Abstract
Introduction:
In the present study we determine the differences observed between 25-gauge-vitrectomy combined with phacoemulsification, and the 20-gauge-vitrectomy combined with pars plana phacofragmentation.
Methods:
A prospective study of a sample of 987 eyes of 661 patients randomly divided into two groups. 25-gauge-vitrectomy plus phacoemulsification included 486 eyes, and 20-gauge-vitrectomy plus phacofragmentation 501 eyes. We evaluated the differences at the time of the surgery, the intra-and postoperative complications, and the variations in intraocular pressure.
Results:
The final visual outcome was similar in both groups. The most important differences between groups were: surgical time was faster in group 1 than in group 2, (mean time: 35.16 ± 3.49, 44.74 ± 5.30 minutes). Intraoperative complications were more numerous in group 1. In group 1, postoperative low levels of intraocular pressure are present in all patients with 2.77% of patients with hypotension (<8 mmHg), and three choroidal effusion. In group 2, intraocular lens decentration and retinal detachment are more frequent (2.38% and 1.39%, respectively).
Conclusions:
In the present study, both techniques have a similar number of complications and have a similar postoperative outcome, and are valid for the management of the pathologies selected.
Introduction
Vitrectomy has been used to treat vitreoretinal disorders for more than 25 years.Citation1,Citation2 The management of the lens during vitrectomy has come full circle since the first vitrectomy interventions.Citation3,Citation4 The lens is removed frequently during surgery even if clear, and the lens status affects the management of retinal pathologies by pars plana vitrectomy. In many cases, it is advantageous to remove the lens, such as in patients with proliferative diabetic retinopathy, which permits better dissection of the anterior vitreous.Citation5
If the lens is not removed during the vitrectomy, one of the most likely outcomes is cataract formation. In young patients, a cataract may take many years to develop, but in older patients, especially those aged over 60 years, with pre-existing nuclear sclerosis, visual loss may develop from a cataract much sooner, forcing cataract extraction in a short time after vitrectomy.Citation6–Citation8 If the cataract develops after vitrectomy, subsequent cataract surgery is more challenging than when the lens is removed at the time of combined surgery. Difficulties and risks involved cataract extraction as zonular dehiscence, increased mobility of the posterior capsule, extremely deep anterior chamber during phacoemulsification, and loss of the nuclear fragments inside the vitreous cavity.Citation9–Citation13
Classically, lens removal in conjunction with vitrectomy can be managed with pars plana lensectomy by phacofragmatome, the retention of the anterior lens capsule and the introduction of a posterior chamber lens in front of the capsule. This technique is available with 20-gauge vitrectomy, and the combined surgery hastens and simplifies the visual recovery of patients in whom cataracts will predictably develop after vitrectomy.
However, there is a new vitrectomy technique, transconjunctival sutureless vitrectomy (TSV 25), introduced in 2001, which has become widely adopted for vitreoretinal surgery. With this new technique there is no 25-gauge phacofragmentation tip, so a combination of phacoemulsification and 25-gauge vitrectomy is needed in these cases.Citation14,Citation15
The aim of the present study is to determine the differences observed between two groups of patients, the first submitted to 25-gauge vitrectomy combined with clear corneal phacoemulsification, and the second submitted to 20-gauge vitrectomy combined with pars plana phacofragmentation (lensectomy).
Methods
Design
A prospective study of a sample of 987 eyes of 661 patients who needed a pars plana vitrectomy in combination with lens extraction, selected according to the inclusion criteria, and randomly divided into a two groups; the first including 486 eyes of 331 patients submitted to 25-gauge transconjunctival vitrectomy combined with clear corneal phacoemulsification, and the second including 501 eyes of 320 patients submitted to 20-gauge vitrectomy and phacofragmentation via pars plana. The recruitment dates extended from January 1st, 2003 to December 31st, 2008.
