Abstract
Purpose
To emphasize the effect of photodynamic therapy (PDT) on the size and progression of the neovascular lesion (NL) and evolution of the disciform scar (DS) in predominantly classic subfoveal choroidal neovascularization (SFCNV).
Methods
A retrospective study of 62 eyes treated with PDT for SFCNV was performed. The greatest linear dimension (GLD) before and at last follow-up after treatment and the size of the DS post-PDT were analyzed. A subgroup of patients with DS in their fellow eye at presentation without prior PDT was also studied. The size of the scar in these eyes was compared to that following PDT.
Results
After an average follow-up at 9 months, the size of the NL was stabilized or reduced in 64% of the study eyes with absence of fluorescein leakage in 45%. Only 3 eyes (5%) developed DS. At presentation, 14 patients already had DS in their fellow eye, the size of which was significantly larger than that post-PDT (p = 0.044). It was also significantly larger than that of the potential scar in the study eyes of the same subgroup of patients (p = 0.002) and of the rest of the patients (p = 0.0001).
Conclusion
This study demonstrates a beneficial effect for PDT on the size of the NL and DS in SFCNV, which might be of great significance, particularly when PDT fails to prevent severe vision loss.
Introduction
Age-related macular degeneration (AMD) is the leading cause of legal blindness in the elderly (CitationKlein et al 1995). The main reason for visual loss in these patients is the development of choroidal neovascularization (CNV) in the macular area, mainly in the subfoveal region (CitationFerris et al 1984; CitationMPSG 1993). Subfoveal choroidal neovascularization (SFCNV) is also a common cause of vision loss in pathologic myopia (CitationSoubrane and Coscas 2001) and the presumed ocular histoplasmosis syndrome (POHS) (CitationOlk et al 1984). Since its introduction, PDT has had a great impact on the management of SFCNV (CitationBressler and Bressler 2000; CitationMargherio 2000) and its benefit in reducing the risk of vision loss has been documented in large multicenter clinical trials in the case of AMD (CitationTAP 1999; CitationBressler 2001, Citation2002; CitationVIP 2001b) and myopia (CitationVIP 2001a). Some evidence for its benefit in other causes of SFCNV is also available (CitationSickenberg et al 2000; CitationSaperstein et al 2002; CitationBusquets et al 2003). Although these trials included data regarding the fluorescein angiographic outcomes and progression of the CNV lesion following PDT, the main emphasis was on visual outcome and the reduction in the risk of vision loss. However, these studies, and data from other studies (CitationSchmidt-Erfurth 1999; CitationMichels et al 2000), suggest that PDT could limit progression of and reduce fluorescein leakage from CNV lesions. Our study exclusively discusses the beneficial effect of PDT on the progression and size of the CNV lesion, and on the evolution of the disciform scar (DS) in patients with predominantly classic SFCNV. We also comment on why this effect may be desirable even when PDT fails to reduce the risk of vision loss.
Methods
Following Institute Review Board approval, we performed a retrospective chart review of all patients who received verteporfin-mediated PDT for SFCNV at the Department of Ophthalmology at the University of Virginia Health System during a 2-year period. All patients were treated by two of us (JST and BPC). Patients with AMD and non-AMD-related predominantly classic (SFCNV) (area of classic CNV occupies 50% or more of the area of the entire lesion) were included. All cases that demonstrated ophthalmoscopic and fluorescein angiographic evidence of “retinal angiomatous proliferation” (RAP) (CitationYannuzzi 2001) were excluded.
To be included in this study, each treated eye had to complete at least 6 months of follow-up after the first PDT treatment. In addition, the CNV lesion had to meet the eligibility criteria set in the TAP and VIP trials (CitationTAP 1999; CitationVIP 2001a). Also, the treatment, re-treatment and follow-up methods had to adhere to the standard protocol of verteprofin-mediated PDT used in these trials. Only two minor deviations were allowed. First, eyes with initial best-corrected visual acuity worse than 20/200 for AMD and 20/100 for myopia were included. Second, only rarely eyes whose follow up did not precisely adhere to the CitationTAP (1999) and CitationVIP (2001a) follow-up protocol were included. The breach to the protocol in such eyes was that fluorescein angiography (FA) was not necessarily performed on all follow-up visits. These were stable eyes that had dramatic and sustained improvement in symptoms with visual stabilization following PDT, in which the treating physician found no funduscopic evidence of active CNV on later follow up visits.
