Abstract
Purpose:
To compare the intraocular pressure- (IOP-) lowering efficacy of fixed combinations travoprost 0.004%/timolol 0.5% and dorzolamide 2%/timolol 0.5% in patients with ocular hypertension or open-angle glaucoma.
Methods:
In this prospective, multicenter, double-masked, randomized clinical trial, 319 qualifying patients received either travoprost/timolol once daily in the morning (n = 157) or dorzolamide/timolol twice daily (n = 162). IOP was assessed morning and evening at 2 and 6 weeks. The primary outcome measure was mean diurnal IOP.
Results:
Baseline mean IOP values were similar between groups. Mean pooled diurnal IOP was significantly lower in the travoprost/timolol group (16.5 mmHg ± 0.23) than in the dorzolamide/timolol group (17.3 mmHg ± 0.23; P = 0.011). Mean IOP was significantly lower in the travoprost/timolol group compared to the dorzolamide/timolol group at the 9 AM time point both at Week 2 (P = 0.006) and Week 6 (P = 0.002). The travoprost/timolol combination produced mean IOP reductions from baseline of 35.3% to 38.5%, while the dorzolamide/timolol combination produced mean IOP reductions from baseline of 32.5% to 34.5%.
Conclusions:
The fixed combination travoprost 0.004%/timolol 0.5% dosed once daily in the morning demonstrated superior mean diurnal IOP-lowering efficacy compared to dorzolamide 2%/timolol 0.5% dosed twice daily in patients with ocular hypertension or open-angle glaucoma.
Introduction
Glaucoma is one of the main causes of blindness and irreversible visual deterioration worldwide. To date, the only treatment that can effectively prevent the developmentCitation1 and progressionCitation2 of glaucoma is reduction of intraocular pressure (IOP). The most commonly used way of achieving IOP reduction is the use of topical IOP-lowering medications. Monotherapy is frequently not sufficient for reaching the preset target IOP; therefore, many patients require more than one medication to achieve adequate IOP reduction.Citation1
Several fixed combinations of commonly used IOP-lowering medications have been developed and are available in various markets worldwide. Most fixed combinations contain timolol, as it can be dosed either once or twice daily and can be combined with prostaglandin analogues, adrenergic agonists, and carbonic anhydrase inhibitors. Compared to concomitant dosing with individual constituents, these combinations offer the convenience of fewer drops per day, fewer bottles to handle by the patients, reduced exposure to preservatives, and elimination of the washout effect of multiple drops.Citation3
Travoprost 0.004%/timolol 0.5% (DuoTrav®; Alcon Laboratories, Fort Worth, Texas, USA) and dorzolamide 2%/timolol 0.5% (Cosopt™; Merck and Co., Whitehouse Station, New York, USA) are different fixed combinations, but both contain timolol 0.5%. To date, no comparative clinical studies have been published with these two agents. The purpose of this study was to compare the IOP-lowering efficacy and safety of these two fixed combinations in patients with ocular hypertension or open-angle glaucoma.
Methods
This was a prospective, multicenter, randomized, double-masked clinical trial that was approved by the Ethics Committee of each individual study site and was conducted in accordance with the tenets of the Declaration of Helsinki. All participating patients provided written informed consent before they enrolled in the study.
Patients
Eligible patients were male or female patients 18 years of age or older, of any race, diagnosed with open-angle glaucoma (with or without pseudoexfoliation or pigment dispersion component) or ocular hypertension. The patients had to be under treatment with one or more topical IOP-lowering drugs and, in the opinion of the investigator, would have benefited from treatment with a combination therapy. To be eligible, patients must have had at least one eye that reached an IOP of 24 mmHg at 9 AM and 21 mmHg at 4 PM at both eligibility visits. IOP must not have exceeded 36 mmHg (or 30 mmHg at study sites in Germany, as per a specific local requirement) at any time point. Contact lens wearers were eligible if they removed their lenses before instilling study medication and did not replace the lenses for at least 15 minutes after instillation.
