Abstract
Bimatoprost is the only representative of a novel class of prostaglandin ethanolamide (prostamide) compounds used therapeutically as an efficacious treatment for glaucoma. The pathways through which bimatoprost works to improve uveoscleral outflow to relieve elevated intraocular pressure are similar to those of the conventional prostaglandins used in glaucoma therapy, with some evidence of a preferential action at the trabecular meshwork. The pharmacology of bimatoprost is however, unclear. Pharmacological evidence supports a specific and distinct receptor-mediated agonist activity of bimatoprost at ‘prostamide’ receptors, which is selective to the prostamides as a class. However, other studies have reported either activity of bimatoprost at additional prostanoid and nonprostanoid receptors, or a conversion of bimatoprost to metabolites with agonist activity at prostaglandin FP receptors in the human eye. The formation of endogenous prostamides has been demonstrated in vivo, by a novel pathway involving the cyclooxygenase-2-mediated conversion of endogenous cannabinoid (endocannabinoid) substrates. Irrespective of the pharmacology of bimatoprost and the prostamides in general, further studies are needed to determine the biological role and biochemical pathology of prostamides in the human eye, particularly in glaucoma. Such studies may improve our understanding of uveoscleral flow and may offer new treatments for controlling intraocular pressure.
Glaucoma is a chronic disease of the optic nerve, caused at least in part by a sustained, elevated intraocular pressure. Mechanisms of optic deterioration include direct axonal damage, structural failure, and altered microvascular supply.Citation1 Intraocular pressure is normally maintained at a steady state and in health eyes assumes a relatively narrow range. The intraocular pressure in any given eye is determined by the rate of fluid (aqueous) production within the eye by the ciliary body and the drainage of aqueous humor from the eye through the trabecular meshwork, aqueous flow, and uveoscleral outflow. In glaucomatous eyes, the increased resistance to aqueous humor outflow is due in part to an increase in extracellular matrix deposited in the trabecular meshwork, but also in other outflow structures such as Schlemm’s canal,Citation2 with the amount of extracellular matrix correlated with the loss of axons in the optic nerve.Citation3 One study found that inflammatory genes were upregulated in trabecular meshwork from primary open-angle glaucomatous eyes under conditions of explant culture, compared to nonglaucomatous eyes.Citation4 However, many genes in the trabecular meshwork underwent altered expression under culture conditions, so the pathological relevance of these changes is unclear. While a host of other cellular and interstitial changes also occur in the outflow facility during glaucoma that lead to the loss of normal drainage via this tissue,Citation5 the pathogenic factors underlying glaucoma are still uncertain. This is partly a consequence of the complex physiological determinants of resistance within the outflow facility.Citation6 In addition, there are difficulties in mimicking primary angle open glaucoma in animal models, and the limited translational value of in vitro approaches such as whole organ, anterior segment and cell culture studies (such as studies in trabecular meshwork cells), ensures that the pathogenesis of glaucoma to date remains enigmatic.Citation4,Citation7