In each case, the etiology of the neovascular lesion, the indication for PDT, the number of treatment sessions, and the range of follow up were noted. Also, the size of the neovascular lesion, estimated by the greatest linear dimension (GLD) of the lesion (CitationTAP 1999), was measured before treatment and at the last follow up following treatment. Then, the percentage change in lesion size; the percentage of eyes with stable, reduced, or progressed neovascular lesion (according to TAP grading criteria of fluorescein angiographic assessment at follow up); and the percentage of eyes that developed disciform scarring following PDT were calculated.
A subgroup of 14 cases that received PDT to one eye only (study eye) and already had a DS in the fellow eye at presentation was analyzed. None of the latter eyes received PDT prior to scar development. The size of the DS, as estimated by its GLD, was measured in each case from funduscopic and angiographic data. The average size of these scars was calculated and compared to that of the DS that developed following PDT in some eyes. Also it was compared to the average size of the DS that could have potentially developed following PDT in the study eyes of the same 14 patients, and in the study eyes of the rest of the patients. In each of these comparisons, a double-tailed student’s t-test was performed to assess significance.
The size of the DS that could have potentially developed in the study eyes over the follow-up period after PDT was calculated based on the following. In eyes that had no progression or less CNV leakage by TAP criteria (CitationTAP 1999), the size of the potential DS was approximated by the size of the pre-treatment neovascular lesion. We believe this is appropriate because PDT is believed to cause occlusion of the vascular component of the neovascular lesion without affecting the fibrous component (CitationGhazi et al 2001). So although by TAP criteria (CitationTAP 1999) the size (GLD) of the entire lesion and CNV leakage might angiographically decrease following PDT, the neovascular membrane, with its occluded vessels and fibrous matrix, anatomically persists with no regression. In eyes with progression following PDT, the size of the potential DS was approximated by the size of the post-treatment neovascular lesion at the last follow up visit.
Results
One hundred and forty eight eyes of 137 patients were treated with verteporfin-mediated PDT for SFCNV at the Department of Ophthalmology at the University of Virginia Health System over a period of 2 years. Careful review of the charts of these patients disclosed that 62 eyes of 60 patients satisfied the inclusion criteria of this study. The major reasons for exclusion included less than 6 months follow-up after the first PDT session, nonpredominantly classic SFCNV, and/or evidence of “RAP” (CitationYannuzzi et al 2001). The following is a report of the results obtained from analysis of the 62 eyes.
Sixty patients received PDT to only one eye and two patients had PDT performed to both eyes. Ninety-five percent (59/62) of the eyes had predominantly classic SFCNV secondary to AMD. The remaining 3 (5%) were due to pathologic myopia (2 cases) and POHS (1 case). Twelve eyes (19%) had recurrent SFCNV after having previously received laser photocoagulation for extrafoveal or juxtafoveal CNV. The follow up interval following the first PDT session ranged from 6 to 16 months (average 9 months) with 53% of the eyes followed for at least 9 months (equivalent of a maximum of 4 PDT sessions by TAP protocol). The number of treatment sessions over the period of follow-up ranged from 1 to 5 (average of 2.7 sessions). The average pre-treatment GLD was 2585 μm (range: 400–4800 μm) and the average post-treatment GLD was 2103 μm (range: 0–6800 μm).
