Abstract
Purpose:
The objective of the study was to compare the intraocular penetration levels of the newer fluoroquinolones, moxifloxacin, gatifloxacin, and levofloxacin in the rabbit’s cornea, aqueous humor, and conjunctiva after topical instillation.
Methods:
0.5% moxifloxacin, 0.3% gatifloxacin, and 0.5% levofloxacin were instilled in random sequence in both eyes of nine New Zealand White rabbits at two-minute intervals. Instillation was repeated every 15 minutes for a total of three drops of each fluoroquinolone per eye. Three additional animals had only moxifloxacin instilled bilaterally using the same schedule. Sixty minutes after the final instillation, the rabbits were sacrificed for determination of corneal, aqueous humor, and conjunctival fluoroquinolone concentrations using high-performance liquid chromatography.
Results:
Moxifloxacin achieved significantly higher concentrations than levofloxacin and gatifloxacin in the cornea (P = 0.0102 and P = 0.0006, respectively), aqueous humor (P = 0.0015 and P < 0.0001, respectively), and conjunctiva (P = 0.0191 and P = 0.0236, respectively).
Conclusions:
0.5% moxifloxacin eyedrops provided superior intraocular penetration in rabbits’ eyes compared with the two other fluoroquinolones, 0.5% levofloxacin and 0.3% gatifloxacin.
Introduction
Prophylactic antibiotic eye drops are routinely used to prevent postoperative infections. Fluoroquinolones in particular have been used extensively due to their excellent activity against ocular pathogens, including both Gram-positive and Gram-negative bacteria.Citation1,Citation2 Selection of the most appropriate antibiotic should be based on considerations of pharmacodynamic and pharmacokinetic properties; namely, an agent with a broader spectrum, lower minimum inhibitory concentration (MIC) against pathogens, and better achievable concentration levels in the intraocular tissues should be the agent of choice to achieve optimal efficacy. However, there are few studies that have clearly assessed the superiority of one drug over another among the newer fourth-generation fluoroquinolones, including moxifloxacin and gatifloxacin, when used in humans.
The current study was conducted to compare the penetration levels of new fluoroquinolones, including the fourth-generation fluoroquinolones (moxifloxacin and gatifloxacin) and a third-generation fluoroquinolone (levofloxacin) in the cornea, aqueous humor, and conjunctiva after topical instillation in New Zealand White rabbits. This animal study was performed as a preliminary step toward conducting a similar ocular penetration study in patients scheduled for keratoplasty.
Materials and methods
Twelve New Zealand White rabbits were used to determine the concentration levels of three topically instilled fluoroquinolones in the cornea, aqueous humor, and conjunctiva. Topical preparations of 0.5% moxifloxacin (Vegamox, Alcon Japan, Tokyo, Japan), 0.3% gatifloxacin (Gatiflo, Senju Pharmaceuticals, Osaka, Japan) and 0.5% levofloxacin (Cravit, Santen Pharmaceuticals, Osaka, Japan) were obtained from the respective manufacturers.
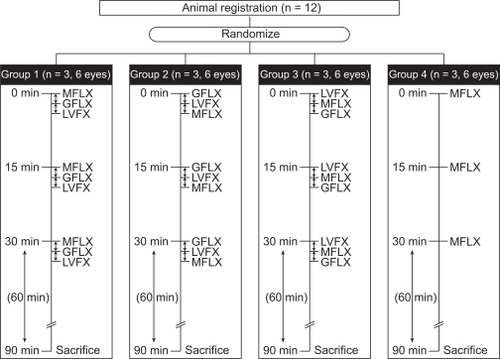
The rabbits were randomly assigned to four treatment groups in accordance with the analytical method reported by Diamond and colleagues and Yamada and colleagues.Citation3–Citation5 One drop of each topical preparation was instilled in both eyes of each animal at two-minute intervals (). In group 1, moxifloxacin was instilled first, followed by gatifloxacin two minutes later, and finally, levofloxacin after another two minutes. In group 2, gatifloxacin was instilled first, followed by levofloxacin, then moxifloxacin, and in group 3, levofloxacin was instilled first, followed by moxifloxacin, then gatifloxacin. In group 4, only moxifloxacin was instilled to compare the concentration levels with those in animals instilled with three different fluoroquinolones in order to verify that the levels were similar. Instillation was repeated every 15 minutes for a total of three drops of each fluoroquinolone per eye. Sixty (60) minutes after the final instillation, the rabbits were sacrificed and corneal, aqueous humor, and conjunctival samples were collected from each eye.
Figure 1 Treatment schedule.
‡ 2 minutes.