Setting
The Hospital St. Joan is the only public ophthalmic center in Reus, Spain, with a dependent population of 389,471 inhabitants.
Ethical adherence
The study was carried out in keeping with local legal requirements (approval by the local ethical committee of Hospital Universitario Sant Joan – Reus) and in accordance with the revised guidelines of the Declaration of Helsinki. The nature of the study was explained and all patients gave written consent to participate.
Diagnostic criteria
The time for phacoemulsification/lensectomy is defined as the time that the surgeon needed between opening the lens and the end of cataract extraction. We divided the time of vitrectomy into three steps. The time for surgical opening was defined as the interval between the first instrument contacting the conjunctiva through to the placement of all cannulae and the infusion line. The closing time was defined as the time required for removing the cannulae and infusion line. The operative time was defined as the interval between the opening and closing times.
The main outcome measures were the incidence of intraoperative or postoperative complications. Persistent vitreous postoperative hemorrhage was defined as the presence noted within the first postoperative week. Hypotony was defined as an intraocular pressure of less than 8 mmHg. Lens opacities were classified according to the lens opacities classification system III.Citation16 Postoperative ocular irritation has been evaluated subjectively, by questionnaire, at 24 hours, seven days, and 15 days. All patients were asked the following question: “Do you have a sensation of foreign body in your eye?” The answer could be none or mild, moderate, or severe.
Also, an objective evaluation of the conjunctival hyperemia was performed in the following way:
None or mild hyperemia, if none or minimal conjunctival vessels are dilated.
Moderate, if vessels are more ingurgitated.
Severe, if hemorrhages are present.
Inclusion criteria for the cataract extraction.
Patients with a cataract that makes retina visualization difficult, with a nuclear opacity ≥NO3, NC3, and/or cortical opacity ≥C3, and/or subcapsular opacity ≥P3
All patients aged ≥60 years.
Exclusion criteria for the study.
Patients that had previously undergone intraocular surgery.
Patients who presented surgical diagnosis of proliferative vitreoretinopathy associated detachments, extensive diabetic retinal proliferation or tractional detachment.
Patients with retinal detachment, or giant retinal tears.
Patients with glaucoma (chronic open angle, neovascular, or previous closed-angle glaucoma).
Patients who needed silicone oil infusion at the end of surgery.
The distribution of diagnosis indicating surgical intervention for both surgical groups is listed in .
Table 1 Description of previous pathology of the eyes submitted to 25-gauge and 20-gauge vitrectomy combined with cataract extraction
Patient examination
Visual acuity was measured using the standard Snellen methods. All visual acuity values were converted to logarithm of the minimum angle of resolution (logMAR) scores, where each 0.1 logMAR unit represents one line of visual acuity. Off the chart visual acuity designated as counting fingers, hand motions or light perception were assigned logMAR values of 1.6, 1.9, or 2.2, respectively, according to previously established conventions.Citation17 Intraocular pressure (IOP) values were measured the day before surgery by Goldman tonometer.
Anterior segment photography was obtained in order to evaluate the lens opacity, according to the LOCS III system.
Before cataract surgery all patients underwent a fundus retinography, if possible, using a fundus camera. The macular area was examined with a noncontact 70-diopter fundus lens. Fluorescein angiography and optical coherence tomography was performed in patients with macular epiretinal membrane, macular diabetic edema and macular hole if the lens are clear enough. The interpretation of these explorations was made by an independent investigator.
There was a follow-up control of patients after surgery at 24 hours, one week, 15 days, one month, three months, and every six months thereafter. As a control, patients were submitted to the visual acuity and IOP measures, anterior segment biomicroscopic exploration, and fundus exploration with retinography and non contact lens macular exploration.
Surgery
Both 25-gauge + phacoemulsification and 20-gauge + phacofragmentation surgery were performed by one of two consultants (PR-A, MB-B) in standard fashion at a single centre, both surgeons had previously performed at least 50 25-gauge vitrectomies. At the end of the study the differences between the number and type of procedure performed by the surgeons were not statistically significant.
