Abstract
Retinal hypoxia is the potentially blinding mechanism underlying a number of sight-threatening disorders including central retinal artery occlusion, ischemic central retinal vein thrombosis, complications of diabetic eye disease and some types of glaucoma. Hypoxia is implicated in loss of retinal ganglion cells (RGCs) occurring in such conditions. RGC death occurs by apoptosis or necrosis. Hypoxia-ischemia induces the expression of hypoxia inducible factor-1α and its target genes such as vascular endothelial growth factor (VEGF) and nitric oxide synthase (NOS). Increased production of VEGF results in disruption of the blood retinal barrier leading to retinal edema. Enhanced expression of NOS results in increased production of nitric oxide which may be toxic to the cells resulting in their death. Excess glutamate release in hypoxic-ischemic conditions causes excitotoxic damage to the RGCs through activation of ionotropic and metabotropic glutamate receptors. Activation of glutamate receptors is thought to initiate damage in the retina by a cascade of biochemical effects such as neuronal NOS activation and increase in intracellular Ca2+ which has been described as a major contributing factor to RGC loss. Excess production of proinflammatory cytokines also mediates cell damage. Besides the above, free-radicals generated in hypoxic-ischemic conditions result in RGC loss because of an imbalance between antioxidant- and oxidant-generating systems. Although many advances have been made in understanding the mediators and mechanisms of injury, strategies to improve the damage are lacking. Measures to prevent neuronal injury have to be developed.
Introduction
The structural and functional integrity of the retina depends on a regular oxygen supply. Being one of the most metabolically active tissues, retina consumes oxygen more rapidly than other tissues (CitationCohen and Noell 1965) such as the brain (CitationAnderson and Saltzman 1964; CitationAmes 1992). The presence of a dual circulation (CitationOsborne et al 2004) makes retinal oxygenation unique. The photoreceptors and the greater portion of the outer plexiform layer receive nourishment from the choriocapillaris indirectly whereas the inner retinal layers are supplied by the superficial and deep capillary plexuses formed by branches of the central artery of the retina. Inner layers of the retina are known to show highest sensitivity to hypoxic challenges (CitationJanáky et al 2007), whereas the outer retina is more resistant to a hypoxic stress (CitationTinjust et al 2002).
Retinal hypoxia occurs in ocular conditions such as central retinal artery occlusion and ischemic central retinal vein thrombosis. Hypoxia is also implicated in the development of glaucoma (CitationFlammer 1994; CitationTielsch et al 1995; CitationChung et al 1999; CitationOsborne et al 1999b; CitationCosta et al 2003; CitationTezel and Wax 2004), diabetes (CitationLinsenmeier et al 1998), and is thought to underlie many of the sight-threatening complications of diabetic eye disease including retinal and optic nerve head neovascularization. Systemic causes of retinal hypoxia include the cardiovascular effects of chronic obstructive airways disease, the ocular ischemic syndrome associated with arterial obstructive conditions such as carotid artery stenosis (CitationBrown and Magargal 1988) and Takayasu’s arteritis (CitationShelhamer et al 1985), hyperviscosity syndromes (CitationAshton et al 1963) or following trauma (Purtscher’s retinopathy; CitationPurtscher 1912; Buckley and James 1997). Retinal hypoxia associated with the above conditions is a common cause of visual impairment and blindness (CitationOsborne et al 2004). Retinal ganglion cells (RGCs) have been reported to be particularly sensitive to acute, transient, and mild systemic hypoxic stress (CitationKergoat et al 2006). Loss of RGCs occurs in many ophthalmic conditions such as glaucoma and diabetes (CitationSucher et al 1997; CitationAbu-El-Asrar et al 2004), hypoxia being implicated in such a loss (CitationWax and Tezel 2002; CitationTezel and Wax 2004; CitationChen et al 2007). This review details some of the molecular and cellular mechanisms which may be involved in RGC death in ocular conditions associated with hypoxia-ischemia. A better understanding of the mechanisms causing hypoxic damage to RGCs may aid the development of therapies aimed at reducing blindness from retinal hypoxic-ischemic visual loss.
A number of systemic and cellular responses such as glycolysis, angiogenesis, vasodilation, and erythropoiesis enable the organisms to respond to hypoxia (CitationHarris 2002). The neural tissue is capable of inducing protective mechanisms under hypoxic-ischemic conditions (CitationKitagawa et al 1990) which are induced within minutes and are of putative importance for limiting the damage. However, these protective mechanisms are lost within hours of the hypoxic-ischemic insult (CitationPrass et al 2003) following which cell death and tissue damage occur. Transcriptional activator hypoxia-inducible factor-1α (HIF-1α) is a master regulator of cellular O2 homeostasis (CitationIyer et al 1998). Hypoxia is known to induce HIF-1α and its target genes (CitationBernaudin et al 2002) such as vascular endothelial growth factor (VEGF) and nitric oxide synthase (NOS) in many tissues. Overproduction of these factors has been implicated in neuronal death in hypoxic-ischemic conditions. In addition, enhanced extracellular accumulation of glutamate and inflammatory cytokines damage the neurons. Upregulated expression of HIF-1α, VEGF, and various isoforms of NOS has been reported in the retina following hypoxic injury (CitationKaur et al 2006) and in the glaucomatous retina (CitationTezel and Wax 2004).
Retinal ganglion cell death in hypoxia ischemia
RGC death has been reported to occur in many experimental studies using different methods to induce retinal ischemia (CitationAdachi et al 1996; CitationGoto et al 2002; CitationLafuente et al 2002; CitationWang et al 2002; CitationChidlow and Osborne 2003). Neuronal degeneration resulting from retinal hypoxia-ischemia, caused by oxygen and substrate deprivation, may be mediated by free oxygen radicals (CitationBlock and Schwarz 1997; CitationMuller et al 1997; CitationSzabo et al 1997), glutamate excitotoxicity (CitationLouzada-Junior et al 1992; CitationOsborne et al 2004; CitationKaur et al 2006), inflammation (Hayashi et al 1996) as well as disruption of the blood retinal barrier (CitationKuroiwa et al 1985; CitationKaur et al 2007).
Based on morphological, histochemical, and biochemical criteria, cell death has been classified as apoptotic or necrotic in hypoxic-ischemic conditions (CitationMehmet et al 1994; CitationCharriaut-Marlangue et al 1996a, Citation1996b; CitationChopp and Li 1996; CitationMacaya 1996; CitationYue et al 1997; CitationMacManus and Linnik 1997; CitationBanasiak and Haddad 1998; CitationPulera et al 1998; CitationRenolleau et al 1998; CitationNakajima et al 2000). In necrotic death, swelling of cell body, disruption of plasma membrane, and irregularly scattered condensation of nuclear chromatin occur (CitationDessi et al 1993; CitationGwag et al 1997; CitationSohn et al 1998). In apoptosis, on the other hand, nuclear condensation and contraction occurs early with the membrane and organelles remaining intact until the final stages. Similar necrotic (CitationBuchi 1992; CitationJoo et al 1999) and apoptotic changes in RGCs have been observed in experimental hypoxic-ischemic conditions (CitationBuchi 1992; CitationJoo et al 1996, Citation1999) as well as in elevated intraocular pressure (CitationGarcia-Valenzuela et al 1995; CitationQuigley et al 1995) and glaucoma (Kerrigan et al 1996) where ischemia is involved in retinal damage directly or indirectly.