In order to decrease intraocular pressure, most glaucoma drug treatments alter either the rate of aqueous humor production (eg, beta blockers, carbonic anhydrase inhibitors) or the outflow pathway (eg, prostanoids).Citation8 This review will limit itself to a focus on the pharmacology and biochemical pathways of prostanoid-based therapies, in particular the prototypical therapeutic prostaglandin ethanolamide, bimatoprost. Irrespective of the antiglaucoma drug class, many patients on intraocular pressure-lowering drugs experience limitations in either efficacy or compliance, or display adverse side effects with long term use.Citation9 Some patients will continue to progress to blindness.Citation10
Prostanoids, cannabinoids, and prostamide pharmacology in the eye
Many clinically useful intraocular pressure-lowering agents act at prostaglandin FP receptors, responsive to the endogenous prostaglandin, prostaglandin F2α (PGF2α). These include such drugs as latanoprost and travoprost. Prostanoids play an important role in the control of intraocular pressure, primarily by increasing uveoscleral outflow via remodeling of the ciliary body.Citation11 Therapy with prostaglandin analogues has been shown to effectively lower intraocular pressure over the long term,Citation12 is often superior to other glaucoma therapies and shows fewer side effects.Citation13 While the prostanoid-based treatments have been found to possess similar profiles of efficacy of surveyed drugs within this class,Citation14 other studies have demonstrated that bimatoprost is more efficacious.Citation15,Citation16 While other prostaglandin analogues acting at TP, EP, and DP receptors have been investigated in animal models of raised intraocular pressure, none have advanced clinically so far.Citation17
An increase in trabecular meshwork outflow with prostanoid therapy has also been reported.Citation17 The shape and area of the intertrabecular spaces of the trabecular meshwork normally determines the rate of aqueous outflow through this tissue (and hence intraocular pressure). Traditionally, the size of the pores within the trabecular meshwork was thought to be influenced by the tone of the adjacent ciliary muscle (CM), a smooth muscle component of the ciliary body with tendinous connections to the trabecular meshwork. However, studies have shown that trabecular meshwork has in itself contractile properties similar to smooth muscle.Citation18–Citation20 It has been suggested that this allows the trabecular meshwork to actively change the intertrabecular spaces by an autoregulatory mechanism.Citation21 It is believed that trabecular meshwork contraction decreases aqueous outflow, while relaxation increases outflow.Citation20 The relative contribution of the mesh-work component to the overall reduction in intraocular pressure with prostanoid treatments is not known, although the weak FP receptor agonist docosanoid unoprostone has been demonstrated to have a preferential action on the trabecular meshwork,Citation17 as is also suggested for bimatoprost.Citation22 Unoprostone may affect outflow indirectly or directly via additional cellular mechanisms, including the alteration of ion channel activation in the trabecular meshwork.Citation23,Citation24
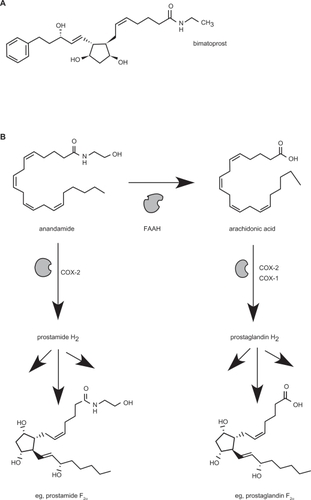
Within this class of prostanoid-based drugs is bimatoprost, which is structurally and chemically similar to the PGF2α analogues used in the treatment of glaucoma (). However, replacement of the carboxylate moiety with an ethanolamide functional group appears to confer to bimatoprost a substantially different pharmacology from the other representative PGF2α agonists.Citation25 It is the first drug of its chemical class, termed the prostaglandin ethanolamides (prostamides), to have a therapeutic application.
Figure 1 A) Chemical structure of bimatoprost B) Pathway for the production of prostaglandin ethanolamides (prostamides) via COX-2 mediated conversion of the major endocannabinoid, anandamide. Only Prostamide F2α is shown for brevity. Comparison is made alongside conventional prostaglandin production via COX enzymes, showing PGF2α as an example.
