Abstract
Since their discovery in the 1950s interferons have been the scope of investigation in many diseases as therapeutic as well as pathogenetic factors. We know they have immune stimulatory and immune regulatory effects. This apparently counter-intuitive mechanism can be summarized as immunomodulatory action and seems to be very effective in a number of ocular inflammatory diseases. We review the current knowledge of interferons in immunity and autoimmunity and show their use in clinical ophthalmologic practice.
Keywords:
What are interferons?
Interferons (IFNs) were discovered in 1957Citation1 as natural antiviral substances produced during viral infection and were characterized for their ability to ‘interfere’ with viral replication, reduce cell proliferation, and alter immunity. They have received attention because of their diverse effects, influencing both innate and adaptive immune responses;Citation2 thus they are involved as well in defense against viral infections, tumor growth, and tolerance induction as well suspected inducers of autoimmune disease, such as sarcoidosis.Citation3,Citation4 At the same time they have been used successfully as treatment for autoimmune diseases such as multiple sclerosis.Citation5 Thus in autoimmunity IFNs appear as double agents, involved in both supportive and suppressive action.
The different IFNs have been classified into type I, with the large group of IFN-α isotypes and IFN-β as the most immunologically relevant, and type II, with the single member IFN-γ, based on their amino acid composition and biologic properties. Type I IFNs share a common receptor, the IFN-α/β receptor, IFNAR, whereas IFN-γ as the only representative of type II IFNs binds to a different receptor, IFNGR.
There is a single IFN-β, while there are at least 13 different IFN-α isotypes. Evidence suggests that they have distinct functions. Their expression seems to depend on the stimulating agent and the cell type.Citation6
In addressing the role of IFN-α/β in autoimmunity, it is important to recognize that in addition to direct action their effect may be mediated by synergistic or antagonistic interactions with other cytokines. As the clinical experience is limited mainly to type I IFNs, for sake of clarity and space restrictions we concentrate on these.
Virtually any cell can produce type I IFNs, but antigen presenting cells, especially plasmacytoid dendritic cells, are the main producers of type I IFNs, already at early stages of the immune response. Thus, type I IFNs, especially IFN-α, may be the pivotal cytokines linking the innate with the adaptive immune system. Low levels of type I IFNs seem to be prerequisites for the upregulation of type I IFNs as a reaction to viral infection and subsequent induction of IFN-γ production. This finally leads to the induction and maintenance of T-helper type 1 cells, CD8+ cytotoxic T cells, and natural killer cells that fight the viral intrusion. In contrast, type I IFNs have also been shown to exert an antiproliferative and proapoptotic effect on T cells. Another IFN effect is the development of tolerance-promoting regulatory T cells. Equally there has been a positive as well as an inhibitory effect described on B-cell development and survival.Citation6,Citation7
IFNs also have been shown to be involved in the pathogenesis of several autoimmune diseases as systemic lupus or type I diabetes.Citation8,Citation9 Systemic lupus is a highly heterogeneous, multiorgan disorder primarily afflicting women and is characterized by the production of diverse autoantibodies, predominantly against nucleosomal and spliceosomal antigens. Type I IFNs, central to both innate and adaptive immunity, have received particular attention for their role in the development of autoimmune responses, and a preponderance of evidence supports their disease-promoting activity in lupus (reviewed in).Citation8,Citation10 In humans with active disease, levels of IFN-α are increased in serum and affected tissues.
Supposed mechanisms of action
IFNs are thought to have immunomodulatory effects rather than immunosuppressive mode of action. This is supported by insights from animal models of uveitis (see below), but also from studies in humans that were treated with IFNs.
IFN in animal models
Experimental autoimmune encephalomyelitis (EAE) is a well-established rodent model of CNS-specific inflammatory disease and is known to be the best animal model for studying the etiology and pathogenesis of encephalomyelitis disseminata (ED). It is mediated by T cells and results in progressive demyelination and paralysis.