Ischemia is known to induce several apoptosis-regulatory genes in cells. Upregulated expression of Bax, a bcl-2 homolog that effects apoptosis in neurons destined to die, after global ischemia (CitationKrajewski et al 1995; Chen et al 1996) and expression of antiapoptotic gene bcl-2 in neurons that survive ischemia (CitationShimazaki et al 1994; CitationChen et al 1997) has been reported suggesting that endogenously induced apoptosis-regulatory genes may play a role in determining the fate of ischemic neurons. Caspases play a key role in cell death by apoptosis (CitationJacobson and Evan 1994). Among the caspases, caspase-3 is activated by many cell death signals and cleaves a variety of important cellular proteins (CitationJänicke et al 1998; CitationNamura et al 1998). Caspase-3-like protease activation is likely to be relevant in neuronal apoptosis in ischemic injury (CitationFink et al 1998; CitationNamura et al 1998). Caspase-2 and -3 (CitationKurokawa et al 1999; CitationLam et al 1999) and Bax (CitationKaneda et al 1999) have been reported to be involved in retinal cell loss after ischemic insult.
Hypoxia-ischemia, retinal edema, and vascular endothelial growth factor
Hypoxia-ischemia underlies various blinding ocular conditions such as diabetic retinopathy and may play a role in the wet form of age-related macular degeneration and in the visual loss from retinal detachment (CitationTso 1982; CitationYanoff et al 1984; CitationMarmor 1999; CitationBressler et al 2001; CitationDavis and Blodi 2001; CitationJackson et al 2003). It is associated with fluid accumulation in the extracellular spaces (vasogenic edema) or intracellulary (cytotoxic edema) in the neural retina (CitationYanoff et al 1984; CitationMarmor 1999). The extracellular spaces in the inner retina consist of the narrow clefts between the tightly packed cellular elements (CitationHamann 2002). Fluid leaking out from damaged capillaries in the inner retina accumulates in the extracellular spaces displacing the retinal cellular elements and disrupting the normal anatomy of the neuronal connections (CitationHamann and La Cour 2005). Factors implicated in pathogenesis of macular edema are retinal ischemia, oxidative stress, and inflammation (CitationBresnick 1983; CitationGuex-Crosier 1999; Citationvan Dam 2002; CitationMiyake and Ibaraki 2002). Increased permeability of blood-retinal barrier (BRB) resulting in fluid accumulation has been reported to contribute to retinal neuronal degeneration by compression (CitationCunha-Vaz and Travassos 1984; CitationAntcliff and Marshall 1999; CitationMarmor 1999; CitationReichenbach et al 2007). Excess production of VEGF, nitric oxide (NO) and aquaporin-4 in hypoxic-ischemic insults causes dysfunction of the BRB in the inner retina resulting in serum leakage into the retinal tissues (CitationMarmor 1999; CitationKaur et al 2007) and retinal edema (CitationHamann and La Cour 2005). In addition to an increase in vascular permeability, hypoxia has also been correlated with endothelial cell death, leukocyte plugging of vessels, and microaneurysms (CitationLinsenmeier et al 1998).
VEGF, also known as vascular permeability factor (Senger et al 1983), is a key player of angiogenesis in health and disease (CitationFerrara 2001; CitationCarmeliet 2003). VEGF binds to two tyrosine kinase receptors, VEGFR-1 or fms-like tyrosine kinase Flt-1 and VEGFR-2 or fetal liver kinase receptor Flk-1 to exert its actions (CitationDe Vries et al 1992; CitationQuinn et al 1993; CitationNeufeld et al 1999; Shibuya 2001). VEGF is inducible by hypoxia-ischemia in vitro and in vivo and has been suggested as a likely candidate for the development of vasogenic brain edema (CitationSchoch et al 2002). A 3–12-fold increase in VEGF gene expression has been reported in hypoxia (CitationIkeda et al 1995; CitationLevy et al 1995; CitationStein et al 1995).
Increased expression of VEGF has been reported in hypoxic brains (CitationSchoch et al 2002; CitationKaur et al 2006), and astrocytes were identified as the cells expressing VEGF (CitationKaur et al 2006). Upregulation of endogenous VEGF in astrocytes in hypoxia-ischemia is believed to interact with receptors for VEGF on the vessels and contribute to the disruption of blood-brain barrier (BBB) resulting in vascular leakage (CitationZhang et al 2000, Citation2002). Inhibition of VEGF is known to reduce the BBB permeability (CitationZhang et al 2000). Similar to the brain, increased production of VEGF and enhanced permeability of BRB was recently reported in the hypoxic retina and inhibition of VEGF production with melatonin reduced BRB permeability (CitationKaur et al 2006, Citation2007).
In addition to its role in increasing vascular permeability, VEGF has also been described as an inflammatory mediator which contributes to inflammatory responses observed in cerebral ischemia (CitationCroll et al 2004). The disruption of BBB by VEGF allows contact of normally sequestered central nervous system antigens with blood-borne immune mediators altering the immune privileged status of the brain (CitationProescholdt et al 1999). VEGF enhances the adhesion of leukocytes to vascular walls and increases intercellular cell adhesion molecule-1(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression in the brain and retina (CitationMelder et al 1996; CitationLu et al 1999; CitationMin et al 2005). Overexposure of normal neural tissue to VEGF has been shown to enhance ICAM-1 and major histocompatibility complex class I and II expression (Proescholdt et al 2004). Many changes induced by diabetes such as ICAM-1 up-regulation, leukocyte adhesion and increased vascular permeability in the retina (CitationMurata et al 1996; CitationAmin et al 1997) have been reported to occur in nondiabetic retinas with intravitreous VEGF injections (CitationTolentino et al 1996; CitationLu et al 1999) supporting the role of VEGF in inflammation. Suppression of inflammation in retina after VEGF inhibition has been reported (CitationJoussen et al 2002).
Intracellular edema has been reported to occur in ischemia through damage to the cell membrane ionic channels (CitationMarmor 1999). Neuronal and/or glial swelling has been considered as a component of retinal edema. The neuronal cells have been reported to become edematous during ischemia and degenerate eventually in the post-ischemic period (CitationJohnson 1974).
Hypoxia-ischemia and nitric oxide
NO is known to play an important role in the pathogenesis of neuronal injury during hypoxia-ischemia. NO is synthesized by the enzyme NOS from L-arginine. NOS exists in three isoforms: neuronal (nNOS) and endothelial (eNOS) which are constitutively expressed and inducible (iNOS). The activities of nNOS and eNOS are stimulated by increases in intracellular calcium whereas iNOS is calcium-independent, and NO generated from this isoform is known to mediate immune functions. Enhanced nNOS, eNOS, and iNOS expression has been reported in the retina in response to hypoxia (CitationKaur et al 2006).
NO has been described to have neuroprotective and neurotoxic roles (Iadeacola 1997). NO produced by the eNOS isoform is a protective response as it maintains retinal perfusion in hypoxic-ischemic conditions (CitationToda et al 2007). Vasodilation occurring after hypoxic-ischemic episodes is mediated by eNOS (CitationBolanos and Almeida 1999) leading to increased blood flow. Blood vessels in retina showed enhanced expression of eNOS following a hypoxic insult (CitationKaur et al 2006). However, it has been proposed that besides its beneficial effects of producing vasodilatation and increasing the blood flow, eNOS is also involved in VEGF-induced vascular hyperpermeability (CitationFukumura et al 2001).
