Abstract
Purpose:
To quantify changes in tear break-up time (TBUT), corneal staining and ocular surface disease index (OSDI) in glaucoma patients after switching therapy from latanoprost with 0.02% benzalkonium chloride (BAK) to travoprost with sofZia™.
Methods:
Prospective consecutive case series evaluating patients before and 8 weeks after switching from latanoprost with BAK to travoprost with sofZia™ in patients with baseline TBUT less than 6 seconds.
Results:
Forty eyes of 20 consecutive patients using latanoprost with BAK were switched to travoprost with sofZia™. Mean TBUT prior to starting travoprost was 2.02 ± 0.71 seconds and increased to 6.34 ± 1.31 seconds 8 weeks after the switch (p < 0.001). Mean inferior corneal staining scores decreased from 2.40 ± 0.87 to 1.38 ± 0.59 (p < 0.001). Mean OSDI scores decreased from 26.31 ± 8.25 to 16.56 ± 6.19 (p < 0.001).
Discussion:
This report focuses on the status of the ocular surface, as documented by TBUT, corneal staining and OSDI, in patients switched from latanoprost with BAK to travoprost without BAK. The switch resulted in a statistically significant increase in TBUT and decreases in corneal staining and OSDI in patients with low baseline TBUT values.
Conclusion:
BAK, a common preservative for glaucoma drops, may increase OSD by disrupting the tear film and increasing conjunctival inflammation. In this study, a change from a BAK-preserved prostaglandin analog (PGA) to a non-BAK-preserved PGA resulted in a measurable improvement of TBUT, corneal staining and OSDI. Further studies are needed to better understand the impact of BAK-preserved medications on the ocular surface.
The US Food and Drug Administration requires manufacturers of multi-dose topical ophthalmic medications to include preservative systems against microbial contamination. Clinicians have long understood that, along with the benefits of safety in prolonged usage and decreased potential of ocular infection, there are proven deleterious side effects with chronic exposure to some preservatives.Citation1 These effects have been demonstrated in animals and humans with both in vitro and in vivo models.Citation2–Citation5 Studies have also demonstrated that when comparing topical medications with preservatives and their non-preserved counterpart, there is less ocular toxicity, suggesting a direct correlation with the presence of preservative and damage to the ocular surface.Citation5–Citation7
One of the more common preservatives used today is benzalkonium chloride (BAK).Citation8 BAK is a quaternary ammonium compound whose antimicrobial activity arises from its ability to disrupt cell membranes and potentiate cell death. Along with its antimicrobial activity, BAK is toxic to the corneal epithelium as well as conjunctival epithelium and stroma.Citation4,Citation9–Citation16 These changes appear to be both dose- and time-dependent. These toxic side effects come to the forefront in the care of glaucoma patients due to the cumulative amount of BAK that can be introduced through a multiple drop regimen lasting decades. Alternative preservatives have been introduced including oxidizing agents, such as sofZia™ (Alcon Laboratories, Inc., Fort Worth, Texas, USA) that contains borate, zinc and sorbitol. Oxidizing agents may lead to less cell damage than detergent preservatives such as BAK.
This study focuses on the effects of topical antiglaucoma medications in patients with pre-existing ocular surface disease. We aim to quantify changes in tear break-up time (TBUT), corneal staining and the ocular surface disease index (OSDI) after switching from latanoprost with 0.02% BAK to travoprost with sofZia™.
Materials and methods
This was a prospective, open label, single-center study involving 40 eyes of twenty consecutive patients presenting to a tertiary care glaucoma practice. The study design was approved by the University of Colorado Internal Review Board. Each patient had been taking latanoprost monotherapy for at least 12 months prior to enrollment. All patients had a baseline tear break-up time (TBUT) of less than 6 seconds. Patients were instructed to avoid adding other topical drops during the study period. Baseline measurements of TBUT, inferior corneal staining and ocular surface disease index (OSDI) were obtained for each enrollee between 7 and 10 AM and in the same examination room at each visit.
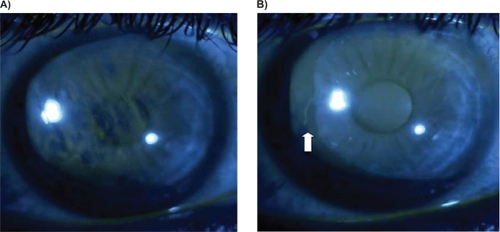
TBUT was obtained by placing 5 μL of 2% preservative-free sodium fluorescein (NaFl) to the inferior fornix using a fixed volume micropipette. To thoroughly mix the NaFl with the tear film, the subject was instructed to blink three times. The slit lamp was set at a magnification of 16 × using cobalt blue illumination and a stopwatch was used to time the occurrence of the first break in the fluorescein stained tear film (Figure ). The timer was started immediately after the last blink and stopped at the first break in fluorescein. This was measured three consecutive times and an average of these measurements was used to calculate the final TBUT. At the 8-week follow-up, each TBUT was obtained by the same observer (MYK) using the same method as described above. Inferior corneal staining was measured on a scale of 0 to 4 (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = confluent) at baseline and final follow-up visits. The ocular surface disease index questionnaire was given to the participants at both the initial visit as well as the eight-week follow-up. We used Student’s t-test to compare data between the two visits.