There are several subtypes of T cells in the immune system. They are distinguished by their surface receptors and cytokine repertoire and their differentiation is influenced by the cytokines in their environment. For quite some time the (dis)balance of TH1 (autoagressive) and Th2 (downregulating) CD4+ T cells was thought to induce autoimmunity. Newer research showed the existence of several more T cell subtypes, as the CD25+, FOXP3+ T “regulatory” T cells, or so-called Th17, IL-17 producing, possibly autoimmunity supporting cells.Citation11
As in ED, EAE is characterized by a breakdown of the blood–brain barrier. The inflammatory response is characterized by T cell infiltrates located around vessels of the CNS white matter, by activation of local microglia and astrocytes, and in the most severe cases it may be eventually followed by demyelination. Anterior uveitis (AU) has been found to coincide with EAE in rabbits, monkeys, in the Lewis rat,Citation12,Citation13 and in mice.Citation14,Citation15 The encephalitogenic T cells are specific for the antigen myelin basic protein (MBP), which is a component of the myelinated sheath surrounding nerve bundles. These myelinated nerve bundles are abundant in the spinal cord and in the iris; thus this “autoantigen” is located at sites of inflammation. In the rodent models of EAE, AU generally persists after the paralysis has subsided. EAE and AU can be induced actively by immunizing with MBP in the presence of adjuvant, or passively by using adoptive transfer of MBP-specific T cells that have been generated against the whole antigen or encephalogenic peptides. Mice deficient in IFN-β showed augmented and chronic EAE.Citation16 Histopathological investigations of CNS in the effector phase revealed an extensive microglia activation and TNF-α production in IFN-β KO mice; this was virtually absent in wildtype littermates. This coincided with an increase in effector functions of T cells in IFN-β KO mice. The authors suggested that that the lack of IFN-β leads to persistent activation of residual antigen presenting cells, which results in prolonged inflammation and extensive demyelination. Another more recent study used EAE as a model to investigate the role of the TRIF-dependent IFN induction pathway of the innate immune system in the regulation of autoimmune inflammation, showing that Th-17 mediated autoimmune inflammation was negatively regulated via IFN-induced IL-27 production in macrophages.Citation17
Another mouse model of intraocular inflammation is experimental autoimmune uveitis (EAU), which is induced by interphotoreceptor retinoid-binding protein (IRBP) in rats or mice. EAU can be mitigated or prevented by IFN-α2a or -β treatment, in the animal model even an oral application was shown to be effective.Citation18–Citation21
IFN in human disease
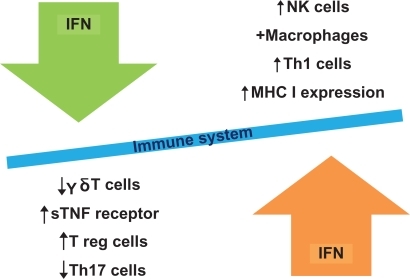
Generally type I IFNs have been shown to have multiple immunostimulatory as well as immunosuppressive effects.Citation6,Citation22 On the one hand, a shift towards Th1 T cell response and related cytokines has been shown, an increase in MHC class I expression and cellular adhesion molecules, an increase in cytotoxicity of T cells, and especially increase in numbers as well as in activity of NK cells.Citation23 This is helpful to eliminate foreign antigen and would explain the anti-viral and anti-tumor effect, but would at the same time indicate an immune-stimulatory capacity that is promoting autoimmunity rather than treating it. IFN is used in treatment of melanoma, and especially in responders autoimmune disease has been described.Citation24 The development of autoimmunity was higher in the extended treatment group. IFNs are also involved in the development of autoimmune diseases as diabetes type I.Citation9 Thus, the immunoregulatory effects of IFNs are harder to grasp. Especially in Behçet’s disease (BD) investigations have been undertaken: Treusch and colleagues have shown that numbers of g-d T cells are normalizedCitation25 and soluble TNF receptor increases.Citation26 These apparently incompatible stimulatory versus regulatory effects (see ) were reconciled by more recent results from human studies showing an upregulation of T regulatory cells in patients with BD treated with IFN.Citation27 Wang and colleagues showed that IFN-α2b upregulates STAT5 and downregulates STAT3, in conjunction with upregulation of Treg and inhibition of IL-17-expressing lymphocytes in melanoma tissues.Citation28 Similarly, it has been shown that IFN-β1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation.Citation29 The discovery of the Th17 cell lineage marked a new era in the studies of the autoimmune response, as it resolved many controversial findings not compatible with the Th1 paradigm of autoimmune response.Citation30 In summary the influence of type I IFNs on the immune system is still not fully elucidated, but seems to be a very complex web of dosage, timing, and type of cell involved.