Exclusion criteria were history of chronic, recurrent, or current inflammatory eye diseases or progressive retinal diseases; history of incisional ocular surgery or ocular trauma within 6 months before the study; ocular laser treatment or ocular surgery, ocular infection within 3 months; history of hypoglycemia or uncontrolled diabetes; contraindication to any study medication; advanced glaucoma (cup-disc ratio > 0.8 or central visual field loss); or any ocular abnormalities precluding accurate applanation tonometry. In addition, patients were excluded if they required systemic glucocorticoid therapy, could not safely discontinue all IOP-lowering therapies for up to 28 days before the first of two eligibility visits conducted 1 week apart, or used any IOP-influencing medication within 30 days before enrollment. Women of childbearing potential (not surgically sterilized at least 3 months prior the study or not postmenopausal for at least 2 years) were not excluded if they were using a reliable form of birth control; a urine pregnancy test was performed after the completion of the second eligibility visit, before randomization, and then repeated at the exit visit in these patients.
Schedule of visits and assessments
Potential candidates for the study received information related to the study and were provided the opportunity to discuss the study requirements with the investigator. Patients who agreed to participate and gave their consent attended a screening visit, at which time demographics and medical history were collected and reviewed, visual acuity was assessed, IOP was measured with Goldmann tonometry, gonioscopy and automated threshold perimetry were performed, and anterior and posterior segment evaluation was conducted. Patients who qualified were requested to discontinue their current IOP-lowering medications according to the following schedule: 5 days for miotics and carbonic anhydrase inhibitors (both topical and oral); 14 days for α and α/β-agonists; and 28 days for β-blockers, prostaglandin analogues, and fixed combination products. Following washout, two separate eligibility visits were performed one week apart. At each of these eligibility visits, the IOP criteria described above had to be met, interim history was recorded, visual acuity assessed, and slit-lamp examination of the anterior segment was performed. At the completion of the eligibility visits, qualified patients were randomized in a 1:1 ratio to receive either travoprost 0.004%/timolol 0.5% fixed combination once daily at 9 AM and timolol vehicle once daily at 9 PM, or dorzolamide 2%/timolol 0.5% fixed combination twice daily at 9 AM and 9 PM, in both eyes. Patients started their study medication on the evening of the second eligibility visit, and continued their treatment for six weeks. Patients were provided with “morning 9 AM bottles” and “evening 9 PM bottles” for masking purposes. The study visits were scheduled 2 weeks and 6 weeks after randomization. At the Week 2 and Week 6 visits, patients arrived at 9 AM and refrained from morning administration of the study medication until visual acuity was assessed, IOP measured, slit-lamp examination of the anterior segment performed, and any adverse event recorded. Study medication was then instilled, and patients returned at 4 PM for the afternoon IOP measurement. Dilated fundus examination for all patients and repeated urine pregnancy testing for patients of childbearing potential were conducted at the Week 6 visit prior to study exit.
Data analysis and statistics
The primary statistical objective of this study was to examine the IOP-lowering efficacy of travoprost 0.004%/timolol 0.5% dosed once daily compared to that of dorzolamide 2%/timolol 0.5% dosed twice daily. One eye per patient was included in the analysis, even if both eyes were dosed. If only one eye was dosed, the dosed eye was selected for the analysis; if both eyes were dosed, the worst evaluable eye was designated study eye and selected for the analysis. If both eyes met eligibility criteria, the eye with the higher IOP at 9 AM averaged over both eligibility visits was selected as the study eye. If IOP of both eyes was equal at 9 AM, the eye with higher IOP measured at 4 PM was used. If IOP was equal for both eyes at 4 PM, the right eye was designated the study eye. The primary efficacy endpoint was mean IOP, which was evaluated at 4 time points: 9 AM and 4 PM at each of the 2 follow-up visits (Week 2 and Week 6). At each time point, at least 2 IOP measurements were taken. If the 2 measurements for the same eye differed by 4 mmHg or less, the average IOP for that eye was used. If the 2 measurements differed more than 4 mmHg, a third measurement was taken and used as the IOP for that eye.