All three types of NOS are produced in the retina in hypoxic-ischemic conditions (CitationKaur et al 2006) and glial cells have been suggested as the major cell types producing them (CitationKobayashi et al 2000; CitationKashiwagi et al 2003). In addition to glial cells, infiltrating leukocytes may also be an important source of iNOS production. The RGCs were also reported to express iNOS and nNOS in the hypoxic retina (CitationKaur et al 2006). NO production from nNOS and iNOS contributes to cytotoxicity resulting in cell death and axonal damage. Other than generation of free radicals, a number of pathways such as N-methyl-D-aspartate (NMDA)-mediated intracellular Ca2+ influx and CREB-mediated transcription of apoptotic proteins such as Bax, Bad, and Bcl-xl are triggered by NO resulting in neuronal death (CitationMishra et al 2002, Citation2006; CitationZubrow et al 2002a, Citation2002b). Increased expression of Bax but not Bcl-2 in hypoxic cerebral tissue thus increasing the Bax/Bcl-2 ratio in favor of hypoxia-induced apoptosis has been reported (CitationMishra et al 2004). In retinal ischemia, RGCs death has been reported to be due to involvement of iNOS as it has been observed that iNOS-positive leukocytes enter the ganglion cell layer and surround the RGCs and cause their degeneration which could be prevented with an inhibitor of iNOS (CitationNeufeld et al 2002).
NO induces the proapoptotic cascade in hypoxic neural tissues by increasing phosphorylation of Bcl-2 (CitationMishra et al 2004). NO-mediated inactivation of MAPK phosphatases has been described as a potential mechanism of activation of ERK and JNK which leads to phosphorylation of the antiapoptotic protein Bcl-2 (CitationMishra et al 2004). The anti-apoptotic potential of phosphorylated Bcl-2 is lost due to its inability to heterodimerize with the proapoptotic protein Bax, resulting in Bax-mediated activation of caspases and initiation of apoptosis (CitationSt. Clair et al 1997, CitationHaldar et al 1996; CitationHu et al 1998). Other mechanisms by which NO contributes to cytotoxicity may be peroxynitrite-mediated oxidative damage, DNA damage, and energy failure (CitationBeckman et al 1990; CitationNguyen et al 1992; CitationZhang et al 1994; CitationGross et al 1996). This observation is supported by recent studies which have suggested that peroxynitrite produced by iNOS is a highly reactive oxidant capable of inducing injury to a number of cell types (CitationLi et al 2005).
Hypoxia-ischemia and excitotoxicity
Excitatory amino acids have been reported to play an important role in the development of hypoxic-ischemic retinal injury. Glutamate, the excitatory neurotransmitter in the retina, is released by photoreceptors, bipolar cells and ganglion cells and mediates the transfer of visual signals from the retina to the brain (CitationMassey 1990). However, augmented release of glutamate and its accumulation in extracellular spaces in hypoxic-ischemic conditions leading to activation of glutamate receptors has been implicated in hypoxic/ischemic neuronal death (CitationBenveniste et al 1984; CitationLu et al 1993). Glutamate neurotoxicity is considered as the underlying problem in retinal neuropathies and neurodegenerative conditions such as glaucoma (CitationDreyer 1998). Elevation of extracellular glutamate concentration in the retina has been shown to mimic hypoxia induced changes in the electroretinogram (CitationIkeda et al 1995). Over-activation of glutamate receptors due to excess glutamate accumulation in the retina can contribute to retinal dysfunction (CitationDreyer 1998; CitationPang et al 1999).
Glutamate exerts its action through ionotropic (amino-methyl-propionic-acid [AMPA], NMDA, and kainate glutamate receptors) and metabotropic receptors (CitationBrandstätter et al 1998; CitationGründer et al 2001). The metabotropic glutamate receptors (mGluRs) have been grouped into three main classes, group I (mGluR1 and 5), Group II (mGluR2, 3), and Group III (mGluR 4, 6, 7, 8) according to their amino acid sequence, pharmacological properties and transduction mechanisms (CitationConn and Pin 1997). RGCs express ionotropic receptors (CitationHartveit et al 1994; CitationBrandstätter et al 1998) as well as mGluRs (CitationHartveit et al 1995). Glutamate receptor-mediated damage has been reported to occur in glaucoma, central, and branch retinal arterial and retinal vein occlusions resulting in loss of retinal ganglion cells (CitationSucher et al 1997).
Neurotoxic effects of glutamate are reported to occur predominantly through activation of ionotropic glutamate receptors (GluR) (Levy et al 1991). NMDA receptors are highly permeable to Ca2+ (CitationMacDermott et al 1986; CitationHollmann et al 1991; CitationRörig and Grantyn 1993), their activation resulting in an increase in the intracellular calcium levels (CitationSiliprandi et al 1992; CitationSucher et al 1990, Citation1991, Citation1997). Ca2+ overload has been reported to be a central event in neuronal death during ischemia (CitationNicotera and Orrenius 1998; CitationSattler and Tymianski 2001). Many cellular functions such as regulation of enzymes require calcium. Abnormal higher concentrations of calcium lead to inappropriate activation of enzymes such as proteases, nucleases, and lipases which are harmful to the cellular constituents, generate free radicals as well as cause mitochondrial failure which results in energy depletion and further free radical production (CitationDugan et al 1995).
Depolarization of neuronal membranes due to energy failure results in Ca2+ influx through the voltage-dependent Ca2+ channels followed by Ca2+-dependent glutamate release (CitationKatsura et al 1994) which further increases the extracellular accumulation of glutamate. Activation of ionotropic glutamate receptors also results in influx of Na+ and Cl− ions, inducing osmotic swelling. Glutamate acting via NMDA receptors activates nNOS (CitationGarthewaite and Garthewaite 1991) and the production of NO (CitationKiss and Vizi 2001). Expression of ionotropic glutamate receptors (GluR2/3 and NMDA) has been reported to be upregulated in the RGCs in hypoxic-ischemic conditions (CitationKaur et al 2006).
Glutamate has also been reported to induce and exacerbate cell death by activating group I mGluRs (CitationAllen et al 2001; CitationHilton et al 2006). Neuronal excitation and excitotoxicity is thought to be potentiated by Group I mGluRs (CitationBuisson and Choi 1995; CitationPin and Duvoisin 1995; CitationBuisson et al 1996), possibly through their interaction with NMDA receptors (CitationFitzjohn et al 1996; CitationBordi and Ugolini 1999). It has been reported that mGluR5 are coexpressed with, and functionally coupled to, NMDA receptors and that activation of mGluR5 enhances NMDA responses in neurons (CitationJia et al 1998; CitationAwad et al 2000; CitationSalt and Binns 2000) contributing to neuronal death (CitationBruno et al 2000).
Glutamate is also known to be involved in the production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) (CitationDe et al 2005). Glutamate-induced activation of AMPA and NMDA receptors has been shown to enhance the production of TNF-α (CitationNoda et al 2000; CitationMatute et al 2001) and interleukin-1 β (IL-1β) (CitationHagan et al 1996) significantly. Co-operation between glutamate receptors and inflammatory cytokines may be one of the mechanisms involved in cell damage.
Glutamate toxicity also results in glutathione depletion and oxidative stress (CitationRatan et al 1994). Glutathione is a major cellular antioxidant which protects the cells against oxidative stress (CitationMeister and Anderson 1983; CitationMizui et al 1992; CitationBobyn et al 2002). Increase in intracellular reactive oxygen species (ROS) generation in response to glutathione depletion has been reported in several studies (CitationCoyle and Puttfarcken 1993; CitationTan et al 1998).
Removal of excess glutamate from the extracellular space by glutamate transporters is crucial to terminate glutamate excitotoxicty. Glutamate transporters are responsible for the removal of glutamate from the extracellular fluid in the retina (CitationDanbolt 2001). It has been suggested that excess glutamate accumulation in the extracellular spaces may result from a failure of the glutamate transporters, such as GLAST, in the vicinity of RGCs (CitationHarada et al 2007). Glutamate transporters have been described as necessary to prevent excitotoxic retinal damage and to synthesize glutathione and their deficiency has been reported to result in RGC degeneration (CitationHarada et al 2007).