At the last follow up visit following PDT, 40 eyes (64%) had stable or decreased GLD of the lesion with no progression of leakage. Twenty-eight of 62 eyes (45%) had no evidence of CNV with absence of leakage (). Twenty-two eyes (36%) had progression. Ten of these (45%) had at least doubling of the GLD of the neovascular lesion compared to pre-treatment. The latter could explain our observation that the average pre-treatment GLD (2585 μm) was not significantly different from the average post-treatment GLD (2103 μm), although 64% of the eyes had stable or decreased GLD. Three study eyes (5%) developed a DS following PDT with an average scar size of 3200 ± 1345 μm (range: 2100–4700 μm) ().
Figure 1 Pre-and post-treatment fluorescein angiogram (FA) of the left eye of a patient with AMD-related predominantly classic SFCNV. A) Pre-treatment, late phase frame. B) Ten months after two sessions of PDT, late phase frame. Note absence of CNV leakage in the post-treatment angiogram.
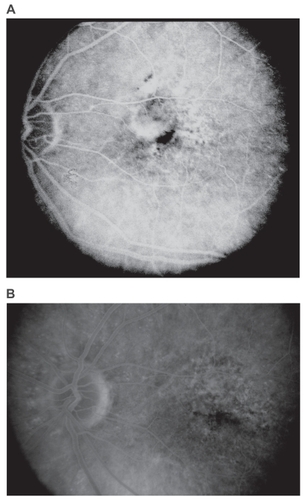
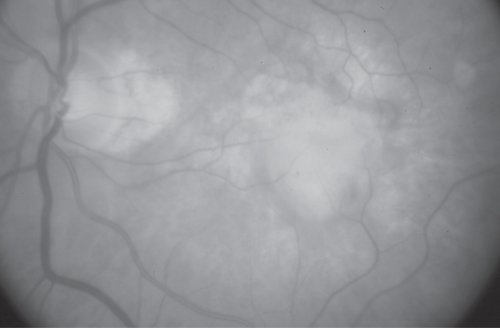
Figure 2 Disciform scar following PDT. Fundus picture of a patient with AMD-related SFCNV that evolved into a disciform scar following two sessions of PDT. This was the largest scar that developed following treatment (GLD: 4700 μm).
Fourteen patients (23%) had already had disciform scarring in their fellow eye at the time of presentation, 6 of these (43%) had previous laser therapy for CNV (). None of these eyes had previously received PDT. At last follow up visit, all but one of the 14 patients had a GLD of the neovascular lesion in the study eye smaller than the size of the scar in the fellow eye; and 7 of them (50%), had no evidence of CNV with absence of leakage in the study eye (). The average size of the DS in these 14 fellow eyes was 5900 ± 1866 μm (range: 2200–9200 μm). The average size of the DS that developed in eyes with previous laser therapy (6/14) was 5500 ± 2365 μm (range: 2200–9200 μm) and that in eyes without previous intervention (8/14; naturally occurring) was 6200 ± 1493 μm (range: 4000–8000 μm) (; ).
Figure 3 Disciform scars in 2 fellow eyes from the subgroup of 14 patients. A) Fundus picture of the largest scar that developed without previous intervention. B) Fundus picture of the largest scar that developed following previous laser treatment. This eye had received 2 sessions of laser photocoagulation for CNV.
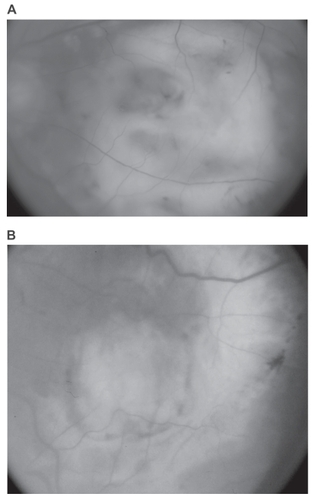
Table 1 Subgroup of 14 patients. GLD of the disciform scar in the fellow eyes and GLD of the neovascular lesion pre- and post-PDT in the study eyes
Table 2 Average size of disciform scars following PDT (actual and potential) and without PDT (with and without prior laser)
The average size of the DS that developed in 3 study eyes following PDT (3200 ± 1345 μm) was significantly smaller than that of the fellow eyes of the subgroup of 14 patients (5900 ± 1866 μm), and than that of the 8 of 14 eyes without previous intervention (6200 ± 1493 μm) (, ). It was not significantly smaller, however, than that of the 6 of 14 eyes that had disciform scaring with prior laser treatment (5500 ± 2365 μm) (, ).
