Abstract
Objective
This retrospective study was designed to investigate the efficacy and tolerability of travoprost 0.004% substituted for latanoprost 0.005% in glaucoma patients at the Manhattan Veterans Administration Hospital.
Research design and methods
We conducted a chart review of patients with stable intraocular pressure (IOP) undergoing a formulary change in regimen from latanoprost 0.005% to travoprost 0.004%. Diagnoses included primary open angle glaucoma, ocular hypertension, pigment dispersion glaucoma, and pseudoexfoliation glaucoma.
Main outcome measures
The primary outcome measures were IOP change between baseline and 6 months and patient-reported adverse events throughout the study.
Results
In the single therapy group (N = 60 eyes), the mean baseline IOP on latanoprost was 15.8 mmHg; after 6 months on travoprost, it was 14.9 mmHg (p < 0.1). In the concomitant therapy group (N = 126 eyes), the mean baseline IOP was 16.7 mmHg; after 6 months on travoprost, it was 15.9 mmHg (p < 0.01). A reduction of IOP ≥ 3 mmHg occurred in 28 eyes of 21 patients at 6 months. An increase of IOP ≥ 3 mmHg occurred in 5 eyes of 4 patients at 6 months. One patient was switched back to latanoprost due to irritation at 3 months. No other patient-reported adverse events, including increased hyperemia, were observed throughout the follow-up period.
Conclusions
A change in therapeutic regimen from latanoprost 0.005% to travoprost 0.004% maintained IOP control in stable patients, and in some produced a further reduction in IOP. A change in therapy from latanoprost to travoprost was effective and well-tolerated for the glaucoma patients in this study.
Introduction
Prostaglandin analogues (PGAs) comprise one of the drug classes used in treating glaucoma. These agents act primarily by increasing uveoscleral outflow and thereby lowering IOP (CitationCrawford and Kaufman 1987; CitationGabelt and Kaufman 1989; CitationNilsson et al 1989; CitationCamras 1995). Frequently, glaucoma studies that have evaluated PGA substitutions have been conducted on patients who were poor responders to the first medication(s) (CitationWilliams 2002; CitationKaback et al 2004; CitationGandolfi and Cimino 2003; CitationHollo et al 2005). A recent study (CitationLaw et al 2005) reported on a mass change in regimen (to bimatoprost) prompted by a formulary change in which patients were not poor responders to the original medication (latanoprost). We used the opportunity of a formulary change in the Veterans Administration (VA) system to review the consequences of a mass change in therapeutic regimen from latanoprost to travoprost in patients with adequate IOP control while on latanoprost. We analyzed medical records from patients in the glaucoma clinic at the Manhattan VA Hospital.
In July 2003, the Veterans Administration (VA) hospital chose to select a single prostaglandin analogue for use within the system. Based on a competitive contracting process, the VA system elected to use travoprost as its primary PGA, and to phase out and eventually totally replace the use of latanoprost. All newly diagnosed glaucoma patients requiring a PGA were to be prescribed travoprost. In order to accommodate potential concerns about tolerability or effectiveness of changing to a new PGA, those patients already on latanoprost were permitted to remain on it at the discretion of their ophthalmologist. No industry support or paid recruitment for a regimen change occurred, however the VA strongly recommended the regimen change to travoprost. In August 2003, the ophthalmologists at the Manhattan VA hospital elected to place all stable glaucoma patients who were prescribed a PGA on travoprost. There was no direct clinical trial experience within the VA hospital system with travoprost. Thus, we had an interest in evaluating if travoprost would be tolerated as well and would be as effective as other PGAs such as latanoprost.
The purpose of this retrospective study was to evaluate the efficacy and tolerability (as defined by changes in IOP and changes in adverse effects, respectively) of travoprost 0.004% substituted for latanoprost 0.005% in a glaucoma population undergoing a formulary change at the VA hospital.