Preoperative dilating regimen included, for both techniques, a mixture of 2.5% phenylephrine and 0.5% tropicamide, instilled three times at 30-minute intervals two hours before surgery. All patients received sedation and local anesthetic consisting of 5 ml of a retrobulbar injection of a 50:50 mixture of 2% mepivacine and 0.75% bupivicaine. Supplementary anaesthetic was administered as necessary. Patients’ skin and lashes and the ocular surface were prepared with 5% povidine-iodine solution.
Group 1 patients submitted to 25-gauge vitrectomy combined to phacoemulsification. In the first step, the trocar of the infusion sclerotomy was located 3.5 mm behind the limbus, and the infusion is turned off. The second step was the phacoemulsification surgical procedure, a technique that included a superior limbal 2.8 mm incision, phacoemulsification of the cataract and in the bag implantation of a hydrophilic acrylic lens (Akreos®). The wound was sutured with a point of 10-0 nylon, and viscoelastic (Amvisc Plus®). The third step was the three port 25-gauge vitrectomy, a technique that consisted of the lateral displacement of conjunctiva in the two sites where the trocars would be placed at the conclusion of the procedure. The trocars were withdrawn and the conjunctiva was pushed laterally with a cotton bud, applying pressure for few seconds over the sclera. Finally the viscoelastic was removed from the anterior chamber by aspiration.
Group 2 patients submitted to 20-gauge pars plana vitrectomy combined with lensectomy (phacofragmentation). A 4 mm infusion cannula was placed through a sclerotomy located 3.5 mm behind the limbus. Once the infusion had been started, two additional sclerotomies were made at the 2:30 and 9:30 clock positions; from each of these sclerotomies, the blade was directed into the nucleus lens through the equator, a fragmentation tip was passed through the nucleus and a classic lensectomy was performed, the anterior capsule was preserved safely. The second step was the posterior vitrectomy and intravitreous manoeuvres that needed the patient. The sclerotomies were sutured at the end of surgery by 7-0 vicryl. Finally a sulcus lens (Meridian HP60M®), was inserted via clear corneal 3.2 mm incision, the corneal wound was sutured by a point of 10-0 Nylon.
Gas-fluid exchange was performed only in the patients with macular hole. A Bausch and Lomb (Rochester, NY) Millennium system was utilized in all cases.
Statistical analysis
For the statistical analysis, two groups of patients were formed:
Group 1 patients submitted to 20-gauge pars plana vitrectomy combined with lensectomy (phacofragmentation).
Group 2 patients submitted to 25-gauge vitrectomy combined with phacoemulsification.
Descriptive statistics were created using SPSS statistical software (version 17.0; SPSS Inc, Chicago, IL, USA). Values are expressed as mean ± standard deviation, and statistical significance was determined using the Student’s t-test for paired data. Student’s paired test was used to compare the quantitative data as:
Visual acuity.
Current age.
Postoperative IOP.
Surgical time.
Best-corrected acuity was converted to LogMAR values to allow for statistical analysis.
When possible, intraoperative and postoperative complications were assessed by statistical analysis. Various statistical tests such as paired Student t-test for quantitative data and chi-squared analysis for qualitative data were used to compare means and assess contingencies between groups.
In all cases, P-values of less than 0.05 were considered statistically significant. We also determined the values of the odds ratio with a confidence interval of 95%.
Results and discussion
Results
Demographic data
All 987 patients completed the study and the mean follow-up was 18.35 ± 1.65 months (7–34 months) for group 1, and 18.17 ± 1.72 for group 2 (8–36 months). The preoperative demographic data of the patients is listed in . Mean age in group 1 was 68.29 ± 9.59 (53–88) years and for group 2 was 69.34 ± 9.21 (55–90) years. Group 1 comprised 54.93% men and 46.07% women, and group 2 comprised 52.87% men and 47.13% women.