Table 3 Comparison between the average size of the disciform scar following PDT (actual and potential) and that without PDT (with and without prior laser). Statistical analysis, p-valueTable Footnote*
The size of the DS that could have potentially developed following PDT was determined from the study eyes of the subgroup of 14 patients, and then also from the rest of the study eyes (45 eyes) excluding the 3 that developed DS. It was found to be 3607 ± 1389 μm and 3579 ± 1473 μm, respectively (). In either case, it was significantly smaller than the average size of the scar in the fellow eyes of the subgroup of 14 patients (5900 ± 1866 μm), and of the 8 of 14 without previous intervention (6200 ± 1493 μm) (, ). However, in both cases, it was not significantly smaller than that of the 6 of 14 eyes that developed the scar following prior laser treatment (5500 ± 2365 μm) (, ).
Discussion
Photodynamic therapy is now one of the first line treatment modalities used in the management of subfoveal CNV (VIP et al 2002). Its benefit in reducing vision loss has been proven in the case of AMD-related predominantly classic SFCNV (CitationTAP 1999; CitationBressler 2001), AMD-related occult SFCNV (CitationVIP 2001b; CitationBressler 2002), and SFCNV related to pathologic myopia (CitationVIP 2001a). Some evidence also exists for a treatment benefit in other cases of SFCNV (CitationSickenberg et al 2000; CitationSaperstein et al 2002; CitationBusquets et al 2003). Although these clinical trials reported on the fluorescein angiographic outcome following treatment and provided evidence that PDT could limit the size and progression of the CNV lesion, the main emphasis was on the reduction in the risk of moderate and severe visual loss associated with PDT. The effect of treatment on the size of the CNV lesion, particularly the evolution of the DS potentially associated with it, was less extensively assessed. Our study exclusively discusses this treatment benefit of PDT regardless of the final visual outcome, and supports the idea that the latter should not be the sole criterion by which treatment success is determined.
Over an average follow-up period of 9 months, we found that 64% of PDT treated eyes had stable or decreased GLD of the CNV lesion with no progression, 45% had no evidence of CNV with absence of leakage, and only 36% had progression at the last follow-up visit. These results compare favorably to those reported in the one-year follow up of the TAP trial (CitationTAP 1999) where 19% of treated eyes had absence of leakage and 46% had progression. The explanation for this favorable outcome is two-fold. First, the majority of our study eyes had a lesion almost entirely composed of classic CNV (data not shown here). Although in a subgroup analysis in the TAP trial, lesion characteristics at baseline did not have a significant effect on the magnitude of treatment benefit on progression and final lesion size more than 6 MPS disc areas, only three broad classes of lesions were analyzed: those with at least 50%, those with less than 50%, and those with no classic CNV. Those with at least 50% classic CNV were not further subdivided. Our group of study eyes constitutes one subgroup of those with at least 50% classic CNV and probably the one with the most favorable response to PDT as far as post-treatment lesion size and progression are concerned. Second, a selection bias, by virtue of the retrospective nature and inclusion criteria of the study, might be partially responsible. It is generally believed that patients with poor response to therapy are more likely to be noncompliant with treatment plans. Thus it is quite possible that the majority of eyes that had significant progression and poor visual outcome early after treatment failed to maintain the desired follow-up criteria of this study with either a short (less than 6 months) or an irregular (did not adhere to the TAP protocol) follow-up, leading to subsequent exclusion. The result of that would be the inadvertent selection of eyes with better response to PDT.