A number of clinical trials and case series have been published on systemic treatment with type I IFNs in uveitis. lists these in detail. The mode of administration generally is subcutaneous. Intravitreal application has been tried in only 2 patients with advanced neovascular age-related macular degeneration with little response and was not pursued any further due to a reversible reduction in the light response on electroretinography after 1 month.Citation31
Table 1 Systemic interferon treatment in ocular inflammatory disease
Behçet’s disease (BD)
BD is a multisystem disorder that can affect basically all organs. It is common in countries along the old silk route from Turkey and Japan. Uveitis in a patient with BD is accompanied by severe inflammation with occlusive vasculitis that can lead to visual impairment. Therefore, many drugs have been investigated for their potential in preventing ocular recurrences in patients with BD, including azathioprine,Citation32 cyclosporine A,Citation33 and infliximab.Citation34
As a viral pathogenesis is thought to play a role in the pathogenesis of BD, IFN-α was introduced in 1986 for its antiviral activity, with encouraging results.Citation35 In 1994 Feron et alCitation36 used IFN-α for the first time in patients with ocular BD. Kotter et alCitation37 have published the widest experience in treating patients with BD with IFN-α. They published an open, noncomparative prospective study including 50 subjects. In this study, the drug was administered initially at a dose of 6 million units daily, with dose and frequency adjusted depending on the clinical response. A response rate of 92% was reported, with significant improvements in both visual acuity and posterior uveitis score. More than one third of patients were off treatment and disease-free at an average of 2.5 years. The IFN treatment was also beneficial for extraocular manifestations of BD, although less so for oral ulcerations.
Alpsoy and colleaguesCitation38 performed a randomized, placebo-controlled, double-blind trial showing IFN-α2a to be superior to placebo in oral and genital ulcer reduction as well as in ocular flare reduction. The study included 50 patients, of whom nine had ocular involvement. Other groups have published case series supporting therapeutic effects of IFN-α in this subtype of uveitis.Citation39–Citation43
There have been 2 meta-analysis of the literature performed.Citation44,Citation45 The first analysis included 32 original reports and 4 selected abstracts. Systemic IFN-α was administered to 338 patients, including 182 with acute ocular disease. Eighty-six percent of the patients with mucocutaneous symptoms, 96% with arthritis, and 94% with uveitis exhibited a partial or complete response. Higher IFN doses were more effective than low-dose regimens. Long-term remissions seem to be associated with higher doses, but not longer duration of treatment. IFN-α2a apparently was superior to IFN-α2b.
The second included 22 studies with 144 participants (some participants were shared between studies). Fourteen studies (70 participants) reviewed treatment with IFN-α2a and 8 studies (74 participants) reviewed treatment with IFN-α2b. Seventy-four percent of patients with mucocutaneous manifestations, 95% of patients with uveitis, and 93% of patients with arthropathy/arthritis exhibited a partial or complete response. IFN-α2a regimens were more effective than IFN-α2b ones on mucocutaneous (47% versus 7% complete response) and ocular (67% versus 8% complete response; P < 0.001) manifestations.
Bodaghi and colleagues presented a retrospective evaluation of 45 patients treated with IFN-α2a.Citation46 About half of the patients had BD. Especially in these patients, the treatment was effective (82.6%), but it was also judged effective in 59% of the patients with other types of uveitis. Subsequently, it was possible to significantly reduce oral prednisone in both groups. The same group published their results for 32 patients with BD and IFN in 2008Citation47 and also for pediatric BD.Citation48 In contrast to the Koetter group, these authors started IFN treatment together with an iv methylprednisolone pulse and subsequent oral taper, reaching a final mean of 7 mg of oral prednisone/day. Colchicine was allowed as adjunct treatment. Another group recently published their long-term results on 45 patients with BD and also started with 100 mg of prednisone and subsequent rapid taper down to 10 mg in 2 weeks.Citation43 See also below for controversial opinions about additional immunosuppressive treatment.