Bimatoprost has demonstrated efficacy comparable to other prostanoids in the reduction of intraocular pressure.Citation25,Citation26 While bimatoprost elicits a similar and clinically significant increase in uveoscleral outflow and possibly an increase in trabecular outflow facility as for the prostaglandins,Citation17,Citation22 whether the mechanism(s) of its intraocular pressure-lowering action are distinctive from that of the prostaglandins is still not known with certainty. It has been suggested that bimatoprost acts solely at the trabecular meshwork to increase aqueous outflow.Citation27 Certainly, long-term changes in vascularization, inflammatory cell infiltrate, and trabecular meshwork morphology are noted both with prostaglandin agonists and bimatoprost.Citation28,Citation29 Bimatoprost therapy is also associated with similar changes to extracellular matrix markers as with conventional prostaglandin treatments,Citation24 indicating that longer term structural changes follow a similar pathway to the prostaglandins. Studies comparing bimatoprost with latanoprost found that the greater efficacy of intraocular pressure reductions with bimatoprost was offset by a higher incidence of conjunctival hyperemia,Citation15,Citation30 although replacing existing latanoprost therapy with bimatoprost was associated with lower rates of hyperemia.Citation31,Citation32 The reasons for enhanced conjunctival hyperemia with bimatoprost therapy are not known, but it is believed that part of the effect of bimatoprost is manifest as vasodilatation occurring independently of inflammation, generated through endothelial-derived nitric oxide formation.Citation33 Hyperemia with long term prostanoid use has been attributed, either wholly or in part, to the benzalkonium preservative in topical prostanoid ophthalmologic preparations.Citation34,Citation35 However, this most likely does not account for the hyperemia associated with bimatoprost formulations, where the comparative benzalkonium concentrations are relatively low.
Other changes noted with prostanoid therapy generally include elongation and darkening of eyelashes, induced iris darkening, and periocular skin pigmentation, which are mostly similarly evidenced with bimatoprost treatment.Citation36 Topical ocular prostanoids evoke increased immune marker expression in the eye, which infers that the prostanoids can evoke a mild inflammatory reaction.Citation37 This altered immune marker expression is a feature that was also shared with bimatoprost therapy.Citation37
Bimatoprost is a stable chemical entity representative of the endogenous prostamides, which themselves are only relatively recent discoveries. Evidence has demonstrated that a major pathway for the production of endogenous prostamides is via the conversion of endogenous cannabinoid molecules, such as anandamide, via the action of cyclooxygenase-2 (COX-2)Citation38 (). Prostamides were subsequently shown to be produced in vivo utilizing knock-out mice for the normal endocannabinoid metabolising enzyme, fatty acid amide hydrolase (FAAH).Citation39 Another major endocannabinoid molecule, 2-arachidonoyl glycerol is also a substrate for COX-2, producing prostaglandin glycerol esters. A recent study also suggested other pathways for prostamide production exist and are yet to be fully characterized.Citation40 While the constitutive COX-1 does not display an affinity for the endocannabinoids as an enzyme substrate, it is also recognized that the endocannabinoids are substrates for other oxidation pathways aside from COX-2, such as via lipoxygenases and cytochrome P450 enzymes.Citation41
The prostamides have been shown to influence intraocular pressure in a similar fashion to conventional prostaglandin PGF2α agonists.Citation42 However, they are believed to act at receptors distinct from conventional prostaglandins, namely via a separate class of ‘prostamide’ receptors.Citation26,Citation43 Indeed, bimatoprost is believed to act at distinct ‘prostamide’ receptors to mediate intraocular pressure reductions in glaucoma.Citation43,Citation44 The precise biological role of the prostamides compared to prostaglandins generally is not known, either in ocular physiology or in other systems.Citation45 Certainly there appear to be functional pharmacological differences between the two prostanoid classes. Studies utilizing in vitro bioassays such as trabecular meshwork, ciliary and iridial muscle preparations, and cell culture expression systems ostensibly preclude a conventional FP receptor component to bimatoprost’s activity.Citation25,Citation44,Citation46
The pharmacological selectivity of the endogenous prostamides has been well characterized. Prostamides D2, E2, and F2α are only weakly active at human prostaglandin DP, EP, FP, IP, or TP receptors.Citation47 The distinctive pharmacology of the prostamides is supported by the recent development of prostamide-selective antagonists.Citation48 These have been demonstrated to block contractile responses in feline iris to prostamides (including bimatoprost), but not the corresponding prostaglandins.Citation48 Prostamide metabolites were generally not believed to be responsible for activity as assessed in vitro using either recombinant cell lines or functional bioassays.Citation47 However, another in vitro study in human eye tissues showed that bimatoprost was rapidly hydrolyzed in cornea, iris, sclera, and ciliary muscle to its corresponding 17-phenyl-PGF2α metabolite, known to be active at FP receptors.Citation49
Other studies that question the unique pharmacological selectivity of bimatoprost include one demonstrating an activity at cannabinoid receptors in ciliary muscle,Citation50 which contrasts to another study that demonstrated a negligible activity of prostamides at cannabinoid receptors.Citation42 Cannabinoid receptor agonists lower intraocular pressure,Citation51 potentially via an increased outflow facility through an action at the trabecular meshwork.Citation52 An action of the cannabinoid CB1 receptor antagonist SR141716A at ‘prostamide-sensitive’ receptors should be excluded to possibly clarify these anomalous findings.
