKR2003Evaluation and prediction of fluoroquinolone pharma-codynamics in bacterial keratitisJ Ocul Pharmacol Ther19493914583139
- WilhelmusKR2003Nontuberculous mycobacterial endophthalmitisArch Ophthalmol121166314609941
- WilhelmusKRAbshireRL2003Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitisArch Ophthalmol12112293312963604
- WinthropKLSteinbergEB2003Epidemic and sporadic cases of nontuberculous mycobacterial keratitis associated with laser in situ keratomileusisAm J Ophthalmol135223412566028
- WiseR2003Maximizing efficacy and reducing the emergence of resistanceJ Antimicrob Chemother51Suppl 1742
- WoodwardMRandlemanJB2007Bilateral methicillin-resistant Staphylococcus aureus keratitis after photorefractive keratectomyJ Cataract Refract Surg33316917276277
- ZhanelGGEnnisK2002A critical review of the fluoroquinolones: focus on respiratory infectionsDrugs62135911790155
- ZhanelGGFontaineS2006A review of new fluoroquinolones: focus on their use in respiratory tract infectionsTreat Respir Med54376517154673
- ZhanelGGNoreddinAM2001Pharmacokinetics and pharmacodynamics of the new fluoroquinolones: focus on respiratory infectionsCurr Opin Pharmacol14596311764770