The effect of PDT on lesion size and progression emphasized in this manuscript might be very significant in determining the size of the DS and central scotoma that may ultimately develop in eyes with SFCNV. Disciform scarring is a frequent complication of neovascular maculopathy, particularly AMD (CitationGreen and Enger 1993). The evolution of a DS secondary to CNV has been described by CitationGass (1967a, Citation1967b) who noted that repeated episodes of hemorrhage and exudation from these abnormal vessels in the subretinal and/or subpigment epithelial space result in retinal pigment epithelium (RPE) metaplasia with vascular and fibroblastic in-growth from the choroid. The end result is a disciform maculopathy. Subsequent studies supported this theory (CitationTeeters and Bird 1973; CitationGreen and Key 1977). Based on this, an intervention that limits the activity (bleeding and exudation) of CNV would be expected to favorably alter the evolution of the DS. PDT has been shown to cause endothelial cell damage and denudation of the vascular basement membrane with subsequent platelet aggregation, thrombus formation and vascular occlusion (CitationGhazi et al 2001). This effect, however, is short-lived because thrombus fragmentation and endothelial cell regeneration with re-population of the denuded basement membranes, result in re-perfusion of the occluded vessels following treatment (CitationGhazi et al 2001). This explains why repeated treatment sessions may be needed to maintain CNV non-perfusion. To understand the beneficial effect of PDT on the evolution of the DS despite its short-lived effect, it is important to examine what happens to the CNV in between treatment sessions.
At the vascular level, during the period that the neovessels are occluded following each treatment session, and before reperfusion and leakage re-occur, mild exudation and hemorrhage, if at all, takes place. At the cellular level, after each treatment, newly regenerated endothelial cells replace the treated necrotic cells. Evidence for this phenomenon can be observed experimentally as early as the third day following PDT and is complete by 2 weeks (CitationRoyster et al 1988). After the necrotic endothelial cells are regenerated, the following PDT session leads to their necrosis again, and the cycle continues with each treatment session. The newly regenerated, immature endothelial cells that replace the treated, mature cells might be less leaky. Evidence for this comes from previous ultrastructural studies which showed that development of endothelial fenestrations and loss of some of the surrounding pericytes are part of the maturation process of endothelial cells and capillaries lined by them (CitationIshibashi and Ryan 1992; CitationIshibashi et al 1995; CitationSuzuki and Yoshida 1998). This may contribute to decreased exudation and hemorrhage in the early period following regeneration of treated capillaries and prior to their maturation. Therefore a logical implication would be that PDT could temporarily occlude active neovessels by virtue of thrombosis, and transform them into inactive vessels for at least a short period following regeneration and reperfusion by virtue of capillary immaturity. Hence, less stimulus for RPE metaplasia and fibroblastic ingrowth as per Gass theory (CitationGass 1967a, Citation1967b), and a favorable influence on the evolution of the DS and central scotoma (CitationSchmidt-Erfurth 1999).
The findings in our study support this hypothesis. The average size of the DS that developed in 3 study eyes following PDT (3200 ± 1345 μm) was significantly smaller than the average size of the scar in the fellow eyes of the subgroup of 14 patients (5900 ± 1866 μm), and particularly the 8 of 14 without previous intervention (6200 ± 1493 μm) (, ). In addition, the average size of the potential DS derived from the study eyes of the subgroup of 14 patients (3607 ± 1389 μm) was significantly smaller than that of the scar in their fellow eyes (5900 ± 1866 μm) (, ). One could argue that this comparison may be biased because patients with a DS and central scotoma in their fellow eye might seek care earlier due to the slightest disturbance of vision in their good eye secondary to increased awareness. To eliminate such bias, we also compared the average size of the DS in the fellow eyes of the subgroup of 14 patients (5900 ± 1866 μm) to that of the potential scar in the study eyes of the rest of the patients who do not have a DS in their fellow eye. The latter was found to be 3579 ± 1437 μm and was also significantly smaller (, ). Therefore, the potential scar in both instances was significantly smaller than the DS in the fellow eyes of the 14 patients. This was also true when comparison was made with the 8/14 fellow eyes that had a DS without previous intervention (6200 ± 1493 μm) (, ).
