Abstract
Objective:
To estimate first-year costs among new initiators of topical prostaglandin analogs in a managed care population.
Research design and methods:
We developed a model to estimate first-year direct medical costs. We derived treatment patterns from a claims database analysis. Published studies were used to estimate visit-related resource use. Costs were obtained from standard sources.
Results:
The database analysis identified 9,063 patients meeting study criteria, 41% (n = 3,672) of whom remained on their initial prostaglandin therapy for 12 months after initiation. Adjunctive intraocular pressure lowering therapy was needed in 20.7%, 16.5%, 13.9%, and 8.9% of bimatoprost, latanoprost, travoprost, and BAK-free travoprost patients, respectively. Median numbers of days to the first prescription filled for adjunctive therapy (if required) were 69.5, 67.0, 123.0, and 158.5 for patients initiating on bimatoprost, latanoprost, travoprost, and BAK-free travoprost. Total estimated first-year costs were $1,457, $1,360, $1,278, and $1,307 for patients initiating therapy with bimatoprost, latanoprost, travoprost, and BAK-free travoprost. Findings were consistent through sensitivity analysis.
Conclusions:
A BAK-free prostaglandin analog may permit longer duration of monotherapy and be associated with lower first-year direct medical costs. Use of a claims database and the selection of new initiators of prostaglandin analogs limit projecting findings to all glaucoma patients.
Patient adherence to glaucoma treatment regimens is essential to maintain maximum intraocular pressure (IOP) reduction and slow disease progression in patients with primary open-angle glaucoma (POAG). This IOP reduction however, may be difficult to achieve.Citation1 A number of factors may influence a patient’s adherence to therapy, including experience with adverse events associated with treatment and understanding of the disease and the risk of reduced vision.Citation2 Additionally, persistence rates vary across classes of glaucoma medications, with prostaglandin analogs associated with significantly higher persistence than other medications.Citation1,Citation3 Even though prostaglandin analogs are widely used as initial therapy, monotherapy cannot always sufficiently lower IOP.Citation4–Citation6 Adjunctive therapies, such as topical ophthalmic beta-blockers, alpha-2 agonists, and carbonic anhydrase inhibitors are quite often required to increase IOP control. The use of adjunctive treatments may result in more complicated dosing regimens, an increase in adverse events, and a greater likelihood of poor patient adherence,Citation7 which could lead to disease progression. Also, a recent study suggests that there are diminishing returns to adding a third and fourth therapy to a glaucoma treatment regimen.Citation8 With the implications of poor adherence, the development of medications that minimize barriers to adherence can have an important impact on the success and costs of treatment.
A series of studies exploring the impact of adjunctive therapies on direct medical costs appears to demonstrate an economic benefit to extended monotherapy treatment. The first study compared treatment patterns and estimated annual costs of patients newly initiating therapy with one of three prostaglandin analogs: bimatoprost, latanoprost, or travoprost.Citation9 Findings suggest that the timing of introducing adjunctive therapy is a key determinant of annual costs. A second, later study using the same methods compared all prostaglandin analogs approved by the US Food and Drug Administration, which included a new formulation of travoprost.Citation10 This newer product, BAK-free travoprost, is comparable in concentration to the conventional travoprost using BAK and may be beneficial to those with ocular surface disease (OSD) or sensitivity to BAK.Citation11 Both preclinical and clinical studies have demonstrated BAK’s toxicity to the ocular surface.Citation12–Citation14 More than half of glaucoma patients experience some signs and/or symptoms of OSD in at least one eye.Citation15 Comparisons of the original and reformulated versions of travoprost show that the two drugs are similar in terms of efficacy but that BAK-free travoprost produces improved tolerability.Citation13,Citation16,Citation17 Use of preservatives with less toxicity may maintain vision-related quality of life and be associated both with better patient acceptance, increased persistence, and with less ophthalmologist follow-up care.Citation11 The present study is the first in which it was anticipated that there would be a sufficient number of patients using BAK-free travoprost to examine their treatment patterns separately.