Results
All 20 patients (40 eyes) enrolled in the study who completed the final evaluation were included in data analysis (Figure , Table ). One patient did not return at the 8-week mark and was excluded from analysis. The patients ranged in age from 42 to 78 years, 16 were Caucasian and 4 African American, 12 were male and all were diagnosed with open angle glaucoma. Prior to starting travoprost, the mean TBUT was 2.02 ± 0.71 seconds for the 40 eyes studied. This increased to 6.34 ± 1.31 seconds 8 weeks after the switch (p < 0.001). Inferior corneal staining decreased from 2.40 ± 0.87 to 1.38 ± 0.59 (p < 0.001). The OSDI initially measured 26.31 ± 8.25 which is within the moderate category. Eight weeks after switching to travoprost, the OSDI fell into the mild category: 16.56 ± 6.19 (p < 0.001) in this same population of 20 patients. There was no statistically significant difference in intraocular pressure (IOP) levels at baseline (14.05 ± 2.78 mmHg) and after the switch to travoprost with sofZia™ (13.95 ± 3.04, p = 0.88).
Figure 2 A) Mean tear break-up time on latanoprost and after switch to travoprost without BAK; B) Mean ocular surface disease index on latanoprost and after switch to travoprost without BAK.
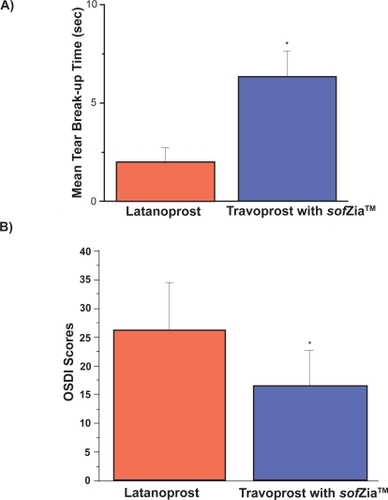
Table 1 Baseline and follow-up results after switching from latanoprost with BAK to travoprost with sofZia™
Discussion
BAK is the most commonly used ophthalmic preservative. Multiple studies have delineated the deleterious effects of BAK on conjunctival epithelium, corneal epithelium and corneal stroma.Citation4,Citation9–Citation20 The microscopic changes seen in patients taking BAK include an increase of pro-inflammatory cytokines, lymphocyte infiltration, fibroblast proliferation, corneal microvilli loss and goblet cell loss in the conjunctival epithelium. In addition, these ocular surface changes may decrease the success rate of glaucoma surgery.Citation21 It is also not surprising that changes to the tear film and increase in inflammation result in a worsening of symptoms in patients with preexisting dry eye who are also treated with preserved topical glaucoma drops.Citation14–Citation16
Yee et alCitation22 found that there was a marked reduction in toxicity when exposing immortalized human corneal epithelial cells to travoprost with sofZia™ compared to latanoprost with BAK. Kahook et alCitation19 described similar findings in both corneal and conjunctival changes in New Zealand white rabbits. Their results concluded that an increase in inflammation, as measured by lymphocytic infiltration, was found in tissue exposed to latanoprost with BAK as compared to travoprost with SofZia. Also, corneal epithelial damage was statistically higher in the latanoprost with BAK group compared to the travoprost with sofZia™ group.
The ocular surface disease index, was reported by Schiffman et alCitation23 as both a valid and reliable means to quantify dry eye syndrome. Using this questionnaire, our study found a statistically significant improvement of OSDI in patients switched from latanoprost with BAK to travoprost with sofZia™. Patients who have less ocular irritation may be more likely to be adherent to their medication regimen. This could lead to more effective lowering of IOP and resultant decrease of glaucomatous vision loss. We also found a statistically significant improvement in tear break-up time (TBUT) after patients were switched to travoprost with sofZia™. For patients with an already reduced TBUT, this change appears to correlate to a significant improvement in dry eye symptoms as reflected by the improvement in OSDI.
While maintaining antimicrobial activity, studies have shown sofZia™ to have less deleterious effects than BAK on ocular epithelium in both in vitro and in vivo studies.Citation19,Citation22,Citation24 In addition, when comparing travoprost with BAK (Travatan®; Alcon Laboratories, Inc., Fort Worth, Texas, USA) to travoprost with sofZia™ (Travatan Z®; Alcon Laboratories, Inc., Fort Worth, Texas, USA), the IOP-lowering efficacy was found to be identical.Citation25 Thus, it appears that BAK is not required for either safety or efficacy of travoprost as an ocular hypotensive agent.