Patients and methods
We conducted a retrospective chart review of patients identified through pharmacy records as being moved from latanoprost 0.005% to travoprost 0.004% from August, 2003 through July, 2004 at the Manhattan VA Hospital. The study was approved by the institutional review board (IRB) of the New York Campus of the New York Harbor Health-care System of the Veterans Administration. In accordance with minimal patient risk, a waiver of consent was obtained from the IRB. A computerized pharmacy dispensing database was accessed, and a printout of those patients was generated. We selected only patients with diagnoses of open angle glaucomas, including primary open angle glaucoma (POAG), pigment dispersion glaucoma (PDG), or pseudoexfoliation glaucoma (PSX), and ocular hypertension (OHT), who were treated in the glaucoma clinic at the Manhattan VA. We excluded uveitis and other disorders that may be associated with IOP fluctuations unrelated to the medication change. Only patients considered stable on the previous medication were included. Patients were considered stable if their IOP values had been within their target range for at least 6 months prior to the switch, and the patients were not being considered for surgery or therapeutic modification outside of the formulary change. Therapeutic regimens prior to the change included latanoprost alone or in combination with other drugs (see Results for specific therapies). No changes in medical regimen, ocular or oral, were permitted during follow-up, other than the change to travoprost. No minimum IOP was required for inclusion in the study.
The primary selection criterion for inclusion into this study was the availability of documentation of patient-reported adverse events for all visits during the study. We used a direct questioning survey designed to capture changes in adverse events. Questions drew upon subjective patient assessment of any changes in adverse events, such as hyperemia and irritation (see Appendix). At the baseline visit, patients were specifically asked if they had experienced any adverse events since beginning latanoprost. At all follow-up visits, they were asked if any changes occurred after changing to travoprost. These responses were documented in the electronic record of each patient. In addition, patients selected for inclusion had to be seen in the VA clinic by one of the doctors and IOP data had to be present in the electronic record for all visits. It should be noted that all patients whose regimen was changed from latanoprost to travoprost were included in the survey, regardless of whether they met the other inclusion criteria which were formulated retrospectively. This was done to ensure the inclusion of all potential patients for subsequent data analysis and help eliminate any inclusion/exclusion bias.
Intraocular pressure was measured by Goldmann tonometry at baseline (prior to the regimen change), at 3 months (± 2 weeks), and at 6 months (± 2 weeks) after the patients received travoprost. Measurements were made by a total of eight to ten observers, as the study was performed within a residency training program. All measurements were performed by third-year residents with training administered by Dr. Farris on the proper method of Goldmann tonometry. The same tonometer was used for every patient at every visit. The tonometer was preset to 20 mmHg prior to any measurements being performed and was calibrated monthly. All measurements were obtained between 12:30 pm and 3:00 pm. We used IOP from both eyes and considered each eye as an independent unit of measurement.
The primary outcome measures were IOP change between baseline and 6 months and patient-reported adverse events throughout the study.
Results
The study consisted of 188 eyes of 97 patients. All patients were male. Demographic data are shown in . The diagnoses for these patients were as follows: POAG (86%), OHT (6%), PSX (6%), and PDG (2%). POAG accounted for 71% of monotherapy patients and 92% of the concomitant therapy group. The monotherapy group included 60 eyes and the concomitant therapy group 128 eyes. One patient (2 eyes) in the concomitant therapy group was switched back to latanoprost at 3 months due to irritation, and thus was not included in final statistical analyses. Due to the small number of patients for whom data were available at 3 months, we elected to omit these data from our final analysis.
Table 1 Demographics and diagnoses
In the concomitant therapy group, medications prescribed in addition to latanoprost included: dorzolamide/timolol in 112 eyes (88%); brimonidine in 94 eyes (73%); timolol in 13 eyes (10%); and pilocarpine in 4 eyes (3%). Regimen details are shown in .