The goals of this study were to evaluate the treatment patterns and annual costs of patients newly initiating therapy with prostaglandin analogs as first-line therapy and to explore the effects of a BAK-free prostaglandin analog formulation on treatment patterns and direct medical costs.
Methods
We used three primary sources of input into the economic model. We first conducted a retrospective cohort study using a prescription benefits database. Data from this analysis were used to identify the population of patients receiving prostaglandin analogs and to explore patterns of use of adjunctive therapies. Second, we reviewed published studies identified through a literature review to estimate the components of a typical outpatient visit (initial evaluation and diagnostics tests and follow-up care). Finally, we consulted standard cost sources to provide costs for each resource (initial treatments, adjunctive therapies, visits) identified.
Database analysis: study population and use of adjunctive therapies
The patients described in the retrospective cohort analysis were receiving prescription benefits and were included in a prescription claims database of a large pharmaceutical benefits manager (PBM). This PBM serves more than 75 million plan participants across the United States. All data were de-identified in accordance with Protected Health Information standards under the Health Information Portability and Accountability Act 1996 so that no individually identifiable information was included in the study database. Therefore, review by an institutional review board was not required.
The study cohort included patients who first initiated therapy with one of four prostaglandin analog products (bimatoprost, latanoprost, travoprost, or BAK-free travoprost) between May 1st, 2007 and October 31st, 2007. To qualify, patients had to have more than one prostaglandin analog prescription claim and six months of prior claims data in which there were no glaucoma therapy claims. Glaucoma therapy claims were defined by therapeutic class plus generic code number (GCN) codes. GCNs are a system of unique numbers assigned to medications according to strength, formulation, route of administration, and size by drug pricing service First DataBank. The study population was further defined by requiring patients to have at least 12 months of uninterrupted use of the index prostaglandin analog following the initial prescription. This was operationalized as not having prescriptions for the other prostaglandin analogs during the year and having at least one prescription for the index prostaglandin analog in the fourth quarter of the follow-up period. Patients who did not meet these requirements (sufficient prior claims data, uninterrupted use, and use of the index prostaglandin analog during the fourth quarter of observation) were excluded from the study database. Patients who switched from one formulation of travoprost to the other were also excluded from the analysis.
We considered that patients added an adjunctive medication, defined by therapeutic class plus GCN codes, if there was a sequential and subsequent refill of an adjunctive agent in the presence of continued refills for the index agent.
Literature review: resource use and cost inputs
The American Academy of Ophthalmology Preferred Practice Patterns for glaucoma suggest that follow-up care should be based on achievement of target IOP and the amount of disease progression,Citation18 neither of which was available in the prescription database used for this study. Therefore, the base case analysis used resource rates from a survey-based studyCitation19 while sensitivity analyses explored visits as recommended by other studies and guidelines.Citation18,Citation20 Since all patients in the model are assumed to have undergone the same procedures and tests at their visits regardless of the prostaglandin analog they received, the relatively small differences across studies in terms of the components of each visit have minimal impact on study findings.
At the initial visit, patients were assumed to have a level 4 (comprehensive) evaluation (CPT 92004). Follow-up visits were assumed to be level 2 (intermediate) visits (CPT 92012), with three follow-up visits during the year (likely but not necessarily at 30 and 90 days following initiation of the index therapy and at 12 months). In addition, two follow-up visits were assumed to be associated with the initiation and monitoring of adjunctive medication among patients for whom it was prescribed. presents the procedures and diagnostic tests that comprised each visit as well as the costs used for each.
Table 1 Unit costs
We use average wholesale price (AWP) for 2008 as the prescription costs.Citation21 The range of published AWPs for the prostaglandin analogs was fairly narrow (US$72.20 to $76.69).Citation21 For the prostaglandin analogs, the average cost for the agents was used in the model because AWP does not take into consideration contract pricing and rebates, which minimize, if not eliminate, differences in cost to the insurer. Further, the number of prescriptions for prostaglandin analogs was normalized to a 2.5 mL bottle, as this was the most common size found in the claims database. Medical costs were estimated using the 2008 Medicare Fee Schedule.Citation22 All the costs used in the model are presented in .