Although the results of both the OSDI and TBUT show a statistically significant improvement when switching from latanoprost with BAK to travoprost with sofZia™, we focused our study only on patients with measurably decreased TBUT at baseline, thus limiting the generalization of our results. Also, the number of patients enrolled is low and we included both eyes of each patient in our analysis. Finally, all participants lived in the low humidity environment of Denver or surrounding suburbs making generalizations to other geographic areas difficult to make.
The results of this study suggest that a change from a BAK-preserved prostaglandin analog to a prostaglandin analog preserved with sofZia™ could improve TBUT, corneal staining and OSDI score while maintaining similar IOP-lowering efficacy. These findings cannot be extrapolated to other BAK-preserved medications owing to the limited nature of this open-label pilot study. Further prospective and randomized studies are needed to better understand and confirm the effect of BAK on the ocular surface of glaucoma patients and to quantify possible effects on patient compliance and overall disease progression.
Disclosures
MYK has received research support and consulting fees from Alcon Laboratories, Inc.
References
- WilconLATo preserve or not to preserve, is that the question?Br J Ophthalmol1996805835848795365
- De Saint JeanMDebbaschCBrignoleFToxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cellsCurr Eye Res200020859410617908
- BursteinNLCorneal cytotoxicity of topically applied drugs, vehicles and preservativesSurv Ophthalmol19802515306998034
- BursteinNLThe effects of topical drugs and preservatives on the tears and corneal epithelium in dry eyeTrans Ophthalmol Soc UK19851044024093898475
- De JongCSolwijkTKuppensETopical timolol with and without benzalkonium chloride; epithelial permeability and autofluorescence of the cornea in glaucomaGraefes Arch Clin Exp Ophthalmol19942322212248034210
- MietzHNiesenUKrieglsteinGKThe effect of preservatives and antiglaucomatous medication on the histopathology of the conjunctivaGraefes Arch Clin Exp Ophthalmol19942325615657959096
- PisellaPJFillacierKElenaPComparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbitsOphthalmic Res2000323810657748
- NoeckerREffects of common ophthalmic preservatives on ocular healthAdv Ther20011820521511783457
- BaudouinCde LunardoCShort term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteersBr J Ophthalmol19988239429536878
- NornMSOpauszkiAEffects of ophthalmic vehicles on the stability of the precorneal tear filmActa Ophthalmol197755233465897
- YalvaçISGedikoğluGKaragözYEffects of antiglaucoma drugs on ocular surfaceActa Ophthalmol Scand1995732462487493237
- KuppensEVde JongCAStolwijkTREffect of timolol with and without preservative on the basal tear turnover in glaucomaBr J Ophthalmol1995793393427742279
- WilsonWSDuncanAJJayJLEffect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and manBr J Ophthalmol1975596676691203224
- HerrerasJMPastorJCCalongeMOcular surface alteration after long-term treatment with an antiglaucomatous drugOphthalmology199299108210881495787
- RolandoMBrezzoVGiordanoGvan BijsterveldOPLempMASpinelliDSymposium on the lacrimal system Singapore, 31990AmsterdamKugler and Ghedini19918791
- HollyFJTear film formation and rupture: an updateHollyFJThe precorneal tear film in health diseases and contact lens wearDry Eye Institute IncLubbock1986634645
- BursteinNLPreservative cytotoxic threshold for benzalkonium chloride and Chlorhexidine digluconate in cat and rabbit corneasInvest Ophthalmol Vis Sci1980193083137358482
- MarquardtRSchubertTBeeinflussung der Tränenfilmaufreisszeit (BUT) durch Betablocker-Augentropfen ohne KonservierungstoffeKlin Monatsbl Augenheilkd199119975781960936
- KahookMNoeckerRComparison of corneal and conjunctival changes after dosing of travoprost preserved with SofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tearsCornea20082733934318362664
- NoeckerRJHerrygersLAAnwaruddinRCorneal and conjunctival changes caused by commonly used glaucoma medicationsCornea20042349049615220734
- BroadwayDCGriersonIO’BrienCHitchingsRAAdverse effects of topical antiglaucoma medication. II. The outcome of filtration surgeryArch Ophthalmol199411211144614547980134
- YeeRWNorcomEGZhaoXCComparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human cornea epithelial cell culture systemAdv Ther20062351151917050493
- SchiffmanRMChristiansonMDReliability and validity of the Ocular Surface Disease IndexArch Ophthalmol200011861562110815152
- WhitsonJTCavanaghHDLakshmanNAssessment of corneal epithelial integrity after exposure to ocular hypotensive agents preserved with and without benzalkonium chlorideAdv Ther20062366367117142200
- LewisRAKatzGJWeissMJTravoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacyJ Glaucoma2007169810317224758