The contractile effect of bimatoprost was also partly attributed to an agonist activity at the FP receptor, as attested to by the sensitivity of bimatoprost’s actions to the FP receptor antagonist AL8810.Citation50 More recently, prostaglandin FP receptor variants forming heterodimers in cell expression systems accounted for the selectivity of bimatoprost, which was reversible with prostamide-selective antagonists such as AGN211335.Citation53 The molecular identification of the prostamide receptor(s) remains elusive, but speculation of an FP receptor splice variant accounting for the pharmacological variation has been proposed.Citation25 The selectivity based purely on pharmacological actions will continue to raise an ambiguity as to the mechanism of bimatoprost’s actions.
It should also be reiterated that the major endocannabinoid molecules act at cannabinoid receptors expressed in the human eye, mainly in the retinaCitation54 but also in the trabecular meshwork.Citation55 Exogenous cannabinoids and endocannabinoids exert functional influences in the eye, including the modulation of aqueous humor production and outflow. Δ9-THC, the active psychotropic component of Cannabis Sativa, has been shown to increase aqueous outflow.Citation56 2-Arachidonoyl glycerol infusion in anterior eye segments increases aqueous humor outflow and alters actin deposition, possibly via an action at the trabecular meshwork.Citation57 Anandamide infusion has also been shown to increase the aqueous humor outflow facility.Citation58 That both endocannabinoid molecules and their COX-2 metabolites, the prostamides, can alter aqueous and uveoscleral outflow independently makes the contribution of their interaction to intraocular pressure difficult to discern in vivo. That both systems are expressed in the eye may be important in eye pathologies, such as glaucoma, where the expression of each system may be altered, with a subsequent modulation of both endocannabinoid and prostamide expression. The functional sequelae of such changes would be intriguing, but has yet to be extensively investigated.
Biochemical pathology in relation to prostamides
There are only a limited number of studies that have separately investigated the expression of the endocannabinoid system and COX-2 in glaucoma, either in animal models or in human glaucoma. None have yet attempted to directly measure prostamide levels in the eye at either normal or elevated intraocular pressure, so potential changes in prostamide generation here are inferred from altered systems involved in their formation.
It is well established that COX-2 expression is inducible under inflammatory conditions; therefore it is attractive to consider that at the tissue level, marked changes in COX-2 activity could dramatically alter the local fate of endocannabinoids. Under such conditions, endocannabinoids may be diverted into the production or prostamides and prostaglandin glycerol esters, which may possess a diverse suite of biological effects that are as yet to be defined.