The sample concentrations of each fluoroquinolone were simultaneously determined by high-performance liquid chromatography (HPLC) at the Mitsubishi BCL laboratory. A HPLC system consisting of a L-7000 solvent delivery system (Hitachi, Tokyo, Japan), a L-7485 fluorescence detector (Hitachi), and a chromatography software (Scientific Software Inc., Pleasanton, CA, USA) was used. The elution was performed using TSKgel ODS-80TM column (Tosoh Inc., Tokyo, Japan) at 40 °C with a mobile phase of 75:25 mixture of pH 3.0, 0.05M phosphoric acid and acetonitrile. Flow rate was 0.8 mL/min and detection was by fluorescence (excitation 290 nm, emission 470 nm) using a fluorescence detector. Drug concentrations were determined against a calibration line constructed from known aqueous drug concentrations.
Tukey’s multiple pair-wise comparison was employed for between-group comparisons. A P value of less than 0.05 was considered statistically significant.
Results
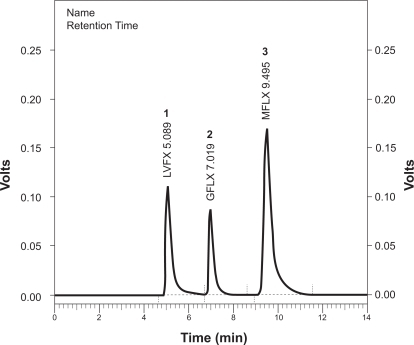
A typical chromatogram is shown in . The mean retention times of levofloxacin, gatifloxacin, and moxifloxacin were 5.089 minutes, 7.019 minutes and 9.495 minutes, respectively.
As shown on , in the 18 corneas of nine animals (excluding the moxifloxacin alone group), the mean concentration of moxifloxacin (12.23 ± 5.33 μg/g) was significantly higher than either levofloxacin (7.34 ± 1.88 μg/g, P = 0.0102) or gatifloxacin (6.32 ± 2.47 μg/g, P = 0.0006). The aqueous humor of 18 eyes of nine animals demonstrated that moxifloxacin had a significantly higher mean concentration (2.677 ± 1.094 μg/mL) compared with levofloxacin (1.511 ± 0.401 μg/mL, P = 0.0015) and gatifloxacin (1.112 ± 0.438 μg/mL, P < 0.0001). In the conjunctiva (18 eyes of nine animals), mean moxifloxacin concentration (3.15 ± 1.60 μg/g) was significantly higher than either levofloxacin (1.77 ± 0.97 μg/g, P = 0.0191) or gatifloxacin (1.84 ± 0.94 μg/g, P = 0.0236). There were no statistically significant differences between levofloxacin and gatifloxacin in any of the samples (cornea, aqueous humor, or conjunctiva).
Table 1 Mean concentration of 0.5% moxifloxacin, 0.5% levofloxacin, and 0.3% gatifloxacin in the cornea, aqueous humor, and conjunctiva
No significant differences were observed among groups of different instillation order. Mean fluoroquinolone concentrations were comparable, regardless of the order of instillation. The mean concentrations of moxifloxacin in each tissue in group 4 (moxifloxacin alone) were nearly identical to those in groups 1, 2, and 3.
Discussion
Perioperative antibiotics should have several therapeutic attributes. Ideal agents should have a broad spectrum of antibacterial activity, high potency against pathogenic organisms, and favorable penetration characteristics to deliver the antibiotic to the target ocular tissues. It is particularly important to consider the ability to achieve a concentration well above the MIC for microorganisms. The present study showed that mean concentrations of moxifloxacin were significantly higher than the two other fluoroquinolones in all three intraocular sites tested. These results are comparable to those previously reported by Yamada and colleagues and others.Citation3–Citation6 The concentration of each fluoroquinolone used in this study, however, was not uniform, given that the gatifloxacin preparation was a 0.3% solution, while moxifloxacin and levofloxacin were administrated as 0.5% solutions. This is because commercially available eyedrops were used in the study to mimic the clinical situation.