Table 2 Concomitant therapy with latanoprost
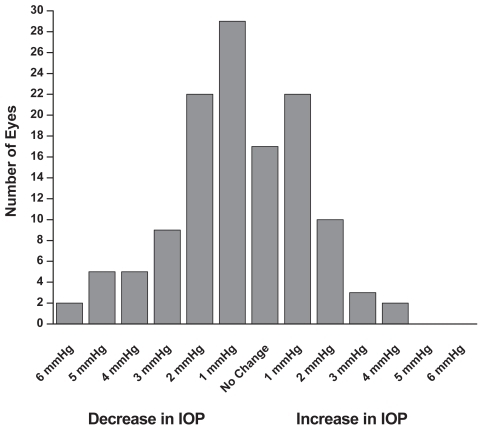
In the single therapy group (N = 60 eyes), the mean baseline IOP on latanoprost was 15.8 ± 3.1 mmHg (). After 6 months on travoprost the mean IOP was 14.9 ± 2.5 mmHg (p < 0.1). In the concomitant therapy group (N = 126 eyes), the mean baseline IOP on latanoprost and concomitant medications was 16.7 ± 2.4 mmHg (). After 6 months on travoprost and the same concomitant medications, the mean IOP was 15.9 ± 2.2 mmHg (p < 0.01). shows the combined data for all patients (both monotherapy and concomitant therapy). Six months after the change in regimen, the IOP was reduced by approximately an additional 1 mmHg (p < 0.001). Reductions from baseline IOP are represented in . Both the monotherapy and concomitant groups showed statistically significant reductions in IOP from the latanoprost baseline (p ≤ 0.001). and are histograms showing responses of individual eyes in terms of decreases/increases in IOP (mmHg). A reduction in IOP of ≥3 mmHg occurred in 28 eyes of 21 patients at 6 months. An increase in IOP of ≥3 mmHg occurred in 5 eyes of 4 patients at 6 months. In our study it was slightly more likely to achieve a 3 mmHg or more additional reduction in the concomitant group (21 of 126 eyes) than in the monotherapy group (7 of 60 eyes).
Figure 1 Monotherapy group: Patients were on latanoprost 0.005% monotherapy prior to the switch to travoprost 0.004% monotherapy. Six months after the switch the IOP was reduced by approximately an additional 1 mmHg (mean + SEM, p < 0.1; n = 60).
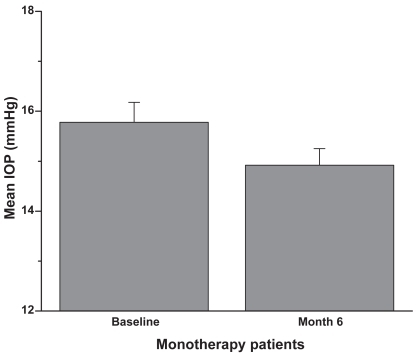
Figure 2 Concomitant therapy group: Patients were on latanoprost 0.005% plus concomitant therapy prior to the switch to travoprost 0.004% plus concomitant therapy. Six months after the switch the IOP was reduced by approximately an additional 1 mmHg (mean + SEM, **p < 0.01; n = 126).
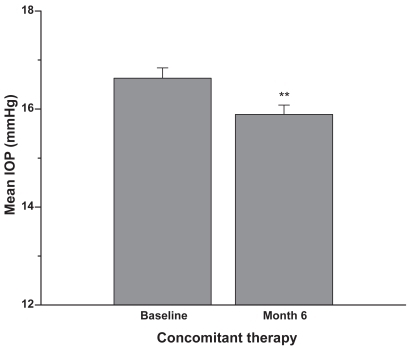
Figure 3 Patients were on latanoprost 0.005% monotherapy or latanoprost plus concomitant therapy prior to the switch to travoprost 0.004%. Six months after the switch the IOP was reduced by approximately an additional 1 mmHg (mean + SEM, **p < 0.001; n = 186).