Elements of both the tissue endocannabinoid system and COX-2 are expressed in the human eye, and perturbations in both endocannabinoid and COX-2 expression seem to follow similar patterns in human glaucoma. Endocannabinoids such as 2-AG are expressed in the human uveoscleral region in reduced levels in human glaucomatous eyes.Citation59 While there is a degree of constitutive COX-2 expression in the human eye, COX-2 expression is significantly reduced in human primary open angle glaucoma.Citation60 Thus, a possible scenario in glaucoma is one of a combination of both reduced COX-2 expression and reduced endocannabinoid substrates for COX-2, impinging upon a potentially important regulatory process for controlling aqueous outflow. The interaction between COX-2 and the cannabinoids is strengthened by evidence that methanandamide, a stable mimic of the endogenous cannabinoid anandamide, directly stimulates COX-2 expression in human nonpigmented ciliary epithelial cells,Citation61 in addition to independently lowering intraocular pressure through an effect on outflow via conventional cannabinioid receptors. The induction of COX-2 in ciliary epithelial cells is a feature also shared by exposure to prostaglandins such as prostaglandin E2 (PGE2).Citation62
In animal studies of the ocular endocannabinoid system, the focus has been on changes in retinal tissue markers as opposed to outflow structures. Nonetheless, as a result of acute elevations in intraocular pressure, rat retina shows enhanced fatty acid amide hydrolase (FAAH) expression and reduced anandamide levels, together with reduced cannabinoid CB1 receptor expression.Citation63 Implications for retinal protection were discussed by Nucci and colleagues.Citation63 However, reduced expression or greater turnover of prostamide substrates in outflow structures as a consequence of such changes in endocannabinoid markers could exacerbate intraocular pressure increases, by impinging on aqueous outflow.
Although ocular COX-2 expression is enhanced in animal models of glaucoma,Citation64 focused studies on humans showing reduced expression of ocular COX-2 in primary open-angle glaucoma and steroid-induced glaucomaCitation60 lend support to a more reliable clinical picture of changes in COX-2 versus animal models. Contrary to the view that COX-2 is proinflammatory, COX-2 expression may be reparative, producing enhanced levels of prostaglandins and prostamides in an effort to restore normal aqueous outflow in acute models of elevated intraocular pressure. The production of prostamides versus prostaglandins in this setting is also an interesting question, where COX-2 expression and activity is altered. Under conditions of cytokine incubation, explants and cell cultures can produce significant proportions of prostamides compared to prostaglandins.Citation65 In some studies of glaucoma, where aqueous humor shows significantly reduced PGE2 levels compared to control,Citation60 there may be a differential reduction in prostamides or prostaglandin concentrations that cannot be distinguished using commercial prostaglandin immunoassays, due to the complete immune cross reactivity between the two classes of prostanoid.Citation65 The implication of such a differential change occurring in the eye is not known, given that both prostamides and prostaglandins mediate increases in uveoscleral outflow and reductions in intraocular pressure. It is interesting to note that prostanoids such as latanoprost directly stimulate COX-2 production in human nonpigmented ciliary epithelial cells and that this is a requirement for matrix metalloproteinase expression.Citation66 Taken together with evidence that human aqueous humor shows significantly reduced PGE2 levels in glaucoma,Citation60 it is conceivable that prostanoids permit the restitution of ocular COX-2 expression, which in turn restores the normal structural and functional components of the outflow facility. As this feature is also shared with endocannabinoid analogues, it will be important to determine if a similar property of the prostaglandins is shared with prostamide therapy.
Studies investigating the effects of long-term use of COX-2 inhibitors (‘coxibs’) with intraocular pressure changes would also be of interest, especially given the reports of ocular side effects associated with their use.Citation67 Nonselective, nonsteroidal anti-inflammatory drugs are used in a variety of ophthalmologic conditions, and coxibs have a demonstrated development potential as new treatments for corneal angiogenesis and diabetic retinopathy.Citation68 Interestingly, administration of the COX-2 selective inhibitor nimesulide was found to enhance the intraocular pressure-lowering effect of latanoprost in patients with primary open-angle glaucoma.Citation69 This likely suggests that the overall COX-2 mediated production of a suite of prostanoids, with sometimes contrasting effects, needs to be considered, rather than just focusing on the role of prostamides in the control of intraocular pressure. Monitoring of intraocular pressure may, in any event, be warranted where COX-2 inhibitors are to be used topically in such settings over an extended period.