Visual acuity
The best preoperative corrected visual acuity was 0.20 ± 0.13 (hand motion to 0.40) in group 1 and 0.22 ± 0.18 (hand motion to 0.40) in group 2.
Mean visual acuity in group 1 at the end of the study was 0.39 ± 0.26 (hand motion to 0.80), LogMAR: +0.41 ± +0.67 (+1.7–0).
Mean visual acuity in group 2 at the end of the study was 0.38 ± 0.28 (hand motion to 0.80), LogMAR: +0.43 ± +0.61 (+1.7–0.1).
Final visual acuity for both groups was well correlated to the diagnostic pathology, and the differences between the two groups are not statistically significant.
Variations in the surgery time ()
The time taken for cataract extraction was similar in both groups of patients and the differences were not significant, but the vitrectomy time was less in patients submitted to 25-gauge vitrectomy. The total time of the surgery was less in group 1 (35.16 ± 3.49 minutes) than in group 2 (44.74 ± 5.33 minutes). These differences were more important in the initial time (sclerotomies versus insertions of trocars) and in the closing time because the extractions of the trocars was faster than closing the sclerotomies by sutures. It is important to observe that the operation time was longer in 25-gauge vitrectomy (23.17 ± 0.97 minutes) than in 20-gauge vitrectomy (20.88 ± 0.45 minutes). These findings were obvious because the aspiration-cutting time in 25-gauge was inferior to the 20-gauge vitrectomy, differences which were significant in the statistical analysis with more advantage for 20-gauge system.
Table 2 Surgical time: Mean time employed in the 25-gauge vitrectomy plus phacoemulsification versus 20-gauge vitrectomy plus phacofragmentation
Intraoperative complications ()
The more frequent intraoperative complication is the 9.26% alteration rate in the depth of the anterior chamber in group 1. These changes may be attributed to the fact that the anterior chamber was not completely closed, because the phacoemulsification had been performed before the vitrectomy, despite a 10-0 nylon suture being leased in the wound and the anterior chamber being filled with viscoelastic. The techniques used during vitrectomy, and possible changes in the vitreous pression may have produced variations in the contents of the anterior chamber. In addition, these variations are the cause of the miosis that has been more frequently observed in group 1. An important finding is that an intraoperative number of retinal tears were observed more frequently in group 2. Perhaps a major traction of the vitreous base by 20-gauge instruments was the cause of this, although we have to bear in mind that the intraoperative tears were more observed in patients with macular hole as diagnostic pathology.
Table 3 Intraoperative and postoperative complications in the two group of patients
Postoperative complications ()
The most important differences in postoperative complications between the two groups of patients correlated well with the major intraoperative complications, such as the alterations of the deep chamber in group 1 and the fact that the sclerotomies in 25-gauge vitrectomy were not sutured at the end of the surgery. As we may observe in group 1, corneal edema is more frequent, such as the postoperative hypotony, and choroidal effusion (which was observed in 1 patient in group 1). These findings in the group 2 presented a more frequent development of retinal detachment, retinal tears, and decentration of the lens.
The other postoperative complications were not statistically different between groups, with a similar rate of postoperative vitreous hemorrhage, rubeosis iridis, and postoperative cystoid macular edema.
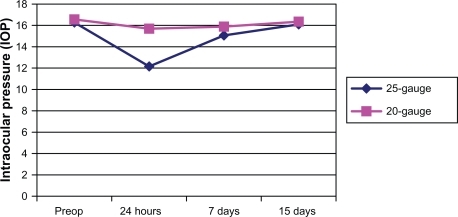
Changes in the IOP (, )
The changes in the of postoperative IOP are greater in the group of patients after 25-gauge vitrectomy, as shown in and after the seventh day we can consider that the IOP has been recovered, and its values are normal. The differences between the two groups are significant in the statistical analysis.