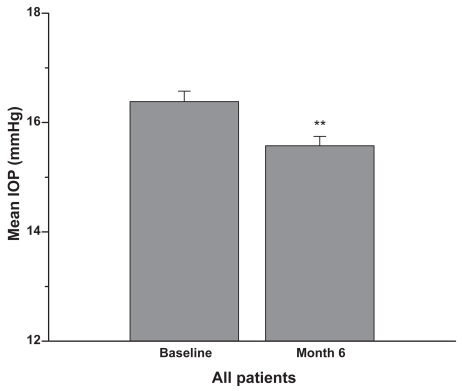
Figure 4 Patients were on latanoprost 0.005% monotherapy or concomitant therapy prior to the switch to travoprost 0.004%. Data presented are based on the number of eyes with IOP measurement available from the specific visit. Changes = Follow-up IOP – Baseline IOP of the same eye. A negative number indicates a reduction in IOP. The results indicate that in each group, the within-eye IOP changes from pre-switch to 6 months (n = 60 monotherapy; n = 126 concomitant therapy) after switch were statistically significant (mean + SEM, ***p < 0.001, †p < 0.0001).
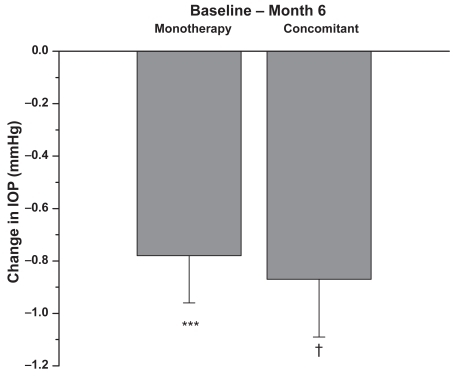
Figure 5 At 6 months, a few patients previously on monotherapy experienced an increase in IOP while several experienced a decrease in IOP of 3 mmHg or more (n = 60).
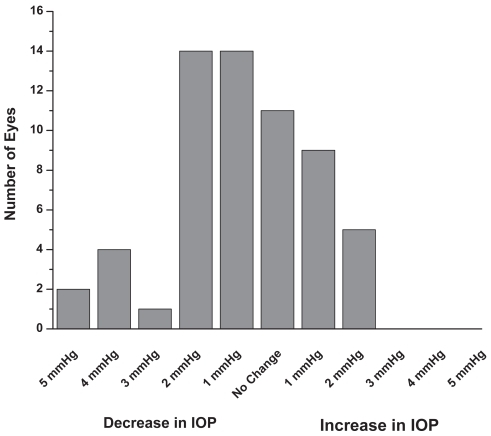
Figure 6 At 6 months, a few patients previously on concomitant therapy experienced an increase in IOP while several experienced a decrease in IOP of 3 mmHg or more (n = 126).
As noted above, one patient (2 eyes) was switched back to latanoprost at 3 months due to irritation. As evaluated by direct questioning survey as described above (see Appendix), no other patients reported changes in adverse events compared to the latanoprost baseline, including increased hyperemia, throughout the 6-month follow-up period.
Discussion
Our study incorporates several features which distinguish it from previous studies. First, the patients in our study were considered stable (with regard to IOP) on latanoprost prior to the change to travoprost therapy. Previous studies have evaluated patients deemed nonresponders or poor responders to latanoprost prior to a regimen substitution (CitationWilliams 2002; CitationKaback et al 2004; CitationGandolfi and Cimino 2003; CitationHollo et al 2005).
Second, in many glaucoma and OHT studies, sample size is limited by the exclusion of fellow eye results. There is current debate as to whether or not fellow eyes in glaucoma exhibit concordant IOP changes, response to treatment, and disease progression. Some have used fellow eyes as an internal control (CitationWilliams 2002). Others have observed independent risk factors and disease progression between fellow eyes (CitationLevine et al 2006; CitationChen and Bhandari 2000). Realini and associates have observed variable fellow eye concordance in different studies. Observations from a 2002 study led Realini et al to conclude that the frequency and magnitude of spontaneous asymmetric fluctuations in IOP between fellow eyes in glaucoma patients could potentially confound the interpretation of monocular drug trials (CitationRealini et al 2002). A 2004 study by Realini and colleagues concluded that the response of the second eye to glaucoma medication could not be adequately predicted from the response of the first eye (CitationRealini et al 2004). In contrast, a 2005 study by Realini and Vickers observed marked symmetry in fellow eye IOP response to glaucoma medications (CitationRealini et al 2005).