The role of endocannabinoid system in glaucoma may thus be of importance, not only because of the direct positive effects on outflow of the endocannabinoid molecules, but also for the provision of substrates for prostamide production, in addition to a potential neuroprotective contribution to the optic nerve.Citation70 The reduced endocannabinoid levels demonstrated in outflow pathways in human glaucomatous eyesCitation59 may exert a marked effect on outflow, both directly through reduced cannabinoid-induced outflow and indirectly, via reduced prostamide production. Irrespective of the final mediator (endocannabinoid or prostamide), there is a potential to investigate the inhibition of endogenous cannabinoid metabolism as a potential antiglaucoma therapy. Anandamide and 2-AG are broken down by the FAAH enzyme. Inhibitors of FAAH or FAAH gene knockouts increase tissue endocannabinoid concentrations,Citation39 including in the trabecular meshwork of the eye.Citation57 Studies will firstly need to directly measure the formation of prostamides to verify their place in the control of intraocular pressure and altered expression in the setting of glaucoma.
Disclosure
The author reports no conflicts of interest in this work.
References
- MackenziePCioffiGHow does lowering of intraocular pressure protect the optic nerve?Surv Ophthalmol200853Suppl 1S39S4319038623
- TianBGabeltBTGeigerBKaufmanPLThe role of the actomyosin system in regulating trabecular fluid outflowExp Eye Res20098871371718793636
- Lutjen-DrecollEFunctional morphology of the trabecular meshwork in primate eyesProg Retin Eye Res199918911199920500
- LitonPBLunaCChallaPEpsteinDLGonzalezPGenome-wide expression profile of human trabecular meshwork cultured cells, non-glaucomatous and primary open angle glaucoma tissueMol Vis20061277479016862071
- Lutjen-DrecollEMorphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the diseaseExp Eye Res2005811415978248
- OverbyDRStamerWDJohnsonMThe changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endotheliumExp Eye Res20098865667019103197
- JohnsonDHTrabecular meshwork and uveoscleral outflow modelsJ Glaucoma20051430831015990614
- WoodwardDFGilDWThe inflow and outflow of anti-glaucoma drugsTrends Pharmacol Sci20042523824115120486
- SchwartzGFQuigleyHAAdherence and persistence with glaucoma therapySurv Ophthalmol200853Suppl 1S57S6819038625
- Sharts-HopkoNCGlynn-MilleyCPrimary open-angle glaucomaAm J Nurs20091094047; quiz 48.19299999
- SchachtschabelULindseyJDWeinrebRNThe mechanism of action of prostaglandins on uveoscleral outflowCurr Opin Ophthalmol20001111211510848216
- EasthopeSEPerryCMTopical bimatoprost: a review of its use in open-angle glaucoma and ocular hypertensionDrugs Aging20021923124812027782
- HodgeWGLachaineJSteffensenIThe efficacy and harm of prostaglandin analogues for IOP reduction in glaucoma patients compared to dorzolamide and brimonidine: a systematic reviewBr J Ophthalmol20089271218156371
- BeanGWCamrasCBCommercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differencesSurv Ophthalmol200853Suppl 1S69S8419038626
- ChengJWWeiRLMeta-analysis of 13 randomized controlled trials comparing bimatoprost with latanoprost in patients with elevated intraocular pressureClin Ther20083062263218498911
- van der ValkRWebersCALumleyTHendrikseFPrinsMHSchoutenJSA network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressureJ Clin Epidemiol2009621279128319716679
- TorisCBGabeltBTKaufmanPLUpdate on the mechanism of action of topical prostaglandins for intraocular pressure reductionSurv Ophthalmol200853Suppl 1S107S12019038618
- StumpffFWiederholtMRegulation of trabecular meshwork contractilityOphthalmologica2000214335310657743
- FerrerETrabecular meshwork as a new target for the treatment of glaucomaDrug News Perspect20061915115816804567