Hypoxia-ischemia and reactive oxygen species
Hypoxia-ischemia results in perturbation of the cellular pro-oxidant-antioxidant balance by accumulation of ROS, known as oxidative stress, which has been implicated as an important mechanism of cytotoxicity. In vitro studies have shown that ROS generation in hypoxic-ischemic conditions in neurons occurs from three sources: mitochondria generating an initial burst of ROS followed by a second phase of ROS generation due to xanthine oxidase activation and a third phase of Ca2+-dependent ROS generation (CitationAbramov et al 2007).
ROS are known to cause lipid peroxidation, protein oxidation, and DNA oxidation, which contributes to neuro-degeneration (CitationHall and Braugher 1989; CitationChan 1994, Citation1996). ROS can also stimulate ischemic cells to secrete inflammatory cytokines and chemokines which induce cell damage and disruption of BBB (CitationWang et al 2007). ROS have been reported to be cytotoxic to RGCs (CitationTezel and Yang 2004) causing necrotic cell death by direct oxidative damage to cellular constituents and apoptotic death by participating in the signal transduction pathway for apoptosis (CitationKortuem et al 2000; CitationLevkovitch-Verbin et al 2000; CitationLieven et al 2003, Citation2006; CitationNguyen et al 2003).
NO, a free radical is produced by the endothelial cells and serves as a vasodilator (CitationGarthwaite et al 1988; CitationLamas et al 1992; CitationSoutham and Garthwaite 1993; CitationIadecola et al 1994). However, NO, as mentioned above, can also be neurotoxic causing neuronal death in hypoxic and excitotoxic insults (CitationDawson et al 1991; CitationMoncada et al 1991; CitationBoje and Arora 1992; CitationLees 1993). It has been shown that NO can react with the superoxide anion (O2−) to form peroxynitrite (OONO−) (CitationBeckman et al 1990) which is neurotoxic (CitationLipton et al 1993). NO alone, even at high levels, has been reported as nontoxic to cortical neurons, but becomes neurotoxic after its reaction with O2− to form ONOO− (CitationLipton et al 1993). In vitro studies have shown that formation of OONO− increases the VEGF-induced permeability of retinal microvascular endothelial cells (CitationMarumo et al 1999) and tissue damage through DNA damage, lipid peroxidation, and reduced cellular anti-oxidant defenses (CitationSalgo et al 1995; CitationSalvemini et al 1998).
Hypoxia-ischemia and inflammation
Hypoxia is known to regulate expression of many genes modulating inflammation (CitationHedtjärn et al 2004). An acute inflammatory reaction, characterized by increased expression of proinflammatory mediators (CitationSzaflarski et al 1995; CitationBona et al 1999), a rapid microglial/monocytic response (CitationIvako et al 1996) and gliosis (CitationBurtrum et al 1994), have been reported to be elicited in the brain by hypoxia-ischemia (CitationCowell et al 2002). Many cell types including injured neurons have been reported as a major source of chemokines such as monocyte chemoattractant protein (MCP-1) (CitationIvako et al 1997) whereas expression of macrophage inflammatory protein-α has been reported in monocytes and activated microglial cells (CitationCowell et al 2002) in hypoxic-ischemic brain injury. Chemokine receptors CCR2 and CCR5 have also been reported to be upregulated (CitationHedtjärn et al 2004). Chemokine expression may play a role in leukocyte recruitment and infiltration in the inner retina, leading to RGC damage (CitationJo et al 2003). Leukocytes are known to play a central role in post-ischemic tissue damage (Citationdel-Zoppo et al 1991; CitationHeinel et al 1994; CitationZhang et al 1994) by producing free radicals (CitationWerns et al 1985) and inflammatory cytokines (CitationGhezzi et al 1991).
Hypoxia-ischemia is known to attract macrophages to hypoxic areas through expression of MCP-1. The hypoxia-activated macrophages and microglia, the immune effector cells in the retina, release TNF-α which has been reported as a triggering factor to activate production of interleukin-8 (IL-8), VEGF, or MCP-1 in retinal vascular cells and/or glial cells adjacent to microvessels (CitationYoshida et al 2004). Expression of TNF-α and cyclooxygenase-2 (COX-2) was reported recently in the ischemic retina (CitationZheng et al 2007). Several inflammatory molecules including ICAM-1, TNF-α, IL-1β, iNOS, and COX-2 released by activated inflammatory cells and glial elements play a major role in degeneration of retinal capillaries (CitationJoussen et al 2004; CitationZheng et al 2007) and subsequently the RGCs.
Expression of adhesion molecules, ICAM-1 and VCAM-1, on the endothelial cells facilitating leukocyte adhesion and infiltration into the areas of damage has been reported to be induced by TNF-α (CitationWong and Dorovini, 1992; CitationHess et al 1994; CitationMcHale et al 1999). In vitro studies have shown that IL-1β and TNF-α induce ICAM-1 expression in endothelial cells (CitationFeuerstein et al 1998). ICAM-1 is important for establishing adhesion of leukocytes before their movement across the endothelium into the tissue (CitationWang et al 1994).
IL-1β and TNF-α may also be involved in transcriptional activation of the iNOS gene (CitationLopez-Figueroa et al 2000; CitationKadhim et al 2006). Endothelial cells of brain microvessels are known to express iNOS and produce large amounts of NO under inflammatory conditions as IL-1β has an important role in iNOS expression and NO generation (CitationBetz et al 1996; CitationBonmann et al 1997). Induction of IL-1 β gene expression in the vascular wall, accompanied by perivascular induction of iNOS mRNA was observed in the rat brain during systemic inflammation (CitationWong et al 1996). Increased release of IL-1β and TNF-α in the retina in hypoxic-ischemic conditions may have a similar action.
Conclusion
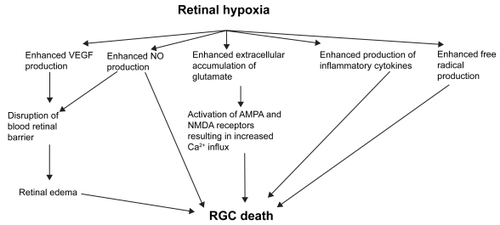
Retinal hypoxia results in increased release VEGF, NO, glutamate, inflammatory cytokines and ROS (). These processes result in RGC loss through various mechanisms such as disruption of BRB, excitotoxicity and increased accumulation of intracellular Ca2+. Understanding of the processes outlined in this review may provide new strategies to minimize RGC loss and possibly counteract or prevent it.
Acknowledgments
This study was supported by a research grant (R181-000-098-112) from the National University of Singapore, Singapore. The help provided by Dr. V. Sivakumar in the preparation of this manuscript is gratefully acknowledged. The authors report no conflicts of interest in this work.