We also observed that there was no significant difference between the average size of the scar in the 6/14 fellow eyes in which the scar developed following previous laser treatment (5500 ± 2365 μm) and that of the scar following PDT in the 3 study eyes that developed scarring (3200 ± 1345 μm), the study eyes of the subgroup of 14 patients (3607 ± 1389 μm), or the study eyes of the rest of the patients (3579 ± 1473 μm), respectively (, ). So it also appears that laser treatment, like PDT, might have a beneficial effect on the evolution of the DS. This, again, we believe is the result of the interruption of the active exudative phase of the CNV that leads to the development of disciform maculopathy.
One drawback of the subgroup analysis of our study is that the DS in the fellow eye of each of these 14 patients was already present at presentation. It was not prospectively observed to evolve; therefore, we could not tell with certainty whether the process started with SFCNV, or non-SFCNV with secondary subfoveal spread. Thus comparing disciform scars in these fellow eyes to actual and potential disciform scars secondary to documented SFCNV in the study eyes might be biased towards smaller scars in the study eyes. However, there is evidence that SFCNV is usually larger than non-SFCNV. Bressler and colleagues (1993) found that 66% of CNV involving the subfoveal region were bigger than 1500 μm as opposed to 12% of CNV that did not. In addition, to our knowledge there is no evidence that the rate of CNV progression differs according to the location of the CNV. Therefore the subsequent scar is expected to be larger for SFCNV which is the location of CNV that all our study eyes had at presentation. Our results suggest that PDT may alter this expectation with a beneficial effect on the evolution of the DS secondary to SFCNV. This appears to be related to the effect of PDT on the activity of the CNV as discussed above. Another drawback is that the DS that was already present in the fellow eye of these 14 patients at the time of initial examination might have developed over years. Thus, comparison with actual and potential disciform scars that developed or could have developed over the finite follow-up period of this study might be also biased. The best way to address this factor is by prospectively following a study group and a control group for a long period of time. However, the availability of treatment options such as PDT for SFCNV nowadays makes studies with a control group unfeasible. In addition, to our knowledge, these comparisons have not been performed in such prospective trials to date (CitationTAP 1999; CitationVIP 2001a, Citation2001b; CitationBressler 2001, Citation2002). A third drawback is that no measures of the central visual function other than visual acuity were performed on our patients. As has been demonstrated, central visual field function and scotoma characteristics as demonstrated by microperimetry may yield valuable information regarding final visual function and visual rehabilitation (CitationSchmidt-Erfurth 2004).
This study shows evidence that the DS and potentially the subsequent central scotoma that might develop following PDT is expected to be smaller than that which develops naturally secondary to predominantly classic SFCNV. In microperimetric analysis studies of the central scotoma, PDT was associated with an improvement in the central visual field and reduction in scotoma size and intensity (CitationSchmidt-Erfurth 1999, Citation2004). This might prove to be a very significant effect of PDT in neovascular maculopathy particularly in patients who fail the benefit of PDT in preventing visual loss. The literature has accumulating evidence that visual rehabilitation of patients with a smaller DS and central scotoma might be easier and more rewarding (CitationTimberlake et al 1986, Citation1987; CitationWhittaker et al 1988; CitationFletcher and Schuchard 1997; CitationSchuchard and Fletcher 2000). This is because the size of the central scotoma is thought to be the most important factor in determining both the functional characteristics of the preferred retinal locus/loci (PRL(s)) that these patients develop for fixation, and the anatomic proximity of that locus/loci to the fovea (Citationvon Noorden and Mackensen 1962; CitationSunness et al 1996). Both factors may be significantly important in rehabilitating patients who lost central vision (Citationvon Noorden and Mackensen 1962; CitationFletcher and Schuchard 1997; CitationSchuchard and Fletcher 2000). In recent studies, reading speed, macular sensitivity, macular electrophysiology, and fixation patterns correlated with the extent of macular impairment in choroidal neovascularization and appeared to improve following PDT (CitationErgun et al 2003; CitationMidena et al 2004; CitationVarano et al 2005; CitationYodoi et al 2007).