Hypothesis tests were performed using a repeated measures analysis of variance and the primary inference was based on the comparisons of mean IOP between the two treatment groups across the four on-therapy visits and time points using the intent-to-treat dataset. A chi square test of independence (or Fisher’s exact test if one or more expected cell frequencies were <5) was used to assess differences between treatment groups for each demographic characteristic. Mean IOP change from baseline was estimated using a repeated measures analysis of variance. Descriptive statistics were calculated for IOP, IOP change from baseline, and IOP percent change from baseline. To evaluate the IOP-lowering efficacy throughout the day, IOP was pooled across both time points at each of the two follow-up visits, and the mean value obtained was defined as combined diurnal IOP. The term diurnal is used here to indicate the awake period during the day, rather than a 24-hour period of time, as would be indicated by a diurnal IOP curve.
With 150 patients per group planned, this study had a 96% power to detect a 1.5 mmHg difference between groups, based on an assumed common standard deviation of 3.5 mmHg and a Type I error of α = 0.05.
Results
A total of 319 adult patients were enrolled in this study and randomized to receive either travoprost/timolol (n = 157) or dorzolamide/timolol (n = 162). All 319 participants were included in the safety analysis. Three patients were excluded from the intent-to-treat (ITT; n = 316) analysis due to lack of data on-therapy, and 6 additional patients were excluded from the per-protocol (PP; n = 310) analysis, due to protocol deviations (inadequate time interval from dosing to IOP reading, no qualifying IOP at entry, and intraocular surgery less than 6 months prior to study entry). Results of ITT analyses are presented in this report and were confirmed in PP analyses. Baseline patient demographic information for the safety population is provided in . No significant differences were observed between treatment groups in any of the demographic characteristics; the same was true for the ITT and PP population data sets. The age (mean ± SD) of patients in the safety population was 61.7 ± 10.8 years. The IOP-lowering medications taken by patients prior to study enrollment are presented in , with both treatment groups showing a similar breakdown among the therapy classifications.
Table 1 Baseline demographics – safety population (n = 319)
Table 2 Intraocular pressure- (IOP-) lowering medication at screening – safety population (n = 319)
Mean diurnal IOP values (pooled across 9 AM and 4 PM time points) are illustrated in . Baseline mean diurnal IOP values were similar in the travoprost/timolol (26.0 ± 0.18 mmHg) and the dorzolamide/timolol (26.1 ± 0.18 mmHg) groups (P = 0.818). Both treatments reduced diurnal IOP at Weeks 2 and 6 from baseline; however, treatment with travoprost/timolol resulted in 0.8 mmHg lower mean diurnal IOP than that with dorzolamide/timolol at each of the two on-therapy visits (P < 0.05). A similar difference was observed for travoprost/timolol compared with dorzolamide/timolol when pooled across the two visits (combined; 16.5 ± 0.23 mmHg vs 17.3 ± 0.23 mmHg, respectively; P = 0.011).
Figure 1 Mean diurnal intraocular pressure (IOP) (± standard error) across visits.