References
- AbramovAYScorzielloADuchenMR2007Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenationJ Neurosci2711293817267568
- Abu-El-AsrarAMDralandsLMissottenL2004Expression of apoptosis markers in the retinas of human subjects with diabetesInvest Ophthalmol Vis Sci452760615277502
- AdachiMTakahashiKNishikawaM1996High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in ratsGraefes Arch Clin Exp Ophthalmol234445518817288
- AllenJWViciniSFadenAI2001Exacerbation of neuronal cell death by activation of group I metabotropic glutamate receptors: role of NMDA receptors and arachidonic acid releaseExp Neurol1694496011358458
- AmesA1992Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retinaCan J Physiol Pharmacol70SupplS158641295666
- AminRHFrankRNKennedyA1997Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathyInvest Ophthalmol Vis Sci3836479008628
- AndersonBSaltzmanHA1964Retinal oxygen utilization measured by hyperbaric blackoutArch Ophthalmol72792514205438
- AntcliffRJMarshallJ1999The pathogenesis of edema in diabetic maculopathySemin Ophthalmol142233210758223
- AshtonNKokDAFouldsWS1963Ocular pathology in macroglobulinaemiaJ Pathol Bacteriol864536114068954
- AwadHHubertGWSmithY2000Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleusJ Neurosci207871911050106
- BanasiakKJHaddadGG1998Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell deathBrain Res7972953049666152
- BeckmanJSBeckmanTWChenJ1990Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxideProc Natl Acad Sci U S A871620242154753
- BenvenisteHDrejerJSchousboeA1984Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysisJ Neurochem431369746149259
- BernaudinMNedelecASDivouxD2002Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brainJ Cereb Blood Flow Metab2239340311919510
- BetzALSchielkeGPYangGY1996Interleukin-1 in cerebral ischemiaKeio J Med4523078897766
- BlockFSchwarzM1997Effects of antioxidants on ischemic retinal dysfunctionExp Eye Res64559649227274
- BobynPJFranklinJLWallCM2002The effects of dietary sulfur amino acid deficiency on rat brain glutathione concentration and neural damage in global hemispheric hypoxia-ischemiaNutr Neurosci54071612509070
- BojeKMAroraPK1992Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell deathBrain Res58725061381982
- BolanosJPAlmeidaA1999Roles of nitric oxide in brain hypoxia-ischemiaBiochim Biophys Acta14114153610320673
- BonaEAnderssonALBlomgrenK1999Chemokine and inflammatory cell response to hypoxia–ischemia in immature ratsPediatr Res45500910203141
- BonmannESuschekCSprangerM1997The dominant role of exogenous or endogenous interleukin-1 beta on expression and activity of inducible nitric oxide synthase in rat microvascular brain endothelial cellsNeurosci Lett230109129259476
- BordiFUgoliniA1999Group I metabotropic glutamate receptors: implications for brain diseasesProg Neurobiol59557910416961
- BrandstätterJHKoulenPWässleH1998Diversity of glutamate receptors in the mammalian retinaVision Res381385979667006
- BresnickGH1983Diabetic maculopathy. A critical review highlighting diffuse macular edemaOphthalmology901301176664669
- BresslerNMBresslerSBFineSLSchachatAP2001Neovascular (exudative) age-related macular degenerationRetinaSt. Louis, MOMosby110035
- BrownGCMagargalLE1988The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic featuresInt Ophthalmol11239513182177
- BrunoVBattagliaGKsiazekI2000Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal deathJ Neurosci2064132010964947
- BuchiER1992Cell death in the rat retina after a pressure-induced ischemia-reperfusion insult: an electron microscopic study, I: Ganglion cell layer and inner nuclear layerExp Eye Res55605131483506
- BuckleySAJamesB1996Purtscher’s retinopathyPostgrad Med J72409128935600
- BuissonAChoiDW1995The inhibitory mGluR agonist, S-4-carboxy-3-hydroxy-phenylglycine selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal deathNeuropharmacology34108178532157
- BuissonAYuSPChoiDW1996DCG-IV selectively attenuates rapidly triggered NMDA-induced neurotoxicity in cortical neuronsEur J Neurosci8138438713457
- BurtrumDSilversteinFS1994Hypoxic-ischemic brain injury stimulates glial fibrillary acidic protein mRNA and protein expression in neonatal ratsExp Neurol12611288157121
- CarmelietP2003Angiogenesis in health and diseaseNat Med96536012778163
- ChanPH1994Oxygen radicals in focal cerebral ischemiaJ Brain Pathol45965
- ChanPH1996Role of oxidants in ischemic brain damageStroke27112498650725
- Charriaut-MarlangueCMargaillIBorregaF1996bNG-nitro-L-arginine methyl ester reduces necrotic but not apoptotic cell death induced by reversible focal ischemia in ratEur J Pharmacol310137408884209
- Charriaut-MarlangueCPollardHBen-AriY1996aIs ischemic cell death of the apoptotic type?Adv Neurol71425308790818
- ChenHLPistollatoFHoeppnerDJ2007Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levelsStem Cells25229130117556599
- ChenJGrahamSHNakayamaM1997Apoptosis repressor genes Bcl-2 and Bcl-x-long are expressed in the rat brain following global ischemiaJ Cereb Blood Flow Metab172108978381
- ChenJZhuRLNakayamaMExpression of the apoptosis-effector gene, Bax, is up-regulated in vulnerable hippocampal CA1 neurons following global ischemiaJ Neurochem6764718667027
- ChidlowGOsborneNN2003Rat retinal ganglion cell loss caused by kainate, NMDA and ischemia correlates with a reduction in mRNA and protein of Thy-1 and neurofilament lightBrain Res96329830612560136
- ChoppMLiY1996Apoptosis in focal cerebral ischemiaActa Neurochir Suppl662168780792
- ChungHSHarrisAEvansDW1999Vascular aspects in the pathophysiology of glaucomatous optic neuropathySurv Ophthalmol43Suppl 1S435010416746
- CohenLHNoellWKGraymoreCN1965Relationships between visual function and metabolismBiochemistry of the RetinaOrlando, FlaAcademic Press Inc3650
- ConnJPPinJP1997Pharmacology and functions of metabotropic glutamate receptorsAnn Rev Pharmacol Toxicol37205379131252
- CostaVPHarrisAStefánssonE2003The effects of antiglaucoma and systemic medications on ocular blood flowProg Retin Eye Res2276980514575724
- CowellRMXuHGalassoJM2002Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brainStroke3379580111872906
- CoyleJTPuttfarckenP1993Oxidative stress, glutamate, and neurode-generative disordersScience262689957901908
- CrollSDRansohoffRMCaiN2004VEGF-mediated inflammation precedes angiogenesis in adult brainExp Neurol18738840215144865
- Cunha-VazJGTravassosA1984Breakdown of the blood-retinal barriers and cystoid macular edemaSurv Ophthalmol28S48592
- DanboltNC2001Glutamate uptakeProg Neurobiol65110511369436
- DavisMDBlodiBASchachatAP2001Proliferative diabetic retinopathyRetinaSt. Louis, MOMosby130949
- DawsonVLDawsonTMLondonED1991Nitric oxide mediates glutamate neurotoxicity in primary cortical culturesProc Natl Acad Sci U S A886368711648740
- DeAKruegerJMSimaskoSM2005Glutamate induces the expression and release of tumor necrosis factor-alpha in cultured hypothalamic cellsBrain Res1053546116040010
- De VriesCEscobedoJAUenoH1992The fms-like tyrosine kinase, a receptor for vascular endothelial growth factorScience255989911312256
- del-ZoppoGJSchmid-SchonbeinGWMoriE1991Polymorpho-nuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboonsStroke221276831926239
- DessiFCharriaut-MarlangueCKhrestchatiskyM1993Glutamate- induced neuronal death is not a programmed cell death in cerebellar culturesJ Neurochem60195358097239