Several case series about the effective use of IFN-α inhibitors in BD have been published (for a review seeCitation49). EULAR recommendations say to introduce either cyclosporine or infliximab as a second line agent in refractory eye involvement; alternatively IFN-α can be used.Citation50 So far no direct comparison of IFN-α inhibitors and IFNs or other immunosuppressive agents and IFN have been performed, but a multicentric national trial is currently ongoing comparing IFN versus cyclosporine (INCYTOB, see clinicaltrials.gov).
Encephalomyelitis disseminata (multiple sclerosis)
Intermediate uveitis is the most frequent form of ED-associated uveitis. Anterior uveitis is rare in patients with ED, but if it occurs is of the granulomatous subtype.Citation51,Citation52 A sign of intermediate uveitis are snowbanks and snowballs. Especially in intermediate uveitis accompanying ED, snowbanks and continous retinal periphlebitis in combination seem to be typical.Citation53,Citation54 In patients with this type of uveitis, secondary changes like the formation of cystoid macular edema (CME) or occlusive vasculitis with vasoproliferations can develop (), which may be complicated by retinal detachments or vitreous hemorrhage.Citation55 Especially macular edema with subsequent epiretinal membrane formation is a challenge and a threat to visual prognosis.
There is increasing evidence that IFN is very effective in treatment of uveitis associated with ED, especially the accompanying macular edema. We used type 1 IFNs to treat uveitis associated with multiple sclerosis that was refractory to corticosteroid treatment in a retrospective, multicenter observational case series. Thirteen patients (8 female, 5 male) with proven multiple sclerosis and associated uveitis in 25 eyes from 5 uveitis centers were treated with IFN-β1a. Visual acuity improved in 17 eyes (71%), 5 did not change (21%), and 2 eyes deteriorated (8%) because of development of cataract. CME resolved after or during IFN treatment in 82% of the eyes. Side effects were noted in three patients (elevation of liver enzymes in 1 patient, depression in 1, and joint pain in 1). At the last visit, 9 patients (69%) had discontinued systemic corticosteroids; 3 were taking 10 mg of prednisone or less. Treatment of multiple sclerosis-associated uveitis with IFN appeared to have beneficial effects on visual acuity, intraocular inflammation activity, and the presence of CME in this study.Citation56,Citation57 First results of a randomized, controlled, clinical trial have been presented at the Association for Research in Vision and Ophthalmology (ARVO) meeting, indicating superiority of IFN over methotrexate in patients with intermediate uveitis with or without ED.Citation58
Inflammatory macular edema
Macular edema is a major cause of vision loss in patients with uveitis.Citation59 Diverse treatments are in use, which include periocular or intravitreal corticosteroid injections, systemic corticosteroids, acetazolamide, immunosuppressive medications, octreotides and even intravitreal bevacizumab injections.Citation60–Citation63 None of these medications has been tested in a randomized, controlled, clinical trial.
Deuter et alCitation57 were the first to show a positive effect of IFN-α on uveitic CME in a prospective case series. The authors treated 8 patients (2 male, 6 female) with IFN-α2a at an initial dosage of 3 or 6 million units daily, depending on body weight. All patients had inactive primary uveitis with CME that had not responded to systemic corticosteroids and acetazolamide previously. In seven patients, a response to IFN-α2a was seen within 3 days, and CME completely disappeared after 2 to 4 weeks in all 13 eyes in these patients. In the nonresponder, anti-IFN-α2a antibodies were discovered. Recently, the authors published their experiences in the long-term treatment of 24 patients.Citation64
Other uveitis subtypes
PlskovaCitation65 and colleagues published their experiences with IFN-alpha 2b in severe posterior or panuveitis. Two of their patients were diagnosed with BD, 1 sympathetic ophthalmia, the rest were idiopathic. A positive clinical response was observed in 83% of their patients. Bodaghi and coauthors published a retrospective evaluation of 45 patients treated with IFN-α2a.Citation46 About half of the patients had BD, but 22 had other forms of uveitis. In 59% of these patients, the treatment was judged effective.