Besides the difference in concentrations of fluroquinolones tested, the present results can be logically explained from the viewpoint of the physical properties of the respective fluoroquinolones. Moxifloxacin is highly hydrophilic and lipophilic; the hydrosolubility of moxifloxacin is more than 6%, while that of gatifloxacin and levofloxacin is 0.21% and 1.85%, respectively. The liposolubility of moxifloxacin is 0.24 (C-7, đ), while that of gatifloxacin and levofloxacin is 0.11 and 0.06, respectively.Citation7,Citation8 Such disparities in the physical properties of these fluoroquinolones lead to differences in their ocular penetration. The corneal permeability of moxifloxacin is 15.8 × 107 cm/sec, while that of gatifloxacin and levofloxacin is 4.6 × 107 cm/sec and 6.95 × 107 cm/sec, respectively. Such properties of moxifloxacin are thought to be due to its chemical structure, which comprises a large pyrrolo-pyridine group chain at the C7 position of the basic quinolone structure, conferring hydrophilicity along with lipophilicity.Citation7
Based on the findings from the current animal study, a similar clinical study using patients scheduled for keratoplasty is planned. The drugs used and the application schedule will be identical to the present study, and samples will be collected during surgery for drug concentration determination. Although animals are typically randomized into three different treatment groups in studies comparing three distinct eye drops, the present study design, in which three different eye drops are nearly simultaneously instilled into the animals’ eyes, offers the advantage that a single specimen can yield three drug concentrations because HPLC can distinguish the different drugs. Individual differences in specimen permeability can make it difficult to compare the concentrations of different eye drops using traditional study design; however, the present method overcomes such problems, and allows the drugs to be compared within one specimen.
Before proceeding to a human study, some issues that may limit the interpretation of the current results should be reviewed. Since the time to maximum concentration in the aqueous humor (AQTmax) differs according to each agent, the timing of instillation is critical.Citation9,Citation10 The present study may have missed AQTmax of one or more of the study medications. The second issue is whether the concentrations of other agents might increase as a consequence of moxifloxacin in a group where moxifloxacin is instilled first since there is substantial penetration of moxifloxacin into the intraocular tissue. The third issue is whether a two-minute interval is optimal, since some investigators recommend a five-minute interval or at least a three-minute interval when investigating the efficacy of a particular agent. Although a two-minute interval is supported by a report showing that concentration levels in the anterior chamber did not differ when steroid eye drops were instilled at two-minute intervals,Citation11 the controversy remains. Our results also support the use of a two-minute interval, as no difference in concentrations was found among all four treatment groups, including the group which received only moxifloxacin.
Conclusion
In this study, topically applied 0.5% moxifloxacin achieved higher concentrations in cornea, aqueous humor and conjunctiva than 0.5% levofloxacin and 0.3% gatifloxacin in rabbit eyes. However, future penetration studies in humans as well as clinical studies using patients with ocular infections would reveal the differences in clinical efficacy among fluoroquinolones.
Acknowledgements
Editorial assistance was provided by Jennifer Klem, PhD, a freelance medical writer. This assistance was supported by Alcon Research, Ltd. The authors report no conflicts of interest in this work.
References
- MatherRKarenchakLMRomanowskiEGKowalskiRPFourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibioticsAm J Ophthalmol2002133446346611931779
- KowalskiRPDhaliwalDKKarenchakLMGatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolatesAm J Ophthalmol2003136350050512967804
- DiamondJPWhiteLLeemingJPBing HohHEastyDLTopical 0.3% ciprofloxacin, norfloxacin, and ofloxacin in treatment of bacterial keratitis: a new method for comparative evaluation of ocular drug penetrationBr J Ophthalmol19957966066097626579
- YamadaMMochizukiHYamadaKKawaiMMashimaYAqueous humor levels of topically applied levofloxacin, norfloxacin, and lomefloxacin in the same human eyesJ Cataract Refract Surg20032991771177514522299
- YamadaMIshikawaKMochizukiHKawaiMCorneal penetration of simultaneously applied topical levofloxacin, norfloxacin and lomefloxacin in human eyesActa Ophthalmol Scand200684219219616637835
- YagciROfluYDincelAPenetration of second, third, and fourth generation topical flouroquinolone into aqueous and vitreous humor in a rabbit endophlamitis modelEye200721799099416732216
- RobertsonSMCurtisMASchlechBAOcular pharmacokinetics of moxifloxacin after topical treatment of animals and humansSurv Ophthalmol200550Suppl 1S32S4516257309
- FukudaMSasakiKIn vitro topically applied fluoroquinolone penetration into the anterior chamberNippon Ganka Gappai Zasshi1995995532536
- FukudaMSasakiHCalculation of AQCmax: comparison of five ophthalmic fluoroquinolone solutionsCurr Med Res Opin200824123479348619032129
- FukudaMSasakiKGeneral purpose antimicrobial ophthalmic solutions evaluated using new pharmacokinetic parameter of maximum drug concentration in aqueousJpn J Ophthalmol200246438439012225816
- LeibowitzHMKupfermanADrug interaction in the eye. Concurrent corticosteroid-antibiotic therapy for inflammatory keratitisArch Ophthalmol1977954682685192185