Table 4 The intraocular pressure values according to the diagnosis of patients
Comfort and inflammation ()
The patients in group 1 had poor sensation of foreign body since the first day postsurgery. These findings agree with the hyperemia visualization of the conjunctiva. Group 2 presented a more frequent sensation of foreign body.
Table 5 Comfort and conjunctival hyperemia in the patients
Discussion
The big advantage of combined surgery is that a single intervention can repair all current and likely significant future intraocular diseases for the patient. If surgery is done sequentially, a likely scenario is for the patient to gradually lose vision due to a formation of cataracts possibly up to 80%.Citation6–Citation8 On the other hand the drawbacks to combined surgery is in young patients with intact accommodation. Other disadvantages include increased opening time, and a tendency to cause more inflammation, especially in patients who require gas and laser.
The introduction of 25-gauge vitrectomy in 2001 represented a revolutionary advance in posterior segment surgery, that simplified opening and closing of the eye, minimized surgical trauma for the patient, and made postsurgical recovery faster and less painful.Citation18–Citation20 Despite these advantages, if we want to combine lens extraction surgical phacofragmentation, the tips do not exist. In order to perform lens extraction via pars plana, the phacoemulsification is the technique of choice,Citation21,Citation22 but there are many difficulties in combining the 25-gauge vitrectomy with phacoemulsification.
In the cataract extraction step, we observed that the mean time of the surgery between the two groups is similar, and the difference was not significant. In the intervention technique it is important to note that in group 1 the insertion of the first trocar (the trocar of the infusion canula, in the inferotemporal) is important in order to facilitate the posterior insertion of the two other trocars after the phacoemulsification step if it hadn’t been implanted before. Insertion after phacoemulsification is difficult because the eyes are hypotense. In group 2, the pars plana lensectomy may lead to loose fragments of the lens entering the vitreous, but in this event in our series this fragments has not made the vitrectomy difficult.
In group 1, the location of the intraocular lens (IOL) implant is important, and the insertion is into the capsular bag, but in group 2 the location of the IOL was in the sulcus. This implantion has caused the decentration observed in 2.38% of the series. This problem can be avoided if the lensectomy is substituted for phacoemulsification by clear cornea in group 2, as described in the literature.Citation23–Citation27
There are many differences between groups in respect to the time taken for the vitrectomy. The most important advantage of group 1 is the reduction of the mean time of the vitrectomy phase. This reduction occurred in the initial and closing phases of the vitrectomy, despite the vitrectomy operation time being greater in group 2. In the operation time the authors should be indicate that one of the major problem with 25-gauge vitrectomy is the flexibility of the instruments that hampers thorough removal of most of the peripheral vitreous.Citation28–Citation30 The introduction of the new 23-gauge technique may avoid this problem.Citation31–Citation35
In our series one of the major problems during the vitrectomy was the change observed in the depth of the anterior chamber. Despite the presence of viscoelastic, these variations worsened the vision of the retina, and may be the cause of postoperative corneal edema observed in 4.93% in group 1 against 1.19% of patients in group 2.
Also the anterior chamber depth may worsen mydriasis. An excellent pupillary dilatation is an essential prerequisite to safe and successful vitreoretinal surgery. An inadequate mydriasis makes the vitrectomy difficult, as we have observed in this series. The patients submitted to phacoemulsification plus 25-gauge vitrectomy had more frequent pupillary constriction (6.17%) than patients of group 2 (1.79%). This finding is probably due to the changes in the depth of the anterior chamber observed in group 1.
One important finding in postoperative time is the presence of IOL decentration observed in patients of group 2. The implantation of a lens in the sulcus and not in the capsular bag is disadvantageous and causes the decentration. Compared with pars plana lensectomy, phacoemulsification offers the benefit of allowing the IOL to be placed in the capsular bag.