Specifics of IFN treatment
Types of IFN available for treatment
Three different IFN-β drugs (Avonex® [β1a], Rebif ® [β1a], Betaseron® [β1b]) and 2 IFN-α drugs (Intron A® [IFN-α2b], Roferon® [IFN-α2a]) are available.
IFN ± other immunosuppressive treatment?
Owing to the particular mode of action of IFNs (immunomodulatory rather than immunosuppressive), it is thought that IFNs need an unsuppressed immune system. In 2006Citation66 we wrote in a similar review on IFNs “Therefore, it is recommended to use additional corticosteroids only in low doses. The concurrent use of immunosuppressives should be avoided during IFN treatment.” This was proposed especially by Kotter and coworkers previously.Citation67 They based this recommendation on their clinical experience,Citation68 backed up by the rationale that the activity of NFκB is reduced by glucocorticoids. NFκB is a transcription factor that is thought to regulate the sensitivity of cells toward IFN-mediated antiviral activityCitation69 and is itself activated by IFN in a signaling pathway that protects cells against apoptosis.Citation70 Recently, several authors/studies put this paradigm to a test: Gueudry et alCitation47 treated BD uveitis with initial methylprednisolone pulse and IFN-α and never aimed to taper corticosteroids completely. In a case series of 2 patients with BD and uveitis Harmuryudan et alCitation71 combined IFN and azathioprine with good results. As can be seen in , different groups have used IFNs in combination with immunosuppressive agents. There is also increasing evidence from the ED literature that adding corticosteroids or other immunosuppressives to IFN reduces formation of neutralizing antibodies and thus improves clinical response.Citation72 Especially one pilot study could show this for a combination of IFN-β1a and mycophenolate mofetil (Cellcept®),Citation73 clinical trials addressing this topic are currently undertaken.
Apparently this is a preventive measure and does not work once neutralizing antibodies have been formed.Citation74
Management of IFN therapy
A close collaboration between other specialists and ophthalmologists is helpful to optimally direct IFN treatment. It is necessary to perform an exact medical history before starting IFN therapy focusing on autoimmune diseases (besides uveitis), impaired thyroid or liver function, and previous or current depressive disorders in particular antecedents of suicidal ideation. Patients should be informed of the most frequent adverse reactions associated with IFN therapy to improve compliance.
Secondary effects
Common side effects of IFN include injection-site reactions, flu-like symptoms (fever, headache, myalgia, arthralgia, sweating, and fatigue), leukopenia, liver enzyme elevations, and alopecia. The flu-like symptoms can be alleviated by concomitant administration of paracetamol and seem to represent a good prognostic marker for a response to IFN treatment, as this may indicate the absence of pre-existing anti-IFN autoantibodies. Usually, severity of these symptoms will decrease in the course of therapy. Patients with cardiac disease, such as angina, congestive heart failure, or arrhythmia, should be closely monitored for worsening of their clinical condition during initiation of therapy with IFNs as symptoms of the flu-like syndrome may prove stressful to patients with cardiac conditions.
Depression and suicidal intentions can occur during therapy with IFN independent of preexisting psychiatric disease.Citation75 Patients treated with IFNs should be advised to immediately report any symptoms of depression and/or suicidal ideation to their prescribing physician.
IFN-α in combination with ribavirin treatment in hepatitis C infection has led to development of sarcoidosis in some patients.Citation3 If this is due to HCV itself acting as an antigenic trigger for the development of sarcoidosis in susceptible patients, or the action of ribavirin in combination with IFN-α, or the latter alone is unknown so far. Of interest, no reports of IFN-β leading to sarcoidosis have been published so far, and no patients treated with IFN-α for other diseases such as BD have been diagnosed with sarcoidosis. Still, this may be due to the lower numbers in these cohorts.