Recently, Dinn et al observed that the diurnal variation of IOP in POAG is largely concordant between fellow eyes, but that fellow eye IOP may fluctuate asymmetrically a minority of the time (CitationDinn et al 2007). In view of evidence for independent risk factors and conflicting observations regarding symmetrical response to medication, we chose to consider fellow eyes independently.
The methodology and results of our mass change to travoprost were comparable to those of CitationLaw et al (2005) in several ways. Both were nonrandomized analyses of a change from one prostaglandin analogue to another. The medication switch in both studies occurred because of a formulary change rather than inadequate IOP control or side effects.
Of particular interest is our observation that in our patient population IOP control was maintained after switching patients with stable IOP. Furthermore, some patients exhibited a lower IOP on travoprost at the 6-month visit despite previous adequate control with latanoprost. An IOP decrease of an additional 3 mmHg or more occurred in at least one eye of greater than 20% of patients after switching from latanoprost to travoprost. In our study it was slightly more likely to achieve a 3 mmHg or more additional reduction in the concomitant (21 of 126 eyes) than in the monotherapy (7 of 60 eyes) group. This finding contrasts with the results observed by Law and colleagues, where most patients with ≥3 mmHg additional reduction were in the monotherapy group. An increase in IOP ≥ 3 mmHg occurred in 5 eyes of 4 patients, all in the concomitant therapy group. No patient in the monotherapy group showed an increase in IOP ≥ 3 mmHg at 6 months. These observations may support the theory that individual patients will have different responses to the medications in the prostaglandin class. Specifically, travoprost has been shown to be a full agonist at the FP receptor while latanoprost is a partial agonist (CitationGriffin et al 1997; CitationHellberg et al 2002; CitationSharif et al 2002). This difference in agonist activity could account for the differential responses seen in our study.
Another salient result in our study was that no significant increase in side effects was observed in the 6 month period following the switch to travoprost. In a 12-week trial involving more than 1000 patients, CitationPrzydryga et al (2004) found that 8% of patients experienced conjunctival hyperemia when switched to travoprost. In contrast, no increase in conjunctival hyperemia was reported by patients at any time after the switch to travoprost (compared to latanoprost baseline) in our study. One patient (2 eyes) chose to switch back to latanoprost due to irritation at the 3-month visit.
The Early Manifest Glaucoma Trial (EMGT) (CitationLeske et al 2003, Citation2004) suggested that each mean increase of 1 mmHg in IOP increases by 10% the risk of visual field deterioration. Further studies are needed to assess the clinical relevance of such small incremental changes in individual patients. Our firesults include data on decreases of up to 6 mmHg after 6 months on travoprost (see and ). The change seen in mean IOP in both groups is statistically significant, and may also be clinically relevant. However, we recognize that the most likely cause of the statistically significant mean IOP reduction is the number of patients whose IOP was lowered greater than 3mmHg compared to those whose IOP rose greater than 3mmHg. Therefore, in our study, a change in regimen from latanoprost to travoprost resulted in minimal change in IOP for most patients; however, a significant lowering of IOP was seen in some patients at 6 months. Few to none exhibited a significant rise in IOP which would prompt a further change in regimen.
The occurrence of further IOP decreases with travoprost in patients who were already controlled is intriguing. Maintenance of control and the absence of significant side effects (notably the absence of increased hyperemia) conferred no disadvantage to a substitution to travoprost in our study. In addition, the change in therapeutic regimen provided the opportunity to optimize control.