Hypotony in our series was experienced by 2.47% of the patients and choroidal effusion appeared in one patient (0.02%). We defined hypotony as the presence of IOP < 8 mmHG in the postoperative period. The majority of the patients recovered normal IOP without any intervention; only one required a suture of the sclerotomies. Only one sclerotomy patient presented a wound leakage in the superotemporal sclerotomy after the surgery, this patient was also the patient with choroidal effusion, which recovered after the suture of sclerotomy. In our series there is a greater reduction in the IOP after the 25-gauge vitrectomy than after 20-gauge vitrectomy, as shown in and . For all diagnostic pathology, the IOP decreased more in the first group of patients. The IOP got back to normal after one month and the differences between the groups were not significant.Citation19,Citation20,Citation30,Citation36 Hypotony, even if transient, is not a benign condition and may increase the risks of serious complications postoperation, including retinal or vitreal incarceration, suprachoroidal hemorrhage, and endophthalmitis.Citation18,Citation37 There is evidence in the literature that 25-gauge sclerotomy frequently does not self-seal.
No endophthalmitis has been observed in our series, despite there being a theoretical possibility that sutureless sclerotomy may serve as a conduit for the entry of bacteria into the eye postoperation. Covering the sites of sclerotomies by conjunctiva should serve as a barrier to the entry of these germs.Citation38–Citation41 In the literature there is a description of a high rate of endophthalmitis in 25-gauge against 20-gauge vitrectomy.Citation42 Kunimoto and colleagues reported one case of endophthalmitis in 5,498 eyes (0.018%) vitrectomized by 20-gauge against seven cases in 3,103 eyes (0.23%) who underwent 25-gauge vitrectomy. In the literature, the presence of postoperative retinal tears or retinal detachment has more frequently been reported in patients submitted to 25-gauge vitrectomy. Ibarra and colleaguesCitation43 reported a prevalence of 4.4% in their series, and Fujii and colleaguesCitation15 reported a prevalence of 2%. In spite of these published studies,Citation15,Citation43–Citation45 in our series the presence of intraoperative and postoperative retinal tears are not more frequent in 25-gauge vitrectomy. In fact we may observe a major presence of retinal tears in the 20-gauge vitrectomy group. The authors think that minor traction over the vitreous base carried out in the 25-gauge system may prevent the appearance of retinal tears, and the small size of the postoperative sclerotomy performed in 25-gauge may be the cause of the minor postoperative contraction of the vitreous base around the wound. It is important to indicate that in the literature a high number of postoperative retinal tears and detachments have been observed in patients diagnosed with macular hole, in agreement with our series.Citation43,Citation44
The results of the present study show that visual acuity outcomes are similar in both groups. Preoperative and postoperative complications are not excessively distinct in both groups, and the differences may be attributed to the technique used in each case. Thus group 1 presents more complications attributed to the extraction of cataract via clear cornea, with the consequent loss of the anterior chamber depth. Group 2 has more frequent complications in the IOL decentration. It is important that in our series no cases of endophthalmitis have been observed, but this may be because of the small sample size, in other series with more patients the endophthalmitis appeared and is more frequent than in 20-gauge vitrectomy.Citation45,Citation46 In postoperative retinal tears, we did not show an increase in group 1 as we may expect according to the published data. It is important to remark that the mean time of surgery and postoperative inflammation are advantages for group 1. The authors think that careful patient selection is critical to a successful combined surgery. In the present study, both techniques have a similar number of complications and have similar postoperative outcomes, and are valid for the management of the pathologies selected.
This study is limited because it is a case series study, despite its prospective and randomized technique, with a limited number of eyes that may become a handicap. This factor may affect the low prevalence of some complications such as, endophthalmitis or retinal detachment. This study was not designed to assess comparative visual change nor standardized best-corrected ETDRS measurements made. Finally, long-term complications may appear and may not be detected with follow-up. In the present study, the follow-up of 18.35 ± 1.65 months may have been insufficient depending on the postoperative complication. Further studies including a cumulative multicenter analysis is called for. A case for selection of surgical pathology remains important in order to select the best surgical method for each patient.