- WiederholtMThiemeHStumpffFThe regulation of trabecular meshwork and ciliary muscle contractilityProg Retin Eye Res20001927129510749378
- WiederholtMDirect involvement of trabecular meshwork in the regulation of aqueous humor outflowCurr Opin Ophthalmol19989464910180513
- WanZWoodwardDFCornellCLBimatoprost, prostamide activity, and conventional drainageInvest Ophthalmol Vis Sci2007484107411517724194
- ThiemeHSteinhausenKOttleczAEffects of unoprostone and endothelin 1 on L-type channel currents in human trabecular meshwork cellsOphthalmic Res20053729330016118512
- OoiYHOhDJRheeDJEffect of bimatoprost, latanoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human ciliary body smooth muscle cellsInvest Ophthalmol Vis Sci2009505259526519443729
- WoodwardDFLiangYKraussAHProstamides (prostaglandin-ethanolamides) and their pharmacologyBr J Pharmacol200815341041917721551
- CantorLBClinical pharmacology of bimatoprostExpert Opin Drug Metab Toxicol2005115115716922657
- WanZWoodwardDFStamerWDEndogenous bioactive lipids and the regulation of conventional outflow facilityExpert Rev Ophthalmol2008345747019381354
- RussHHCostaVPFerreiraFMConjunctival changes induced by prostaglandin analogues and timolol maleate: a histomorphometric studyArq Bras Oftalmol20077091091618235898
- RichterMKraussAHWoodwardDFLutjen-DrecollEMorphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamideInvest Ophthalmol Vis Sci2003444419442614507888
- HowACKumarRSChenYMA randomised crossover study comparing bimatoprost and latanoprost in subjects with primary angle closure glaucomaBr J Ophthalmol20099378278619336424
- KurtzSMannOIncidence of hyperemia associated with bimatoprost treatment in naive subjects and in subjects previously treated with latanoprostEur J Ophthalmol20091940040319396785
- KammerJKatzmanBAckermanSHollanderDEfficacy and tolerability of bimatoprost versus travoprost in patients previously on latanoprost: a 3-month, randomized, masked-evaluator, multicenter studyBr J Ophthalmol200991 [Epub ahead of print].
- ChenJDinhTWoodwardDFBimatoprost: mechanism of ocular surface hyperemia associated with topical therapyCardiovasc Drug Rev20052323124616252016
- HenryJCPeaceJHStewartJAStewartWCEfficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapyClin Ophthalmol2008261362119668762
- GuenounJMBaudouinCRatPPaulyAWarnetJMBrignole-BaudouinFIn vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cellsInvest Ophthalmol Vis Sci2005462444245015980234
- AlmAGriersonIShieldsMBSide effects associated with prostaglandin analog therapySurv Ophthalmol200853Suppl 1S93S10519038628
- Rodrigues MdeLFelipe CrostaDPSoaresCPImmunohistochemical expression of HLA-DR in the conjunctiva of patients under topical prostaglandin analogs treatmentJ Glaucoma20091819720019295371
- KozakKRCrewsBCMorrowJDMetabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamidesJ Biol Chem2002277448774488512244105
- WeberANiJLingKHFormation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometryJ Lipid Res20044575776314729864
- MoriuchiHKodaNOkuda-AshitakaEMolecular characterization of a novel type of prostamide/prostaglandin F synthase, belonging to the thioredoxin-like superfamilyJ Biol Chem200828379280118006499
- PatrignaniPTacconelliSSciulliMGCaponeMLNew insights into COX-2 biology and inhibitionBrain Res Brain Res Rev20054835235915850674
- WoodwardDFCarlingRWCornellCLThe pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 productsPharmacol Ther2008120718018700152
- BurkRMWoodwardDFA historical perspective and recent advances in prostamide research and therapeuticsCurr Opin Drug Discov Devel200710413421
- WoodwardDFKraussAHChenJThe pharmacology of bimatoprost (Lumigan)Surv Ophthalmol200145Suppl 4S337S34511434936
- SmidSDGastrointestinal endocannabinoid system: multifaceted roles in the healthy and inflamed intestineClin Exp Pharmacol Physiol200835111383138718671715