Ophthalmologists and researchers should not overlook the benefit of PDT emphasized in this paper. This important benefit has been probably underestimated and overshadowed because of the emphasis that ophthalmologists invest in the direction of final visual acuity outcome following treatment. Long-follow-up, controlled prospective studies with emphasis on the size of the DS and central scotoma, with utilization of microperimetry with scanning laser ophthalmoscopy could demonstrate the value of PDT in limiting the size of the eventual DS and central scotoma in patients with predominantly classic SFCNV. Also they could possibly determine any beneficial effect of PDT in the rehabilitation process of treated patients. However, prospectively following a control group with SFCNV might not be feasible nowadays due to the availability of treatment options such as PDT. Re-evaluating patients enrolled in previous clinical trials (CitationTAP 1999; CitationVIP 2001a, Citation2001b; CitationBressler 2001, Citation2002) is one potential way for testing our hypothesis.
Disclosure
None of the authors have any proprietary or conflict of interest related to the manuscript.
References
- BresslerNMBresslerSB2000Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciencesInvest Ophthalmol Vis Sci41624810711673
- BresslerNM2001Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials – TAP Report 2Arch Ophthalmol11919820711176980
- BresslerNM2002Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy report 2Am J Ophthalmol133168911755871
- BresslerSBBresslerNMFineSL1983Subfoveal neovascular membranes in senile macular degeneration. Relationship between membrane size and visual prognosisRetina3711
- BusquetsMAShahGKWickensJ2003Ocular photodynamic therapy with verteporfin for choroidal neovascularization secondary to ocular histoplasmosis syndromeRetina2329930612824828
- ErgunEMaarNRadnerW2003Scotoma size and reading speed in patients with subfoveal occult choroidal neovascularization in age-related macular degenerationOphthalmology11065912511348
- FerrisIII FLFineSLHymanL1984Age-related macular degeneration and blindness due to neovascular maculopathyArch Ophthalmol102164026208888
- FletcherDCSchuchardRA1997Preferred retinal loci: relationship to macular scotomas in a low-vision populationOphthalmology10463289111255
- GassJDM1967aPathogenesis of disciform detachment of the neuroepithelium. III. Senile disciform macular degenerationAm J Ophthalmol6361744
- GassJDM1967bPathogenesis of disciform detachment of the neuroepithelium. IV. Fluorescein angiographic study of senile disciform macular degenerationAm J Ophthalmol6364559
- GhaziNGJabbourNMDe La CruzZC2001Clinicopathologic studies of age-related macular degeneration with classic subfoveal choroidal neovascularization treated with photodynamic therapyRetina214788611642377
- GreenWREngerC1993Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman LectureOphthalmology1001519357692366
- GreenWRKeySN3rd1977Senile macular degeneration: a histopathologic studyTrans Am Ophthalmol Soc75180254613523
- IshibashiTInomataHSakamotoT1995Pericytes of newly formed vessels in experimental subretinal neovascularizationArch Ophthalmol113227317864757
- IshibashiTRyanSJ1992Maturation of newly-formed subretinal vesselsEXS6159631377575
- KleinRWangQKleinBE1995The relationship of age-related maculopathy, cataract, and glaucoma to visual acuityInvest Ophthalmol Vis Sci36182917822146
- [MPSG] Macular Photocoagulation Study Group1993Laser photocoagulation of subfoveal neovascular lesions of age-related macular degenerationArch Ophthalmol111120097689827
- MargherioRRMargherioARDeSantisME2000Laser treatment with verteporfin therapy and its potential impact on retinal practicesRetina203253010950407
- MichelsSBarbazettoISchmidt-ErfurthU2000Choroidal changes after photodynamic therapy (PDT). A two year follow-up study of 38 patientsKlin Monatsbl Augenheilkd21794911022663