Retinopathy (cottonwool spots and/or hemorrhages) has been reported as an ocular complication of hepatitis C treatment with IFN, with frequencies varying from 3.8% to 24% in studies, but generally with a good outcome.Citation76–Citation79 There are also about 6 single case reports of multiple sclerosis-related IFN-associated retinopathy.Citation80–Citation82
IFN therapy may induce formation of autoantibodies and other immune-mediated diseases as auto-immune thyroiditis.Citation24
Laboratory exams
Laboratory abnormalities are associated with the use of IFNs. Basic lab tests include complete and differential blood count, platelets, and liver enzymes (especially ALT). Lab tests should be repeated at months 1, 3, and 6 on therapy and periodically thereafter. Dose reduction of IFN therapy should be considered if ALT rises above 5 times the upper normal limit. Patients being treated with IFNs may occasionally develop new or worsening thyroid abnormalities. Thyroid function testing is recommended at baseline and if abnormal, every 6 to 12 months after initiation of therapy. If tests are normal at baseline, routine testing is not needed but should be performed if clinical findings of thyroid dysfunction appear (ref: Summary of Product Characteristics).
Conclusion
IFNs have been shown highly effective in the treatment of uveitis. Most data exist for IFN-α2a in BD, from 1 placebo controlled trial and 2 meta-analyses. IFN-α may be given for ocular BD not responsive to a first-line immunosuppressive, as per EULAR recommendations.Citation50 Evidence is emerging for the use of IFN in the treatment of inflammatory macular edema, but final results from a prospective clinical trial are lacking.
Disclosures
The authors are performing a clinical trial on interferon beta in patients with uveitic macular edema, partially supported by Merck Serono GmbH.
References
- IsaacsALindenmannJVirus interference. I. The interferonProc R Soc Lond B Biol Sci195714792725826713465720
- BaccalaRKonoDHTheofilopoulosANInterferons as pathogenic effectors in autoimmunityImmunol Rev200520492615790347
- AdlaMHepatic sarcoidosis associated with pegylated interferon alfa therapy for chronic hepatitis C: case report and review of literatureDig Dis Sci200853102810281218320314
- DoychevaDInterferon-alpha-associated presumed ocular sarcoidosisGraefes Arch Clin Exp Ophthalmol2009247567568019034483
- LublinFHistory of modern multiple sclerosis therapyJ Neurol2005252Suppl 3iii3iii916170498
- TheofilopoulosANType I interferons (alpha/beta) in immunity and autoimmunityAnnu Rev Immunol20052330733615771573
- BaccalaRTLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunityNat Med200713554355117479100
- BanchereauJPascualVType I interferon in systemic lupus erythematosus and other autoimmune diseasesImmunity200625338339216979570
- DevendraDEisenbarthGSInterferon alpha – a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunityClin Immunol2004111322523315183143
- BennettLInterferon and granulopoiesis signatures in systemic lupus erythematosus bloodJ Exp Med2003197671172312642603
- SteinmanLA brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damageNat Med200713213914517290272
- AdamusGMyelin basic protein specific T-Helper cells induce experimental anterior uveitisJ Neurosci Res19964465135188794942
- VerhagenCT cell immunity to myelin basic protein induces anterior uveitis in Lewis ratsJ Neuroimmunol199453165717519633
- ShaoHInduction of autoimmune encephalomyelitis and uveitis in B6 and (B6 × SJL) mice by peptides derived from myelin/oligodendrocyte glycoproteinJ Neuroimmunol20021321–211712212417441
- ConstantinescuCSConstantinescuLEAnterior uveitis in murine relapsing experimental autoimmune encephalomyelitis (EAE), a mousemodel ofmultiple sclerosis (MS)Curr Eye Res2000201717610611718
- TeigeIIFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitisJ Immunol20031709477648412707359
- GuoBThe type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in miceJ Clin Invest200811851680169018382764
- OkadaAAEffect of type I interferon on experimental autoimmune uveoretinitis in ratsOcul Immunol Inflamm1998642152269924918
- StubigerNInterferon alpha 2a in IRPB-derived peptide-induced EAU – part IAdv Exp Med Biol200352853754012918759
- SuzukiJOral administration of interferon-beta suppresses experimental autoimmune uveoretinitisGraefes Arch Clin Exp Ophthalmol2002240431432111981647
- MizuguchiJType I interferons as immunoregulatory molecules; implications for therapy in experimental autoimmune uveoretinitisArch Immunol Ther Exp (Warsz)200250424325412371620
- BrassardDLInterferon-alpha as an immunotherapeutic proteinJ Leukoc Biol200271456558111927642
- PfefferLMBiological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferonsCancer Res19985812248924999635566