The nature of data collection subjects this study to several limitations. First, patients were sequentially, not randomly selected. However, patients selected for inclusion in the study are typical of those seen in clinical practice in regard to such factors as age, disease state, and potential for benefiting from medical intervention. In addition, circumstances of the therapeutic substitution such as the lack of a washout phase of the previous medication are typical of everyday clinical practice. Because the study was conducted at the VA, a predominantly male population, no female patients were included. No data on gender variation of PGA response are available, however there is no reason to suspect that such variation exists. Thus, the study patients are in many ways representative of the population to which the results will be applied.
In addition to non-randomization, the study was open-label, thus presenting the possibility that participants were influenced toward better compliance. However, such assessment bias was likely minimal because patients knew their medication was being substituted purely due to a change in formulary. In accordance with minimal patient risk, a waiver of consent had been obtained from the IRB, and patients were aware that the new medication was not required due to medication failure or advancing disease. Thus patient motivation for better compliance seems unlikely. Another limitation posed by our evaluation is the inclusion of multiple observers. Inter-observer differences in technique could account for some changes in IOP, and these potential differences are difficult to analyze separately. However, intra- and inter-observer variation have been reported to range from less than 1 mmHg to more than 3 mmHg (CitationSudesh et al 1993; CitationDielemans et al 1994; Kaufman et al 2004; CitationTonnu et al 2005). In addition, inter-observer differences were at least partially offset by the fact that all observers were third-year residents with similar training administered by Dr. Farris on the proper method of Goldmann tonometry. The same tonometer was used for every patient at every visit. The tonometer was preset to 20 mmHg prior to any measurements being performed and was calibrated monthly. Moreover, it is unlikely that most differences introduced by inter-observer variation would fall in the same direction and account for the statistically significant results obtained in our study. A further limitation of the study was observation of a single IOP at all study points. Diurnal variation was minimized, however, by measuring all IOP within a 3-hour window in the afternoon. It seems unlikely that any of the above limitations could account for the high percentage of patients with a decrease in IOP versus the low percentage with an increase after the regimen change.
Conclusions
A large scale change in regimen from latanoprost 0.005% to travoprost 0.004% maintained IOP control in stable patients and in some produced a further reduction in IOP. Reductions from baseline occurred at 6 months in the monotherapy and concomitant groups. There were no patient-reported events of increased hyperemia throughout 6 months of treatment. A change in therapy from latanoprost to travoprost was effective and well-tolerated for the glaucoma patients in this study.
Acknowledgments
The author acknowledges the contributions of Rhonda R. Porterfield, MD, Julie Y. Crider, PhD, and Jenny Song, MS for medical writing, editing, and statistical analyses, respectively. Publication support was provided by Alcon Laboratories, Inc.
References
- CamrasCB1995Mechanism of the prostaglandin-induced reduction of intraocular pressure in humansAdv Prostaglandin Thromboxane Leukot Res23519257732900
- ChenPPBhandariA2000Fellow eye prognosis in patients with severe visual field loss in 1 eye from chronic open-angle glaucomaArch Ophthalmol118473810766132
- CrawfordKKaufmanPL1987Pilocarpine antagonizes prostaglandin F2 alpha-induced ocular hypotension in monkeys. Evidence for enhancement of uveoscleral outflow by prostaglandin F2 alphaArch Ophthalmol1051112163477218
- DielemansIVingerlingJRHofmanA1994Reliability of intraocular pressure measurement with the Goldmann applanation tonometer in epi-demiological studiesGraefes Arch Clin Exp Ophthalmol23214148188062
- DinnRBZimmermanMBShubaLM2007Concordance of diurnal intraocular pressure between fellow eyes in primary open-angle glaucomaOphthalmology1149152017467528