Notes: *P < 0.05 for difference in mean diurnal IOP. Travoprost/Timolol = travoprost 0.004%/timolol 0.5%. Dorzolamide/Timolol = dorzolamide 2%/timolol 0.5%
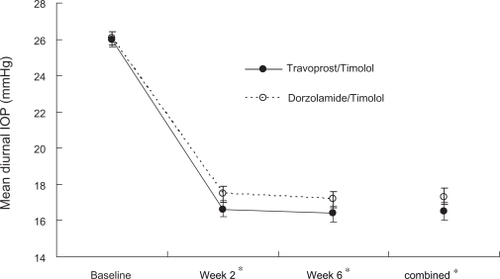
Mean IOP values at the individual time points at baseline, Week 2, Week 6 and Combined (Week 2 + Week 6) are presented in . Baseline mean IOP values were similar in the travoprost/timolol and the dorzolamide/timolol groups both at 9 AM (26.9 ± 0.19 mmHg and 27.0 ± 0.19 mmHg, respectively, P = 0.652) and 4 PM (25.1 ± 0.19 mmHg in both groups, P = 0.987). At 9 AM (approximately 24 hours after dosing with travoprost/timolol), mean IOP was significantly lower in the travoprost/timolol group than in the dorzolamide/timolol group, both at Week 2 (17.0 ± 0.26 mmHg vs 18.0 ± 0.25 mmHg; P = 0.006) and Week 6 (16.6 ± 0.26 mmHg vs 17.7 ± 0.25 mmHg; P = 0.002), as well as when combining the 2 visits (16.8 ± 0.24 mmHg vs 17.9 ± 0.24 mmHg; P = 0.001). Although IOP-lowering values favored the travoprost/timolol group compared to the dorzolamide/timolol group at all 4 PM time points, the differences did not reach statistical significance.
Table 3 Mean intraocular pressure (IOP) (mmHg) across time points – intent-to-treat population (n = 316)
As shown in , travoprost/timolol produced a mean IOP reduction from the untreated baseline ranging from 8.8 ± 0.23 mmHg to 10.4 ± 0.25 mmHg, whereas dorzolamide/timolol produced a mean IOP reduction ranging from 8.2 ± 0.21 mmHg to 9.3 ± 0.25 mmHg. shows that the mean diurnal IOP reductions in the travoprost/timolol group were significantly greater than those in the dorzolamide/timolol group at Week 2 and Week 6 (P < 0.05 for each).
Figure 2 Mean diurnal intraocular pressure (IOP) (± standard error) reduction from baseline.
Notes: *P < 0.05 for difference in IOP reduction from baseline. Travoprost/Timolol = travoprost 0.004%/timolol 0.5%. Dorzolamide/Timolol = dorzolamide 2%/timolol 0.5%
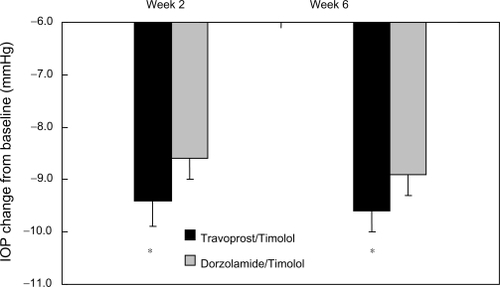
Table 4 Descriptive mean intraocular pressure (IOP) and mean IOP change from baseline (mmHg) – intent-to-treat population (n = 316)
Safety
The adverse events, related to the use of the study drugs, observed at an incidence of greater than 1% in the two treatment arms are described in . The most common treatment-related ocular adverse event was mild eye irritation, occurring in 5.7% of patients in the travoprost/timolol group and in 4.3% of patients in the dorzolamide/timolol group. More patients in the travoprost/timolol group than in the dorzolamide/timolol group experienced hyperemia, generally of mild severity and described as conjunctival (5.7% vs 0.6%, respectively; P = 0.0096) or ocular hyperemia (5.1% vs 0.6%, respectively; P = 0.0182). No serious treatment-related adverse events were reported in either group.
Table 5 Ocular treatment-related adverse events occurring at an incidence of greater than 1% – safety population (n = 319)
In the travoprost/timolol group, 4 patients (2.5%) stopped the study medication and were discontinued from the study due to ocular treatment-related events (hyperemia, eye pain, conjunctivitis, and hypersensitivity). In the dorzolamide/timolol group, 1 patient (0.6%) stopped the study medication and was discontinued from the study due to a nonocular event (hallucination, considered not related to treatment).