- ChenJLuRTLaiRBimatoprost-induced calcium signaling in human T-cells does not involve prostanoid FP or TP receptorsCurr Eye Res20093418419519274525
- MatiasIChenJDe PetrocellisLProstaglandin ethanolamides (prostamides): in vitro pharmacology and metabolismJ Pharmacol Exp Ther200430974575714757851
- WoodwardDFKraussAHWangJWIdentification of an antagonist that selectively blocks the activity of prostamides (prostaglandin-ethanolamides) in the feline irisBr J Pharmacol200715034235217179945
- DaviesSSJuWKNeufeldAHAbranDChemtobSRobertsLJ2ndHydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitroJ Ocul Pharmacol Ther200319455412648303
- RomanoMRLogranoMDEvidence for the involvement of cannabinoid CB1 receptors in the bimatoprost-induced contractions on the human isolated ciliary muscleInvest Ophthalmol Vis Sci2007483677368217652738
- SzczesniakAMKellyMEWhynotSShekPNHungOOcular hypotensive effects of an intratracheally delivered liposomal delta9-tetrahydrocannabinol preparation in ratsJ Ocul Pharmacol Ther20062216016716808676
- McIntoshBTHudsonBYegorovaSJollimoreCAKellyMEAgonist-dependent cannabinoid receptor signalling in human trabecular meshwork cellsBr J Pharmacol20071521111112017922024
- LiangYWoodwardDFGuzmanVMIdentification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexesBr J Pharmacol20081541079109318587449
- WeiYWangXWangLPresence and regulation of cannabinoid receptors in human retinal pigment epithelial cellsMol Vis2009151243125119547718
- StamerWDGolightlySFHosohataYCannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissuesEur J Pharmacol200143127728611730719
- CrandallJMatragoonSKhalifaYMNeuroprotective and intraocular pressure-lowering effects of (−)Delta9-tetrahydrocannabinol in a rat model of glaucomaOphthalmic Res200739697517284931
- NjieYFHeFQiaoZSongZHAqueous humor outflow effects of 2-arachidonylglycerolExp Eye Res20088710611418597752
- NjieYFQiaoZXiaoZWangWSongZHN-arachidonylethanolamide-induced increase in aqueous humor outflow facilityInvest Ophthalmol Vis Sci2008494528453418539938
- ChenJMatiasIDinhTFinding of endocannabinoids in human eye tissues: implications for glaucomaBiochem Biophys Res Commun20053301062106715823551
- MaihofnerCSchlotzer-SchrehardtUGuhringHExpression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyesInvest Ophthalmol Vis Sci2001422616262411581208
- RoschSRamerRBruneKHinzBR(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cellsJ Pharmacol Exp Ther20063161219122816330497
- RoschSRamerRBruneKHinzBProstaglandin E2 induces cyclooxygenase-2 expression in human non-pigmented ciliary epithelial cells through activation of p38 and p42/44 mitogen-activated protein kinasesBiochem Biophys Res Commun20053381171117816256948
- NucciCGasperiVTartaglioneRInvolvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in ratsInvest Ophthalmol Vis Sci2007482997300417591864
- MarshallJLStanfieldKMSilvermanLKhanKNEnhanced expression of cyclooxygenase-2 in glaucomatous dog eyesVet Ophthalmol20047596214738509
- GlassMHongJSatoTAMitchellMDMisidentification of prostamides as prostaglandinsJ Lipid Res2005461364136815863842
- HinzBRoschSRamerRTammERBruneKLatanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanismFASEB J2005191929193116076963
- FraunfelderFWSolomonJMehelasTJOcular adverse effects associated with cyclooxygenase-2 inhibitorsArch Ophthalmol200612427727916476901
- RadiZARenderJAThe pathophysiologic role of cyclo-oxygenases in the eyeJ Ocul Pharmacol Ther20082414115118355129
- CostagliolaCParmeggianiFCaccavaleASebastianiANimesulide oral administration increases the intraocular pressure-lowering effect of latanoprost in patients with primary open-angle glaucomaAm J Ophthalmol200614137938116458700
- NucciCBariMSpanoAPotential roles of (endo)cannabinoids in the treatment of glaucoma: from intraocular pressure control to neuroprotectionProg Brain Res200817345146418929127