- DranceSMDouglasGRWijsmanK1988Response of blood flow to warm and cold in normal and low-tension glaucoma patientsAm J Ophthalmol1053593337192
- DreyerEB1998A proposed role for excitotoxicity in glaucomaJ Glaucoma76279493118
- DuganLLSensiSLCanzonieroLM1995Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartateJ Neurosci156377887472402
- FerraraN2001Role of vascular endothelial growth factor in regulation of physiological angiogenesisAm J Physiol Cell Physiol280C13586611350730
- FeuersteinGWangXBaroneFC1998Cytokines in brain ischemia – the role of TNF alphaCell Mol Neurobiol186957019876875
- FinkKZhuJNamuraS1998Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activationJ Cereb Blood Flow Metab18107169778183
- FitzjohnSMIrvingAJPalmerMJ1996Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slicesNeurosci Lett20321138742030
- FlammerJ1994The vascular concept of glaucomaSurv Ophthalmol38SupplS367940146
- FukumuraDGohongiTKadambiA2001Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeabilityProc Natl Acad Sci U S A982604911226286
- Garcia-ValenzuelaEShareefSWalshJ1995Programmed cell death of retinal ganglion cells during experimental glaucomaExp Eye Res6133447556468
- GarthwaiteGGarthwaiteJ1991AMPA neurotoxicity in rat cerebellar and hippocampal slices: histological evidence for three mechanismsEur J Neurosci37152812106458
- GarthwaiteJCharkesSLChess-WilliamsR1988Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brainNature33638582904125
- GarthwaiteJ1991Glutamate, nitric oxide and cell-cell signalling in the nervous systemTrends Neurosci146071708538
- GhezziPDinarelloCABianchiM1991Hypoxia increases production of interleukin-1 and tumor necrosis factor by human mononuclear cellsCytokine3189941883957
- GotoWOtaTMorikawaN2002Protective effects of timolol against the neuronal damage induced by glutamate and ischemia in the rat retinaBrain Res95810912468025
- GrossWLBakMIIngwallJS1996Nitric oxide inhibits creatine kinase and regulates rat heart contractile reservesProc Natl Acad Sci U S A93560498643623
- GründerTKohlerKGuentheE2001Alterations in NMDA receptor expression during retinal degeneration in the RCS ratVis Neurosci18781711925013
- Guex-CrosierY1999The pathogenesis and clinical presentation of macular edema in inflammatory diseasesDoc Ophthalmol9729730910896343
- GwagBJKohJYDemaroJ1997Slowly triggered excitotoxicity occurs by necrosis in cortical culturesNeuroscience773934019472399
- HaganPPooleSBristowAF1996Intracerebral NMDA injection stimulates production of interleukin-1β in perinatal rat brainJ Neurochem67221588863535
- HaldarSChintapalliJCroceCM1996Taxol induces bcl-2 phosphorylation and death of prostate cancer cellsCancer Res562535
- HallEDBraughlerJM1989Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidationFree Radic Biol Med6303132663663
- HamannKF2002[Driving ability with vestibulär lesions]HNO501086812474132
- HamannSla CourM2005Water homeostasis in the ischaemic retina: is aquaporin-4 involved?Acta Ophthalmol Scand83523516187986
- HaradaTHaradaCNakamuraK2007The potential role of glutamate transporters in the pathogenesis of normal tension glaucomaJ Clin Invest11717637017607354
- HarrisAL2002Hypoxia – a key regulatory factor in tumour growthNat Rev Cancer2384711902584
- HartveitEBrandstätterJHEnzR1995Expression of the mRNA of seven metabotropic glutamate receptors (mGluR1–7) in the rat retina. An in situ hybridization study on tissue sections and isolated cellsEur J Neurosci71472837551173
- HartveitEBrandstätterJHSassoè-PognettoM1994Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retinaJ Comp Neurol348570827836563
- HedtjärnMMallardCHagbergH2004Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemiaJ Cereb Blood Flow Metab243351
- HeinelLARubinSRosenwasserRH1994Leukocyte involvement in cerebral infarct generation after ischemia and reperfusionBrain Res Bull34137418044688
- HessDCBhutwalaTSheppardJC1994ICAM-1 expression on human brain microvascular endothelial cellsNeurosci Lett16820147913216
- HiltonGDNunezJLBambrickL2006Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiolEur J Neurosci2430081617156362
- HollmannMHartleyMHeinemannS1991Ca2+ permeability of KA-AMPA – gated glutamate receptor channels depends on subunit compositionScience25285131709304
- HuYBenedictMAWuD1998Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activationProc Natl Acad Sci U S A94386919539746
- IadecolaCPelligrinoDAMoskowitzMA1994State of the art review: nitric oxide synthase inhibition and cerebrovascular regulationJ Cereb Blood Flow Meta1417592
- IadecolaC1997Bright and dark sides of nitric oxide in ischemic brain injuryTrends Neurosci2013299061868
- IkedaEAchenMGBreierG1995Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cellsJ Biol Chem2701976167544346
- IvakoJMalinakCMalinakC1997Hypoxic ischemic injury induces monocyte chemoattractant protein 1 expression in neonatal rat brainJ Cereb Blood Flow Metab17759709270493
- IvakoJSilversteinFSSilversteinFS1996Hypoxic ischemic brain injury induces an acute microglial reaction inperinatal ratsPediat Res3939478825384
- IyerNVKotchLEAganiF1998Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alphaGenes Dev12149629436976
- JacksonTLHillenkampJWilliamsonTH2003An experimental model of rhegmatogenous retinal detachment: surgical results and glial cell responseInvest Ophthalmol Vis Sci4440263412939325
- JacobsonMDEvanGI1994Apoptosis. Breaking the ICECurr Biol4337407857398
- JanákyMGrószATóthE2007Hypobaric hypoxia reduces the amplitude of oscillatory potentials in the human ERGDoc Ophthalmol114455117211646
- JänickeRUSprengartMLWatiMR1998Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosisJ Biol Chem2739357609545256
- JiaZLuYHendersonJ1998Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5Learn Mem53314310454358
- JoNWuGSRaoNA2003Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injuryInvest Ophthalmol Vis Sci4440546012939328
- JohnsonNF1974Effects of acute ischemia on the structure of the rabbit retinaTrans Ophthalmol Soc Uk943944054534186
- JooCKChoiJSKoHW1999Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53Invest Ophthalmol Vis Sci407132010067975
- JooCKParkKYParkMS1996Occurrence of neuronal necrosis and apoptosis following the retinal ischemia: induction of p53 and bcl-2 mRNASoc Neurosci Abstr2646415
- JoussenAMPoulakiVLeML2004A central role for inflammation in the pathogenesis of diabetic retinopathyFASEB J181450215231732
- JoussenAMPoulakiVQinW2002Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivoAm J Pathol160501911839570
- KadhimHKhalifaMDeltenreP2006Molecular mechanisms of cell death in periventricular leukomalaciaNeurology67293916864823
- KanedaKKashiiSKurosawaT1999Apoptotic DNA fragmentation and upregulation of Bax induced by transient ischemia of the rat retinaBrain Res81511209974117
- KashiwagiKIizukaYMochizukiS2003Differences in nitric oxide production: a comparison of retinal ganglion cells and retinal glial cells cultured under hypoxic conditionsBrain Res Mol Brain Res1121263412670710
- KatsuraKKristianTSiesjoBK1994Energy metabolism, ion homeostasis, and cell damage in the brainBiochem Soc Trans2299167698500
- KaurCSivakumarVFouldsWS2006Early response of neurons and glial cells to hypoxia in the retinaInvest Ophthalmol Vis Sci4711264116505051
- KaurCSivakumarVYongZ2007Blood-retinal barrier disruption and ultrastructural changes in the hypoxic retina in adult rats: the beneficial effect of melatonin administrationJ Pathol2124293917582234