Conclusion
In the present study, both techniques have a similar number of complications and have a similar postoperative outcome, and are valid for the management of the pathologies selected. These complications may be attributed to the different systems employed in each group of patients.
Disclosures
The authors report no conflicts of interest in this work.
References
- MachemerRParelJMNortonEWVitrectamy: a pars plana approacn. Technical improvements and further resultsTrans Am Acad Ophthalmal Otolaryngol197276462466
- MachemerRA new concept for vitreous surgery. Two instrument techniques in pars plana vitrectomyArch Opnthalmal197492407412
- DowasNGPars plana vitrectomy microsurgical pars plana lensecomyTrans Sect Ophthalmol Am Acad Ophthalmol Otorlaryngol197681371381
- BlankenshipGWCortezRMachemerRThe lens and pars plana vitectomy for diabetic retinopathy complicationsArch Ophthalmol19799712631267454259
- BlankenshipGWThe lens influence on diabetic vitrectomy results. Report of a prospective randomized studyArch Ophthalmol198098219621987447772
- BlankenshipGWMachemerRLong-term diabetic vitrectomy results; report of 10 year follow-upOphthalmology1985925035062582329
- de BustrosSThompsonJTMichelsRGNuclear sclerosis after virectomy for idiopatic epiretinal membranesAm J Ophthalmol198871601643341433
- NovakMARiceTAMichelsRGAuerCThe crystalline lens after vitrectomy for diabetic retinopathyOphthalmology198491148014846521988
- BiroZKovacsBResults of cataract surgery in previously vitrectomized eyesJ Cataract Refract Surg2002281003100612036644
- McDermottMLPuklinJEAbramsGWEliottDPhacoemulsification for cataract following pars plana vitrectomyOphthalmic Surg Lasers1997285585649243658
- GrushaYOMasketSMillerKMPhacoemulsification and lens implantation after pars plana vitrectomyOphthalmology19981052872949479289
- AhfatFGYuenCHGroenewaldCPPhacoemulsification and intraocular lens implantation following pars plana vitrectomy: a prospective studyEye200317162012579164
- BraunsteinREAirianiSCataract surgery results after pars plana vitrectomyCurr Opin Ophthalmol20031415015412777934
- FujiiGYde JuanEJrHumayunMSA new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgeryOphthalmology20021091807181212359598
- FujiiGYde JuanEJrHumayunMSInitial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgeryOphthalmology20021091814182012359600
- ChylackLTJrWolfeJKSingerDMThe lens classification system III. The longitudinal study of cataract study groupArch Ophthalmol19931118318368512486
- WestheimerGScaling of visual acuity measurementsArch Ophthalmol197997327330550809
- ChenE25-gauge transconjunctival sutureless vitrectomyCurr Opin Ophthalmol20071818819317435424
- LakhanpalRRHumayunMSde JuanEJrOutcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment diseaseOphthalmology200511281782415878061
- IbarraMSHermelMPrennerJLHassanTSLonger-term outcomes of transconjunctival sutureless 25-gauge vitrectomyAm J Ophthalmol200513983183615860288
- Moreno-MontañesJBarrio-BarrioJGarcia-layanaACombined cataract surgery and 25-gauge sutureless vitrectomy for posterior lentiglobusJ Cataract Refract Surg20073338038217321385
- HwangJUYoonYHKimDSKimJGCombined phacoemulsification, foldable intraocular lens implantation, and 25-gauge transconjunctival sutureless vitrectomyJ Cataract Refract Surg20063272773116765787