- GabeltBTKaufmanPL1989Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkeyExp Eye Res493894022792235
- GandolfiSACiminoL2003Effect of bimatoprost on patients with primary open-angle glaucoma or ocular hypertension who are nonresponders to latanoprostOphthalmology1106091412623831
- GriffinBWWilliamsGWCriderJY1997FP prostaglandin receptors mediating inositol phosphates generation and calcium mobilization in swiss 3T3 cells: a pharmacological studyJ Pharmacol Exp Ther281845549152393
- HellbergMRMcLaughlinMASharifNA2002Identification and characterization of the ocular hypotensive efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist, and AL-6598, a DP pros-taglandin receptor agonistSurv Ophthalmol47Suppl 1S133312204698
- HolloGVarghaPKothyP2005Influence of switching to travoprost on intraocular pressure of uncontrolled chronic open-angle glaucoma patients compliant to previously-used topical medicationCurr Med Res Opin211943816368044
- KabackMGeanonJKatzGSTART Study Group2004Ocular hypotensive efficacy of travoprost in patients unsuccessfully treated with latanoprostCurr Med Res Opin201341515383181
- KaufmannCBachmannLMThielMA2004Comparison of dynamic contour tonometry with goldmann applanation tonometryInvest Ophthalmol Vis Sci4531182115326129
- LawSKSongBJFangE2005Feasibility and efficacy of a mass switch from latanoprost to bimatoprost in glaucoma patients in a prepaid Health Maintenance OrganizationOphthalmology11221233016225924
- LeskeMCHeijlAHusseinM2003Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol121485612523884
- LeskeMCHeijlAHymanL2004Factors for progression and glaucoma treatment: the Early Manifest Glaucoma TrialCurr Opin Ophthalmol15102615021220
- LevineRADemirelSFanJOcular Hypertension Treatment Study Group2006Asymmetries and visual field summaries as predictors of glaucoma in the ocular hypertension treatment studyInvest Ophthalmol Vis Sci47389690316936102
- NilssonSFSamuelssonMBillA1989Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2 alpha-1-isopropylester in the cynomolgus monkeyExp Eye Res48707162737263
- PrzydrygaJTEgloffCSwiss Start Study Group2004Intraocular pressure lowering efficacy of travoprostEur J Ophthalmol144162215506604
- RealiniTBarberLBurtonD2002Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucomaOphthalmology10913677112093664
- RealiniTFechtnerRDAtreidesSP2004The uniocular drug trial and second-eye response to glaucoma medicationsOphthalmology111421615019313
- RealiniTVickersWR2005Symmetry of fellow-eye intraocular pressure responses to topical glaucoma medicationsOphthalmology11259960215808250
- SharifNAKellyCRCriderJY2002Agonist activity of bimatoprost, travoprost, latanoprost, unoprostone isopropyl ester and other pros-taglandin analogs at the cloned human ciliary body FP prostaglandin receptorJ Ocul Pharmacol Ther183132412222762
- SudeshSMoseleyMJThompsonJR1993Accuracy of Gold-mann tonometry in clinical practiceActa Ophthalmol (Copenh)7118588333263
- TonnuPAHoTSharmaK2005A comparison of four methods of tonometry: method agreement and interobserver variabilityBr J Ophthalmol898475015965164
- WilliamsRD2002Efficacy of bimatoprost in glaucoma and ocular hypertension unresponsive to latanoprostAdv Ther192758112665048
Appendix
Patient Survey
Patients were asked the following questions before and after the change in regimen from latanoprost to travoprost. Answers were recorded in the patients’ medical record.
Questions at baseline:
Do you experience any stinging/burning/irritation when placing your latanoprost drop?
Yes/No
Have you noticed an increase in the length of eyelashes since beginning latanoprost?
Yes/No
Have you noticed an increase in the darkness around your eyes since beginning latanoprost?
Yes/No
Has there been any change in the color of your eyes since beginning latanoprost?
Yes/No
Have you noticed any increase in eye redness since beginning latanoprost?
Yes/No
Questions at follow-up:
Have you noticed any increase or decrease in stinging/burning/irritation since the switch to travoprost? Identify/None
Have you noticed any increase in eyelash length since the switch to travoprost?
Yes/No
Have you noticed any increase in darkness around your eyes since the switch to travoprost?
Yes/No
Have you noticed any change in the color of your eyes since the switch to travoprost?
Yes/No
Has there been any increase in eye redness since the switch to travoprost?
Yes/No