- GogasHPrognostic significance of autoimmunity during treatment of melanoma with interferonN Engl J Med2006354770971816481638
- TreuschMInfluence of human recombinant interferon-alpha2a (rhIFN-alpha2a) on altered lymphocyte subpopulations and monocytes in Behcet’s diseaseRheumatology (Oxford)200443101275128215252211
- KotterICytokines, cytokine antagonists and soluble adhesion molecules in patients with ocular Behcet’s disease treated with human recombinant interferon-alpha2a. Results of an open study and review of the literatureClin Exp Rheumatol2005234 Suppl 38S20S2616273760
- YangDSInterferon-alpha in the management of patients with Behcet’s diseaseBr J Hosp Med (Lond)2008691057557918949947
- WangWEffects of high-dose IFNalpha2b on regional lymph node metastases of human melanoma: modulation of STAT5, FOXP3, and IL-17Clin Cancer Res200814248314832019088050
- ZhangXIFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulationJ Immunol200918263928393619265172
- IwakuraYIshigame H. The IL-23/IL-17 axis in inflammationJ Clin Invest200611651218122216670765
- KertesPJIntravitreal interferon alpha-2b for the treatment of neovascular age-related macular degeneration: a pilot studyCan J Ophthalmol19973231851889131284
- YaziciHA controlled trial of azathioprine in Behcet’s syndromeN Engl J Med19903222812852404204
- NussenblattRBEffectiveness of cyclosporin therapy for Behcet’s diseaseArthritis Rheum19852866714004976
- Tugal-TutkunIEfficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behcet’s disease: an open-label trialArthritis Rheum20055282478248416052571
- TsambaosDBehcet’s syndrome: treatment with recombinant leukocyte alpha-interferonArch Dermatol Res198627843353363740944
- FeronEJInterferon-à2b for refractory ocular Behcet’s diseaseLancet1994343891014287910903
- KotterIDifferential efficacy of human recombinant interferon-alpha2a on ocular and extraocular manifestations of Behcet disease: results of an open 4-center trialSemin Arthritis Rheum200433531131915079762
- AlpsoyEInterferon alfa-2a in the treatment of Behcet disease: a randomized placebo-controlled and double-blind studyArch Dermatol2002138446747111939808
- CalguneriMEffects of interferon alpha treatment on the clinical course of refractory Behcet’s disease: an open studyAnn Rheum Dis200362549249312695172
- WechslerBEfficacy of interferon alfa-2a in severe and refractory uveitis associated with Behcet’s diseaseOcul Immunol Inflamm20008429330111262659
- KrauseLInterferon alfa-2a in the treatment of ocular Adamantiades-Behcet’s diseaseAdv Exp Med Biol200352851151912918754
- Tugal-TutkunIResults of interferon-alfa therapy in patients with Behcet uveitisGraefes Arch Clin Exp Ophthalmol2006244121692169516673135
- KrauseLLong term visual prognosis of patients with ocular Adamantiades-Behcet’s disease treated with interferon-alpha-2aJ Rheumatol200835589690318412306
- KotterIThe use of interferon alpha in Behcet disease: review of the literatureSemin Arthritis Rheum200433532033515079763
- ZouboulisCCOrfanosCETreatment of Adamantiades-Behcet disease with systemic interferon alfaArch Dermatol19981348101010169722733
- BodaghiBEfficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patientsBr J Ophthalmol200791333533917050581
- GueudryJLong-term efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behcet diseaseAm J Ophthalmol2008146683784419027420
- Guillaume-CzitromSEfficacy and safety of interferon-alpha in the treatment of corticodependent uveitis of paediatric Behcet’s diseaseRheumatology (Oxford)200746101570157317702770
- SfikakisPPAnti-TNF therapy in the management of Behcet’s disease – review and basis for recommendationsRheumatology (Oxford)200746573674117403712
- HatemiGEULAR recommendations for the management of Behcet diseaseAnn Rheum Dis200867121656166218245110
- LimJIAnterior granulomatous uveitis in patients with multiple sclerosisOphthalmology1991981421452008270
- MeislerDMAnterior uveitis and multiple sclerosisCleve Clin J Med1989565355382766545
- BregerBCLeopoldIHThe incidence of uveitis in multiple sclerosisAm J Ophthalmol1966625405455920437
- BiousseVMultiple sclerosis associated with uveitis in two large clinic-based seriesNeurology19995211791819921871
- ValentincicNVitreous hemorrhage in multiple sclerosis-associated uveitisOcul Immunol Inflamm2007151192517365802
- BeckerMDInterferon as a treatment for uveitis associated with multiple sclerosisBr J Ophthalmol200589101254125716170111
- DeuterCMInterferon alfa-2a: a new treatment option for long lasting refractory cystoid macular edema in uveitis? A pilot studyRetina200626778679116963852
- BeckerMFirst results of a randomized controlled clinical trial comparing interferon beta with methotrexate for the treatment of intermediate uveitis with macular edemaInvest Ophthalmol Vis Sci200849E-Abstract 1118.