- KergoatHHérardMELemayM2006RGC sensitivity to mild systemic hypoxiaInvest Ophthalmol Vis Sci475423717122132
- KerriganLAZackDJQuigleyHA1997TUNEL-positive ganglion cells in human primary open-angle glaucomaArch Ophthalmol11510315
- KissJPViziES2001Nitric oxide: a novel link between synaptic and nonsynaptic transmissionTrends Neurosci24211511250004
- KitagawaKMatsumotoMTagayaM1990Ischemic tolerance’ phenomenon found in the brainBrain Res5282142245337
- KobayashiMKuroiwaTShimokawaR2000Nitric oxide synthase expression in ischemic rat retinasJpn J Ophthalmol442354410913641
- KortuemKGeigerLKLevinLA2000Differential susceptibility of retinal ganglion cells to reactive oxygen speciesInvest Ophthalmol Vis Sci4131768210967081
- KrajewskiSMaiJKKrajewskaM1995Upregulation of bax protein levels in neurons following cerebral ischemiaJ Neurosci156364767472401
- KuroiwaTTingPMartinezH1985The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusionActa Neuropathol (Berl)6812293907257
- KurokawaHNishioKFukumotoH1999Alteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cellsOncol Rep63379864397
- LafuenteMPVillegas-PerezMPSells-NavarroI2002Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insultNeuroscience1091576811784707
- LamTTAblerASTsoMO1999Apoptosis and caspases after ischemia-reperfusion injury in rat retinaInvest Ophthalmol Vis Sci409677510102294
- LamasSMarsdenPALiGK1992Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoformProc Natl Acad Sci U S A896348521378626
- LeesGJ1993The possible contribution of microglia and macrophages to delayed neuronal death after ischemiaJ Neurol Sci114119228445391
- Levkovitch-VerbinHHarris-CerrutiCGronerY2000RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotectionInvest Ophthalmol Vis Sci4141697411095611
- LevyAPLevyNSWegnerS1995Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxiaJ Biol Chem27013333407768934
- LiTQMathewsVPWangY2005Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocolAnn N Y Acad Sci10641849216394156
- LievenCJSchlieveCRHoeggerMJ2006Retinal ganglion cell axotomy induces an increase in intracellular superoxide anionInvest Ophthalmol Vis Sci4714778516565382
- LievenCJVrabecJPLevinLA2003The effects of oxidative stress on mitochondrial transmembrane potential in retinal ganglion cellsAntioxid Redox Signal5641614580321
- LinsenmeierRABraunRDMcRipleyMA1998Retinal hypoxia in long-term diabetic catsInvest Ophthalmol Vis Sci391647579699554
- LiptonSAChoiYBPanZH1993A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compoundsNature364626328394509
- López-FigueroaMODayHELeeS2000Temporal and anatomical distribution of nitric oxide synthase mRNA expression and nitric oxide production during central nervous system inflammationBrain Res8522394610661521
- Louzada-JuniorPDiasJJSantosWF1992Glutamate release in experimental ischaemia of the retina: an approach using microdialysisJ Neurochem59358631351929
- LuMPerezVMaN1999VEGF increases retinal vascular ICAM-1 expression in vivoInvest Ophthalmol Vis Sci4018081210393052
- LuYLuBFZhaoFQ1993Accumulation of glutamate is regulated by calcium and protein kinase C in rat hippocampal slices exposed to ischemic statesHippocampus322178102581
- MacayaA1996[Apoptosis in the nervous system]Rev Neurol241356608974737
- MacDermottABMayerMLWestbrookGL1986NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neuronesNature321519223012362
- MacManusJPLinnikMD1997Gene expression induced by cerebral ischemia: an apoptotic perspectiveJ Cereb Blood Flow Metab17815329290580
- MacManusJPLinnikMD1997Gene expression induced by cerebral ischemia: an apoptotic perspectiveJ Cereb Blood Flow Metab17815329290580
- MarmorMF1999Mechanisms of fluid accumulation in retinal edemaDoc Ophthalmol972394910896337
- MarumoTNollTSchini-KerthVB1999Significance of nitric oxide and peroxynitrite in permeability changes of the retinal microvascular endothelial cell monolayer induced by vascular endothelial growth factorJ Vasc Res365101510629427
- MasseySC1990Cell types using glutamate as a neurotransmitter in the vertebrate retinaProgr Retinal Res9399425
- MatuteCAlberdiEDomercqM2001The link between excitotoxic oligodendroglial death and demyelinating diseasesTrends Neurosci242243011250007
- McHaleJFHarariOAMarshallD1999TNF-alpha and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone miceJ Immunol1633993400010491002
- MehmetHYueXSquierMV1994Increased apoptosis in the cingulate sulcus of newborn piglets following transient hypoxia-ischaemia is related to the degree of high energy phosphate depletion during the insultNeurosci Lett18112157898750
- MeisterAAndersonME1983GlutathioneAnnu Rev Biochem52711606137189
- MelderRJKoenigGCWitwerBP1996During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endotheliumNat Med299278782456
- MinJKKimYMKimSW2005TNF-related activation-induced cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NFB activation in endothelial cellsThe Journal of Immunol17553140
- MishraOPAshrafQMDelivoria-PapadopoulosM2002Phosphorylation of cAMP response element binding (CREB) protein during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibitionNeuroscience1159859112435435
- MishraOPZubrowABAshrafQM2006Nuclear Ca(++)-influx, Ca (++)/calmodulin-dependent protein kinase IV activity and CREB protein phosphorylation during post-hypoxic reoxygenation in neuronal nuclei of newborn piglets: the role of nitric oxideNeurochem Res3114637117091402
- MishraOPZubrowABAshrafQM2004Nitric oxide-mediated activation of extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) during hypoxia in cerebral cortical nuclei of newborn pigletsNeuroscience1231798614667452
- MiyakeKIbarakiN2002Prostaglandins and cystoid macular edemaSurv Ophthalmol47S203812204717
- MizuiTKinouchiHChanPH1992Depletion of brain glutathione by buthionine sulfoximine enhances cerebral ischemic injury in ratsAm J Physiol262H31371539690
- MoncadaSPalmerRMHiggsEA1991Nitric oxide: physiology, pathophysiology, and pharmacologyPharmacol Rev43109421852778
- MullerAPietriSVillainM1997Free radicals in rabbit retina under ocular hyperpressure and functional consequencesExp Eye Res64637439227282
- MurataTNakagawaKKhalilA1996The relation between expression of vascular endothelial growth factor and breakdown of the blood retinal barrier in diabetic rat retinasLab Invest74819258606491
- NakajimaWIshidaALangeMS2000Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn ratJ Neurosci207994800411050120
- NamuraSZhuJFinkK1998Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemiaJ Neurosci183659689570797
- NeufeldAHKawaiSDasS2002Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthaseExp Eye Re755218
- NeufeldGCohenTGengrinovitchS1999Vascular endothelial growth factor (VEGF) and its receptorsFASEB J139229872925
- NguyenSMAlexejunCNLevinLA2003Amplification of a reactive oxygen species signal in axotomized retinal ganglion cellsAntioxid Redox Signal56293414580319
- NguyenTBrunsonDCrespiCL1992DNA damage and mutation in human cells exposed to nitric oxide in vitroProc Natl Acad Sci U S A89303041557408
- NicoteraPOrreniusS1998The role of calcium in apoptosisCell Calcium23173809601613
- NodaMNakanishiHNabekuraJ2000AMPA–kainate subtypes of glutamate receptor in rat cerebral microgliaJ Neurosci20251810627602
- OsborneNNCassonRJWoodJP2004Retinal ischemia: mechanisms of damage and potential therapeutic strategiesProg Retin Eye Res239114714766318
- OsborneNNChidlowGNashMS1999The potential of neuroprotection in glaucoma treatmentCurr Opin Ophthalmol10829210537768