Discussion
In this study, we compared the IOP-lowering efficacy of two fixed combinations, travoprost 0.004%/timolol 0.5% dosed once daily in the morning and dorzolamide 2%/timolol 0.5% dosed twice daily, in patients with open-angle glaucoma or ocular hypertension. These results were expected to show that travoprost/timolol would have superior efficacy to dorzolamide/timolol, based on previous studies of travoprost alone compared to the fixed combination of dorzolamide/timolol, in which travoprost produced superior IOP reductionsCitation10,Citation11 and fewer treatment failures.Citation12
Our results show that during the day, the fixed combination travoprost/timolol produced a higher IOP-lowering efficacy than the fixed combination dorzolamide/timolol dosed twice daily, at each visit as well as when pooling data across visits. The observed differences were both statistically significant and clinically meaningful; there was a consistent difference of 0.8 mmHg (P < 0.05) between the two groups.
Travoprost/timolol produced lower mean diurnal IOP than dorzolamide/timolol. Diurnal reduction of IOP has been associated with a reduced risk of progression of glaucoma in several studies. The Early Manifest Glaucoma Trial showed that every 1 mmHg of IOP reduction was associated with approximately a 10% reduction in risk of glaucoma progression.Citation13
Mean IOP with travoprost/timolol was at least 1.0 mmHg lower than with dorzolamide/timolol at 9 AM; these differences were statistically significant at both the Week 2 and Week 6 visits. These results were not unexpected because at the 9 AM time points (12 hours after dosing dorzolamide/timolol and 24 hours after dosing travoprost/timolol), dorzolamide was likely not to be as effective since its duration of action is less than 8 hours,Citation14 whereas travoprost has been shown to be effective more than 48 hours after dosing.Citation15 A tendency for lower IOP in the travoprost/timolol group was uniformly present both at Week 2 and Week 6 and for the pooled data, with the difference between the two treatment arms ≥ 0.5 mmHg at the 4 PM time points. Because these time points measured IOP control only 7 hours after dosing, both travoprost/timolol and dorzolamide/timolol were likely to be effective and therefore no significant difference between treatments was observed.
Travoprost/timolol produced statistically significant and clinically relevant mean IOP reductions from baseline ranging from 8.8 mmHg (35.3%) to 10.4 mmHg (38.5%). This is consistent with prior studies in which mean IOP reductions ranged from 6.9 to 8.6 mmHg,Citation4 7.4 to 9.4 mmHg,Citation5 and 8.8 to 11.5 mmHg.Citation6 Dorzolamide/timolol also produced significant and relevant mean IOP reductions from baseline, ranging from 8.2 mmHg (32.5%) to 9.3 mmHg (34.5%). This is also consistent with prior data in which mean IOP reductions ranged from 7.7 to 9.0 mmHg.Citation7
IOP values at Weeks 2 and 6 varied between 16.2 and 17.0 mmHg for travoprost/timolol and between 16.6 and 18.0 mmHg for dorzolamide/timolol. The narrower range for travoprost 0.004%/timolol 0.5% (0.8 mmHg) suggests less fluctuation of IOP during the day. Asrani and colleagues suggested that eyes with greater diurnal IOP variation are at increased risk of visual field progression.Citation8 Bergea also found that visual field progression was more likely in eyes with higher versus lower diurnal IOP variation.Citation9 However, since the data on the relationship between diurnal IOP fluctuation and progression of glaucoma are scarce, the clinical relevance of the narrower range observed in this study remains to be confirmed.
Both therapies evaluated in this study offer numerous benefits that have been previously described for fixed combination products.Citation3 Compared to dorzolamide/timolol, the fixed combination travoprost/timolol offers the additional advantage of once-daily dosing. Adherence to therapy improves as the frequency of dosing decreases.Citation16 Once-daily dosing is preferred by glaucoma patients and glaucoma specialists alike.