Days of use per bottle were based on data from this population. Number of bottles required was rounded to the nearest tenth.
Analysis
The analysis identified patients by the prostaglandin whose prescription they first filled during the study period. Statistical comparisons of the demographic characteristics of the four cohorts were conducted in SAS software (v9.2; SAS Institute, Cary, NC). Gender was compared using a Chi-squared test and age was compared using analysis of variance (ANOVA), with Bonferroni’s correction for multiple comparisons. Median and mean number of days to initiation of adjunctive therapy were calculated for each cohort, with the distribution of number of days examined to determine which measure to use in the model. The median numbers of days until patients added adjunctive therapies were compared using the Wilcoxon rank sum test and mean times were compared using ANOVA.
Sensitivity analyses explored potential changes in clinical and cost parameters. These scenarios are detailed in . First, the proportion of patients remaining on monotherapy for the entire year was changed to the lowest and highest values among the initial treatments, that is, the proportion of patients remaining on monotherapy was changed to 79.3% (the base case value for bimatoprost) and then to 91.1% (the base case value for BAK-free travoprost) for all treatments. Second, the days to initiating adjunctive therapy was varied in two ways: a) median number of days to the addition of adjunctive medication was changed to the lowest and highest values among the treatments and b) the mean number of days was substituted for the median. Third, the cost of treatments were varied by a) assuming that the least and most expensive adjunctive therapies were the only therapies used and b) rounding up bottles of index and adjunctive therapies to whole numbers. Fourth, sensitivity analysis was conducted on the cost of visits, using resource use estimates from other published sources.Citation18,Citation20 The preferred practice patterns published by the American Academy of Ophthalmology were used to establish minimum and maximum values for the frequency of visits and resource visits. The minimum values assumed that patients were meeting IOP targets and the maximum values assumed that patients were not meeting IOP targets and that there was progression of disease.
Table 5 Sensitivity analyses during the follow-up period
Results
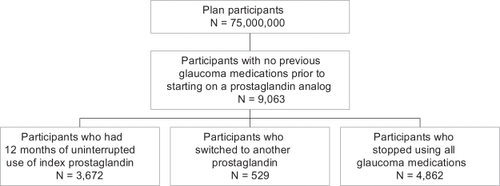
provides a brief flow chart of the patient selection process for the retrospective cohort analysis. Of more than 75 million plan participants, 9,063 initiated treatment with a prostaglandin analog. Approximately 6% of these patients switched from their index prostaglandin analog within the 12-month study period to another glaucoma therapy. This ranged narrowly among the study cohorts from 5.0% to 8.6%. Additionally, about 54% of the patients stopped taking all glaucoma medications within the 12-month period. This also ranged narrowly among the cohorts from 51.3% to 58.2%. The analysis examined only those newly initiating patients who remained on their initial prostaglandin analog therapy for one year, which represents approximately 41% of the glaucoma patients newly initiating with prostaglandin analogs identified from the database.
Demographic characteristics of the 9,063 newly initiating patients are presented in . More than half of the patients were women. The mean age across groups ranged from 64 to 65 years. There were no statistically significant differences among groups in terms of age or gender.
Table 2 Demographic characteristics
In the bimatoprost, latanoprost, travoprost, and BAK-free travoprost treatment groups, 20.7%, 16.5%, 13.9%, and 8.9%, respectively, initiated adjunctive therapy during the course of the year at significantly different proportions (P < 0.0001, see ). Patients on BAK-free travoprost were able to continue without adjunctive therapy longer than patients treated with other prostaglandins. The median numbers of days until patients added adjunctive therapies were 158.5 days for patients initiating therapy with BAK-free travoprost, 123.0 days for travoprost, 69.5 days for bimatoprost, and 67.0 days for latanoprost (P < 0.05, Wilcoxon rank sum test). The mean numbers of days to initiating adjunctive therapy were 156.3 days in the BAK-free travoprost group and 131.5 days in the travoprost group. For bimatoprost and latanoprost, the mean number of days was 120.8 and 108.3, respectively. Mean time to initiating adjunctive therapy was not significantly different across the cohorts.