- MidenaERadinPPPilottoE2004Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry studySemin Ophthalmol19556115590535
- OlkRJBurgessDBMcCormickPA1984Subfoveal and juxtafoveal subretinal neovascularization in the presumed ocular histoplasmosis syndrome. Visual prognosisOphthalmology9115926026084225
- RoysterAJNandaSKHatchellDL1988Photochemical initiation of thrombosis. Fluorescein angiographic, histologic, and ultrastructural alterations in the choroid, retinal pigment epithelium and retinaArch Ophthalmol1061608143190547
- SapersteinDARosenfeldPJBresslerNMVerteporfin in Ocular Histoplasmosis (VOH) Study Group2002Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin in the ocular histoplasmosis syndrome: one-year results of an uncontrolled, prospective case seriesOphthalmology109149950512153802
- Schmidt-ErfurthU1999Indocyanine green angiography and retinal sensitivity after photodynamic therapy of subfoveal choroidal neovascularizationSemin Ophthalmol14354410790573
- Schmidt-ErfurthUMElsnerHTeraiN2004Effects of verteporfin therapy on central visual field functionOphthalmology111931915121371
- SchuchardRAFletcherDCAlbertDMJacobiecFA2000Preferred retinal locus and the scanning laser ophthalmoscopePrinciples and practice of ophthalmologySt. LowisWB Saunders Company2nd Ed2 Ch. 395543843
- SickenbergMSchmidt-ErfurthUMillerJW2000A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks and idiopathic causesArch Ophthalmol1183273610721954
- SoubraneGCoscasGJRyanSJWilkinsonCP2001Choroidal neovascularization in degenerative myopiaRetinaSt. LowisMosby3rd Ed2 Ch. 67113652
- SunnessJSApplegateCAHaselwoodD1996Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt diseaseOphthalmology1031458668841306
- SuzukiHYoshidaK1998Electron microscopic study of capillary in the human renal cell carcinomaNippon Hinyokika Gakkai Zasshi8914229493417
- TeetersVWBirdAC1973A clinical study of the vascularity of senile disciform macular degenerationAm J Ophthalmol7553654684265
- TimberlakeGTMainsterMAPeliE1986Reading with a macular scotoma. I. Retinal location of scotoma and fixation areaInvest Ophthalmol Vis Sci271137473721792
- TimberlakeGTPeliEEssockEA1987Reading with a macular scotoma. II. Retinal locus for scanning textInvest Ophthalmol Vis Sci281268743610545
- [TAP] Treatment of Age-Related Macular Degeneration With Photodynamic Therapy Study Group1999Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP Report 1Arch Ophthalmol11713294510532441
- [VIP] Verteporfin in Photodynamic Therapy Study Group2001aPhotodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial – VIP report no. 1Ophthalmology10884152
- [VIP] Verteporfin In Photodynamic Therapy Study Group2001bVerteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy (VIP) report 2Am J Ophthalmol13154160
- [VIP] Verteporfin Roundtable 2000 and 2001 Participants, Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group Principal Investigators, Verteporfin In Photodynamic Therapy (VIP) Study Group Principal Investigators2002Guidelines for using verteporfin (Visudyne®) in photodynamic therapy to treat choroidal neovascularization secondary to age-related macular degeneration and other causesRetina2261811884872
- VaranoMParisiVTedeschiM2005Macular function after PDT in myopic maculopathy: psychophysical and electrophysiological evaluationInvest Ophthalmol Vis Sci4614536215790915
- von NoordenGKMackensenG1962Phenomenology of eccentric fixationAm J Ophthalmol536426113926696
- WhittakerSGBuddJCummingsRW1988Eccentric fixation with macular scotomaInvest Ophthalmol Vis Sci29268783338884
- YannuzziLANegraoSIidaT2001Retinal angiomatous proliferation in age-related macular degenerationRetina214163411642370
- YodoiYTsujikawaAKamedaT2007Central retinal sensitivity measured with the micro perimeter 1 after photodynamic therapy for polypoidal choroidal vasculopathyAm J Ophthalmol1439849417336913