- PangIHWexlerEMNawyS1999Protection by eliprodil against excitotoxicity in cultured retinal ganglion cellsVis Sci4011706
- PinJPDuvoisinR1995The metabotropic glutamate receptors: structure and functionsNeuropharmacology341267623957
- PrassKScharffARuscherK2003Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietinStroke341981612829864
- ProescholdtMAHeissJDWalbridgeS1999Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brainJ Neuropathol Exp Neurol586132710374752
- ProescholdtMAJacobsonSTresserN2002Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis ratsJ Neuropathol Exp Neurol619142512387457
- PuleraMRAdamsLMLiuH1998Apoptosis in a neonatal rat model of cerebral hypoxia-ischemiaStroke292622309836776
- PurtscherO1912Angiopathia retinae traumatica: lymphorrhagien desaugengrundesGraefes Arch Klin Exp Ophthalmol8234771
- QuigleyHANickellsRWKerriganLA1995Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosisInvest Ophthalmol Vis Sci36774867706025
- QuinnTPPetersKGDe VriesC1993Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endotheliumProc Natl Acad Sci U S A90753378356051
- RatanRRMurphyTHBarabanJM1994Oxidative stress induces apoptosis in embryonic cortical neuronsJ Neurochem6237697903353
- ReichenbachAWurmAPannickeT2007Müller cells as players in retinal degeneration and edemaGraefes Arch Clin Exp Ophthalmol2456273617219109
- RenolleauSAggoun-ZouaouiDBen-AriY1998A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosisStroke291454609660403
- RörigBGrantynR1993Rat retinal ganglion cells express Ca(2+)-permeable non-NMDA glutamate receptors during the period of histogenetic cell deathNeurosci Lett1533268510821
- SalgoMGBermúdezESquadritoGL1995Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytesArch Biochem Biophys32250057574726
- SaltTEBinnsKE2000Contributions of mGlu1 and mGlu5 receptors to interactions with N-methyl-D-aspartate receptor-mediated responses and nociceptive sensory responses of rat thalamic neuronsNeuroscience1003758011008175
- SalveminiDWangZSternM1998Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathologyProc Natl Acad Sci U S A952695703
- SattlerRTymianskiM2001Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell deathMol Neurobiol241–31072911831548
- SchochHJFischerSMartiHH2002Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brainBrain12525495712390979
- SengerDRPerruzziCAFederJ1986A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell linesCancer Res465629323756910
- ShelhamerJHVolkmanDJParrilloJE1985Takayasu’s arteritis and its therapyAnn Intern Med10312162860834
- ShibuyaMItoNClaesson-WelshL1999Structure and function of vascular endothelial growth factor receptor-1 and -2Curr Top Microbiol Immunol23759839893346
- ShimazakiKIshidaAKawaiN1994Increase in bcl-2 oncoprotein and the tolerance to ischemia-induced neuronal death in the gerbil hippocampusNeurosci Res209597527133
- SiliprandiRCanellaRCarmignotoG1992N-methyl-D-aspartate-induced neurotoxicity in the adult rat retinaVisual Neurosci856773
- SohnSKimEYGwagBJ1998Glutamate neurotoxicity in mouse cortical neurons: atypical necrosis with DNA ladders and chromatin condensationNeurosci Lett240149488160
- SouthamEGarthwaiteJ1993The nitric oxide-cyclic GMP signalling pathway in rat brainNeuropharmacology321267777509051
- St ClairEGAndersonSJOltvaiZN1997Bcl-2 counters apoptosis by Bax heterodimerization-dependent and -independent mechanisms in the T-cell lineageJ Biol Chem27229347559361016
- SteinINeemanMShweikiD1995Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genesMol Cell Biol15536387565686
- SucherNJAizenmanELiptonSA1991NMDA antagonists prevent kainate neurotoxicity in rat retinal ganglion cellsJ Neurosci11966711672708
- SucherNJLiptonSADreyerEB1997Molecular basis of glutamate toxicity in retinal ganglion cellsVision Res373483939425525
- SucherNJWongLALiptonSA1990Redox modulation of NMDA receptor-mediated Ca2+ flux in mammalian central neuronsNeuroreport129322151794
- SzaboMEDroy-LefaixMTDolyM1997Direct measurement of free radicals in ischemic/reperfused diabetic rat retinaClin Neurosci424059292250
- SzaflarskiJBurtrumDSilversteinFS1995Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal ratsStroke2610931007762028
- TanSSagaraYLiuY1998The regulation of reactive oxygen species production during programmed cell deathJ Cell Biol1411423329628898
- TezelGWaxMB2004The immune system and glaucomaCurr Opin Ophthalmol1580415021215
- TezelGYangX2004Caspase-independent component of retinal ganglion cell death, in vitroInvest Ophthalmol Vis Sci4540495915505055
- TielschJMKatzJSommerA1995Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessmentArch Ophthalmol113216217864755
- TinjustDKergoatHLovasikJV2002Neuroretinal function during mild systemic hypoxiaAviat Space Environ Med7311899412498547
- TodaNNakanishi-TodaM2007Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathyProg Retin Eye Res262053817337232
- TolentinoMJMillerJWGragoudasES1996Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primateOphthalmology10318201288942877
- TsoMOM1982Pathology of cystoid macular oedemaOphthalmology89902157133637
- van DamPS2002Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectivesDiabetes Metab Res Rev181768412112935
- WangJBeekhuizenHvan FurthR1994Surface molecules involved in the adherence of recombinant interferon-gamma (rIFN-gamma)-stimulated human monocytes to vascular endothelial cellsClin Exp Immunol9526397508346
- WangQTangXNYenariMA2007The inflammatory response in strokeJ Neuroimmunol184536817188755
- WangXNiwaMHaraA2002Neuronal degradation in mouse retina after a transient ischemia and protective effect of hypothermiaNeurol Res24730512392214
- WaxMBTezelG2002Neurobiology of glaucomatous optic neuropathy: diverse cellular events in neurodegeneration and neuroprotectionMol Neurobiol26455512392055
- WernsSWSheaMJLucchesiBR1985Free radicals in ischemic myocardial injuryJ Free Radic Biol Med1103103939137
- WongDDorovini-ZisK1992Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharideJ Neuroimmunol3911211352310
- WongMLBongiornoPBal-ShekhleeA1996IL-1 beta, IL-1 receptor type I and iNOS gene expression in rat brain vasculature and perivascular areasNeuroreport7244588981400
- YanoffMFineBSBruckerAJ1984Pathology of human cystoid macular oedemaSurv Ophthalmol28 Suppl505116463850
- YoshidaSYoshidaAIshibashiT2004Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammationGraefes Arch Clin Exp Ophthalmol2424091315029502
- YueXMehmetHPenriceJ1997Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemiaNeuropathol Appl Neurobiol2316259061686
- ZhangZGZhangLJiangQ2000VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brainJ Clin Invest1068293811018070
- ZhangZGZhangLTsangW2002Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemiaJ Cereb Blood Flow Metab223799211919509
- ZhangRLChoppMChenH1994Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the ratJ Neurol Sci1253107964886
- ZhengLGongBHatalaDA2007Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetesInvest Ophthalmol Vis Sci48361717197555
- ZubrowABDelivoria-PapadopoulosMAshrafQM2002aNitric oxide-mediated Ca2+/calmodulin-dependent protein kinase IV activity during hypoxia in neuronal nuclei from newborn pigletsNeurosci Lett3355812457729
- ZubrowABDelivoria-PapadopoulosmMAshrafmQM2002bNitric oxide-mediated expression of Bax protein and DNA fragmentation during hypoxia in neuronal nuclei from newborn pigletsBrain Res95607