Table 3 Treatment patterns during follow-up period
As shown in , patients had an average of 8.0 to 9.7 prescription claims (normalized to 2.5 ml bottles) for prostaglandin analog therapy during the 12-month follow-up period. Based on the time to initiating adjunctive therapy, anywhere from 4.6 to 10.5 bottles of adjunctive medication were required for the remainder of the year. Reflective of the shorter time to initiation of adjunctive therapy, the number of prescriptions for adjunctive therapies was highest for latanoprost, while fewer bottles were required for BAK-free travoprost patients, who began adjunctive therapy later.
Table 4 Resource use during follow-up period
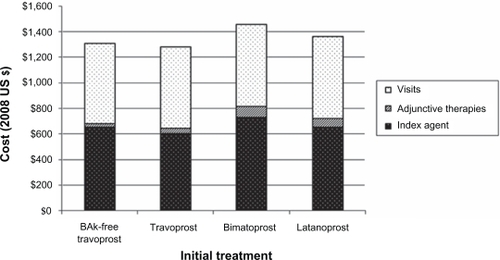
All the costs used in the model are presented in . presents the primary findings from the analysis. Estimated annual costs for patients initiating travoprost were lowest ($1,278), with increasing annual costs for BAK-free travoprost ($1,307), latanoprost ($1,359), and bimatoprost ($1,457). The cost of travoprost was lower than BAK-free travoprost primarily due to slightly fewer 2.5 ml equivalent bottles being used on average during the year (8.0 vs 8.7).
Results of sensitivity analyses are shown in . Findings remained consistent, with travoprost having the lowest annual cost and bimatoprost having the highest cost in all scenarios. The range narrowed (to less than $100) when the number of bottles of index prostaglandin was equalized across cohorts or when the cost for visits was based on guidelines and widened (to more than $200) when the number of bottles of index prostaglandin was rounded up to the nearest whole number.
Discussion
This analysis adds to a growing body of literature finding consistent differences in treatment patterns and costs for glaucoma patients initiating treatment with prostaglandin analogs with varying rates of use of adjunctive therapy. The cohort analysis found that patients receiving BAK-free travoprost remained on monotherapy in greater proportions and for a longer duration compared to other prostaglandin analogs.
Compared to the previous studies in this series,Citation9,Citation10 a number of findings should be highlighted. First, the high rate of monotherapy adherence in the initial study in the seriesCitation9 seems to have been an aberration, with both the secondCitation10 and present studies finding more than half of the patients identified as discontinuing glaucoma therapies within one year, in agreement with other published studies.Citation3,Citation23–Citation25 Second, the advantage travoprost demonstrated in terms of less use of adjunctive therapies associated with new patients starting on this therapy was consistent across studies, and the introduction of the BAK-free formulation only increased this advantage. A possible explanation for longer duration of monotherapy and fewer patients requiring adjunctive therapy is greater patient adherence due to lower rates of OSD associated with BAK-free travoprost.Citation13,Citation16,Citation17
The use of pharmacy claims databases alone to estimate adherence poses methodological challengesCitation24 and is further complicated by the fact that assuming appropriate refill patterns do not imply appropriate instillation of eye drops.Citation26–Citation31 Regardless of the reason, BAK-free travoprost has been shown to be at least as safe and efficacious as travoprostCitation13,Citation17 and if it can also improve adherence by being a more eye-friendly drug, then real-world effectiveness might be enhanced. While the safety of the BAK-free preservative has been widely demonstrated, various reports have identified cystoid macular edema after treatment with prostaglandin analogs.Citation32–Citation35 BAK-free travoprost is associated with less OSD, but high-risk patients must still be monitored for other adverse events, as with any of the medications in the class.