- RothovaACauses and frequency of blindness in patients with intraocular inflammatory diseaseBr J Ophthalmol1996803323368703885
- MackensenFIntravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot studyRetina2008281414518185136
- RothovaAMedical treatment of cystoid macular edemaOcul Immunol Inflamm200210423924612854032
- MissottenTOctreotide long-acting repeatable for the treatment of chronic macular edema in uveitisAm J Ophthalmol2007144683884317916316
- SchillingHLong-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammationRetina200525218218815689809
- DeuterCMEfficacy and tolerability of interferon alpha treatment in patients with chronic cystoid macular oedema due to non-infectious uveitisBr J Ophthalmol200993790691319321469
- PlskovaJInterferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitisAm J Ophthalmol20071441556117601428
- MackensenFInterferon therapy for ocular diseaseCurr Opin Ophthalmol200617656757317065927
- KotterIHuman recombinant interferon alfa-2a for the treatment of Behcet’s disease with sight threatening posterior or panuveitisBr J Ophthalmol200387442343112642304
- DeuterCMBehcet’s disease: ocular effects and treatmentProg Retin Eye Res200827111113618035584
- PfefferLMRole of nuclear factor-kappaB in the antiviral action of interferon and interferon-regulated gene expressionJ Biol Chem200427930313043131115131130
- YangCHIFNalpha/beta promotes cell survival by activating NF-kappa BProc Natl Acad Sci U S A20009725136311363611095741
- HamuryudanVInterferon alfa combined with azathioprine for the uveitis of Behcet’s disease: an open studyIsr Med Assoc J2002411 Suppl92893012455182
- PozzilliCMonthly corticosteroids decrease neutralizing antibodies to IFNbeta1 b: a randomized trial in multiple sclerosisJ Neurol20022491505611954868
- VermerschPCombination of IFN beta-1a (Avonex) and mycophenolate mofetil (Cellcept) in multiple sclerosisEur J Neurol2007141858917222119
- RavnborgMTreatment with azathioprine and cyclic methyl-prednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosisMult Scler200915332332819028832
- PanitchHBenefits of high-dose, high-frequency interferon beta-1a in relapsing-remitting multiple sclerosis are sustained to 16 months: final comparative results of the EVIDENCE trialJ Neurol Sci20052391677416169561
- PanettaJDGilaniNInterferon induced retinopathy and its risk in patients with diabetes and hypertension undergoing treatment for chronic hepatitis C virus infectionAliment Pharmacol Ther2009622 [Epub ahead of print].
- WillsonRAVisual side effects of pegylated interferon during therapy for chronic hepatitis C infectionJ Clin Gastroenterol200438871772215319658
- CuthbertsonFMIs screening for interferon retinopathy in hepatitis C justified?Br J Ophthalmol200488121518152015548803
- d’AlterocheLOphthalmologic side effects during alpha-interferon therapy for viral hepatitisJ Hepatol2006441566116223542
- OhiraMRetinopathy: an overlooked adverse effect of interferon-beta treatment of multiple sclerosisKeio J Med2009581545619398885
- SaitoHRetinopathy in a multiple sclerosis patient undergoing interferon-therapyMult Scler200713793994017468441
- FoldenDVInterferon beta-associated retinopathy in patients treated for multiple sclerosisNeurology200870(13 Pt 2):1153115518362275