- ChangCJChangYHChiangSYLinLTComparison of clear corneal phacoemulsification combined with 25-gauge transconjunctival sutureless vitrectomy and standard 20-gauge vitrectomy for patients with cataract and vitreoretinal diseasesJ Cataract Refract Surg2005311198120716039498
- DemetriadesAMGottschJDThomsenRCombined phacoemulsification, intraocular lens implantation, and vitrectomy for eyes with coexisting cataract and vitreoretinal pathologyAm J Ophthalmol200313529129612614744
- SchorweyKPavlovicSJacobiKWCombind clear corneal phacoemulsification, vitreoretinal surgery, and intaocular lens implantationJ Cataract Refract Surg19992569369810330647
- LaheyJMFrancisRRKearneyJJCombining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: a series of 223 casesOphthalmology20031101335133912867387
- LaheyJMFrancisRRKearneyJJCheungMCombining phacoemulsification and vitrectomy in patients with proliferative diabetic retinopathyCurr Opin Ophthalmol20041519219615118505
- AmatoJEAkdumanLIncidence of complications in 25-gauge transconjunctival sutureless vitrectomy based on the surgical indicationsOphthalmic Surg Lasers Imaging20073810010217396688
- BaharIAxer-SiegelRWeinbergerDPars plana vitrectomy: comparison of three techniques for the treatment of diabetic vitreous hemorrhageOphthalmic Surg Lasers Imaging20063736436917017195
- ByeonSHChuYKLeeSCProblems associated with the 25-gauge transconjunctival sutureless vitrectomy system during and after surgeryOphthalmologica200622025926516785758
- RomeroPSalvatMAlmenaAExperience with 25-gauge transconjunctival vitrectomy compared to a 20-gauge system. Analysis of 132 casesJ Fr Ophtalmol2006291025103217114996
- EckardtCTransconjunctival sutureless 23-gauge vitrectomyRetina20052520821115689813
- HubschmanJPGonzalezCRBourdaDHCombined 25-and 23-gauge surgery: a new sutureless vitrectomy techniqueOphthalmic Surg Lasers Imaging20073834534817674931
- HubschmanJPComparison of different vitrectomy systems (Comparaison des différents systèmes de vitrectomie)Jr Fr Ophtalmol200528606609
- OliveiraLBReisPASilicone oil tamponade in 23-gauge transconjunctival sutureless vitrectomyRetina2007271054105818040244
- PatelliFRadicePZumboGG.25-gauge macular surgery: results and complicationsRetina20072775075417621185
- LiuDTChanCKFanDSChoroidal folds after 25 gauge transconjunctival sutureless vitrectomyEye20051982582715359237
- AcarNUnvearYBAltanTKabranZAcute endophthalmitis after 25-gauge sutureless vitrectomyInt Ophthalmol20072736136317492400
- TaylorSRAylwardGWEndophtalmitis following 25-gauges vitrectomyEye2005191228122915543184
- TabanMUfret-VicentryRLSearsJEEndophthalmitis after 25-gauge transconjunctival sutureless vitrectomyRetina20062683083116963862
- StewartJMWound integrity and the conjunctiva in prevention of endophthalmitis following sutureless 25-gauge vitrectomyEye200620149016878121
- KunimotoDYKaiserRSWills Eye Retina Service. Incidence of endophthalmitis after 20- and 25-gauge vitrectomyOphthalmology20071142133213717916378
- IbarraMSHermelMPrennerJLHassanTSLonger-term outcomes of transconjunctival sutureless 25-gauge vitrectomyAm J Ophthalmol200513983183615860288
- OkudaTNishimuraAKobayashiASugivamaKPostoperative retinal break after 25-gauge transconjunctival sutureless vitrectomy: report of four casesGraefes Arch Clin Exp Ophthalmol200724515515716710667
- KunimotoDYKaiserRSWills Eye ServiceIncidence of endophthalmitis after 20- and 25-gauge vitrectomyOphthalmology20071142133213717916378
- ScottIUFlynnHWJrDevSEndophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomesRetina20082813814218185150