There are some notable study limitations. The patients in this analysis, while generally reflective of the larger glaucoma patient population in terms of demographic characteristics,Citation36 may not reflect the larger population in other facets. The study attempted to identify only patients who were new to therapy, although even a criterion as simple as this is difficult to implement.Citation24 These patients had prescription coverage, and since finances are one reason cited for nonadherence with glaucoma therapy,Citation2 this may be an important difference. Also, based on the study methodology, patients may have had poor adherence; patients in the cohort analysis were required to have a minimum of two glaucoma prescriptions filled during the follow-up period, but no attempt was made to assess medication possession ratio or to evaluate persistence with therapy over time. As shown in multiple other studies, more than half of the patients identified in studies of glaucoma therapy discontinue.Citation10,Citation23–Citation25,Citation37 The subset of patients identified through this analysis may not be representative of glaucoma patients in other aspects.
The use of a large claims database offers an important strength in terms of sample size. However, there are also disadvantages to the use of a pharmaceutical claims database without additional clinical input. Patients are not randomly-assigned to treatment and although sample size should help minimize any bias, it is possible that there are systematic preferences for treatments that cannot be detected without additional clinical information. The reasons for adding adjunctive therapy or switching or discontinuing therapies cannot be surmised from the claims database alone. Knowing more about these reasons might inform the study question however, it is impossible to know these answers. In addition, claims databases report on prescriptions filled, but cannot be used as a definitive statement of adherence.Citation26,Citation28–Citation30 This is further complicated with the study of eye drops, where effective instillation of drops is a problem even among patients who try to adhere to therapy.Citation27,Citation31 The patient population in any given database may not match the overall population of patients with the condition in question nor were patients randomized to treatment, although age and gender distributions here were similar across treatment groups and seem comparable with the glaucoma population. Finally, claims databases also may contain coding biases or errors, although there is no reason to believe that these errors would be different across index treatments also.
Adjunctive therapies were found to be a relatively small contributor to costs in this analysis; however, it is one of the few areas in which products can be differentiated. These findings showed that prostaglandin analogs with a greater proportion of patients remaining on monotherapy and a longer time to initiation of adjunctive agents had lower first-year direct medical costs from a third party payer’s perspective. Accordingly, costs from the patient’s perspective (eg, co-pay for visits and prescriptions) would also be lower with longer duration of monotherapy. Further study is needed to identify to what extent tolerability, specifically the absence of BAK, contributes to use of adjunctive medications among glaucoma patients.
Disclosure
JKS is an employee of Exponent, which received funding from Alcon to conduct this study. DWC is an employee of Alcon Research Ltd. ALR is a consultant for Alcon Laboratories and Alcon Research Ltd.
References
- SchwartzGFQuigleyHAAdherence and persistence with glaucoma therapySurv Ophthalmol200853Suppl 1S57S6819038625
- FriedmanDSHahnSRGelbLDoctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency StudyOphthalmology20081158132013271327.e1318321582
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol2005140459860616226511
- SitAJWeinrebRNCrowstonJGKripkeDFLiuJHSustained effect of travoprost on diurnal and nocturnal intraocular pressureAm J Ophthalmol200614161131113316765686
- CostagliolaCDel PreteAVerolinoMEffect of 0.005% latanoprost once daily on intraocular pressure in glaucomatous patients not adequately controlled by beta-blockers twice daily: a 3-year follow-up. Experience and incidence of side effects in a prospective study on 76 patientsGraefes Arch Clin Exp Ophthalmol2002240537938612073061
- NetlandPALandryTSullivanEKTravoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertensionAm J Ophthalmol2001132447248411589866
- RobinALCovertDDoes adjunctive glaucoma therapy affect adherence to the initial primary therapy?Ophthalmology2005112586386815878067
- NeelakantanAVaishnavHDIyerSASherwoodMBIs addition of a third or fourth antiglaucoma medication effective?J Glaucoma200413213013615097258
- SchmierJKCovertDWRobinALEstimated first-year costs of prostaglandin analogs with/without adjunctive therapy for glaucoma management: a United States perspectiveCurr Med Res Opin200723112867287517922980
- SchmierJKCovertDWRobinALFirst-year treatment patterns among new initiators of topical prostaglandin analogsCurr Med Res Opin200925485185819231912
- BaudouinCDetrimental effect of preservatives in eyedrops: implications for the treatment of glaucomaActa Ophthalmol200886771672618537937
- HorsleyMBKahookMYEffects of prostaglandin analog therapy on the ocular surface of glaucoma patientsClin Ophthalmol2009329129519668581
- HenryJCPeaceJHStewartJAStewartWCEfficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapyClin Ophthalmol20082361362119668762
- KahookMYNoeckerRJComparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tearsCornea200827333934318362664
- LeungEWMedeirosFAWeinrebRNPrevalence of ocular surface disease in glaucoma patientsJ Glaucoma2008817535035518703943
- GrossRLPeaceJHSmithSEDuration of IOP reduction with travoprost BAK-free solutionJ Glaucoma200817321722218414108
- LewisRAKatzGJWeissMJTravoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacyJ Glaucoma20071619810317224758
- American Academy of OphthalmologyPreferred Practice Pattern. Primary Open-Angle Glaucoma. Limited RevisionSan Francisco, CAAmerican Academy of Ophthalmology2003
- FremontAMLeePPMangioneCMPatterns of care for open-angle glaucoma in managed careArch Ophthalmol2003121677778312796247
- QuigleyHAFriedmanDSHahnSREvaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency StudyOphthalmology200711491599160617572498
- Thomson HealthcareRed Book Drug TopicsMontvale, NJThomson Healthcare2008
- Practice Management Information CorporationMedical Fees in the United StatesLos Angeles, CAPractice Management Information Corporation2008
- FiscellaRGGreenAPatuszynskiDHWilenskyJMedical therapy cost considerations for glaucomaAm J Ophthalmol20031361182512834665
- FriedmanDSQuigleyHAGelbLUsing pharmacy claims data to study adherence to glaucoma medications: methodology and find-ings of the Glaucoma Adherence and Persistency Study (GAPS)Invest Ophthalmol Vis Sci200748115052505717962457
- SpoonerJJBullanoMFIkedaLIRates of discontinuation and change of glaucoma therapy in a managed care settingAm J Manag Care2002810 SupplS262S27012188169
- BrownMMBrownGCSpaethGLImproper topical self-administration of ocular medication among patients with glaucomaCan J Ophthalmol1984191256608974
- StoneJLRobinALNovackGDCovertDWCagleGDAn objective evaluation of eyedrop instillation in patients with glaucomaArch Ophthalmol2009127673273619506189
- AshburnFSJrGoldbergIKassMACompliance with ocular therapySurv Ophthalmol19802442372486987764
- KholdebarinRCampbellRJJinYPBuysYMMulticenter study of compliance and drop administration in glaucomaCan J Ophthalmol20084345446118711461
- NorellSEGranstromPASelf-medication with pilocarpine among out-patients in a glaucoma clinicBr J Ophthalmol19806421371417362816
- TsaiTRobinALSmithJP3rdAn evaluation of how glaucoma patients use topical medications: a pilot studyTrans Am Ophthalmol Soc20071052933discussion 25–33.18427591
- EsquenaziSCystoid macular edema in a pseudophakic patient after switching from latanoprost to BAK-free travoprostJ Ocul Pharmacol Ther200723656757018001244
- CarrilloMMNicolelaMTCystoid macular edema in a low-risk patient after switching from latanoprost to bimatoprostAm J Ophthalmol2004137596696815126179
- MoroiSEGottfredsdottirMSSchteingartMTCystoid macular edema associated with latanoprost therapy in a case series of patients with glaucoma and ocular hypertensionOphthalmology199910651024102910328408
- CallananDFellmanRLSavageJALatanoprost-associated cystoid macular edemaAm J Ophthalmol199812611341359683162
- FriedmanDSWolfsRCO’ColmainBJPrevalence of open-angle glaucoma among adults in the United StatesArch Ophthalmol2004122453253815078671
- SchwartzGFReardonGMozaffariEPersistency with latanoprost or timolol in primary open-angle glaucoma suspectsAm J Ophthalmol20041371 SupplS13S1614697910