Aside from eye irritation, which was the most prevalent adverse event in both groups, the most frequent ocular event in the travoprost/timolol subjects was conjunctival hyperemia. Eye pruritus was the most frequent ocular event reported in the subjects receiving dorzolamide/timolol. The statistically significant differences found between treatment groups in the rates of hyperemia do not represent an untoward safety issue. The incidence of these side effects is consistent with the known safety profile of travoprost 0.004%/timolol 0.5%. Hyperemia is not unexpected since it is observed frequently with the use of all prostaglandin analogues. Eye pruritus was also an expected ocular event.
In summary, the fixed combination travoprost 0.004%/timolol 0.5% dosed once daily in the morning demonstrates superior IOP-lowering efficacy compared to dorzolamide 2%/timolol 0.5% dosed twice daily in patients with ocular hypertension or open-angle glaucoma. Both combinations offer the benefits of fixed-combination therapy, but travoprost/timolol offers these benefits with the convenience of once-daily dosing, an attribute valued by both glaucoma patients and their physicians because it encourages patient compliance.Citation16
Study registration number
EudraCT number: 2005-004767-34.
Acknowledgements
The investigators who participated in this study as members of the C-05-25 Study Group: LATVIA: G Laganovska, Stradina Clinical University Hospital and L Volksone, Clinical Hospital Gailezera, Riga. FRANCE: JF Rouland, Hôpital Claude Huriez, Lille, Ch-A Ubaud, Marseille, B Delbosc, Hôpital Jean Minjoz, Besançon and JP Bacquaert, Wattrelos. GERMANY: C Erb, Schlosspark-Klinik, Berlin, I Strempel, Augenklinik am Universitätsklinikum, Marburg and G Duncker, Universitätsklinik und Poliklinik für Augenheilkunde, Halle. HUNGARY: G Holló, Semmelweis University Department of Ophthalmology, A Berta, DEOEC, Debrecen, Á Kerényi, Bajcsy-Zsilinszky Hospital, T Milibák, Uzsoki Hospital, Budapest, B Kovács, University Medical and Health Scientific Center, Pécs, K Korompai, Borsod-Abaúj-Zemplén County Hospital, Miskolc, L Kolozsvári, University of Szeged, B Kovács, Kaposi Mór Teaching Hospital, Kaposvár, J Győri, Cholnoki Ferenc Hospital, Veszprém, I Elek, Bugát Pál Hospital, Gyönygös, Z Öri, Vaszary Kolos Hospital, Esztergom and A Bereczki, Petz Aladar County Teaching Hospital, Gyor. ITALY: R Meduri, Azienda Ospedaliera Policlinico S Orsola Malpigli, Bologna, M Fossarello, Ospedale San Giovanni Di Dio, Cagliari, S Miglior, Università di Milano Bicocca, Monza and A Sebastiani, Arcispedale S Anna, Ferrara. POLAND: B Romanowska-Dixon, SPZOZ Szpital Uniwersytecki w Krakowie, Kraków and R Gos, ”Contact-Med” Sp z o.o.Łódź. SPAIN: M A Teus, Hospital Principe de Astúrias, Madrid, PC Fernández –Vila, Hospital Provincial, Pontevedra and A Arias-Puente Fundación Hospital Alcorcón. TURKEY: K Andac, Ege University Medical Faculty Bornova-Izmir and K Güngör, University Medical Faculty Gaziantep.
Disclosures
The authors would like to acknowledge Jennifer Klein, PhD for medical writing contributions. This assistance was supported by Alcon Research, Ltd. This study was supported by Alcon Research Ltd. as Alcon study C-05-25.
References
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol20021206701713discussion 829–83012049574
- HeijlALeskeMCBengtssonBHymanLBengtssonBHusseinMReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol2002120101268127912365904
- FechtnerRDRealiniTFixed combinations of topical glaucoma medicationsCurr Opin Ophthalmol200415213213515021225
- SchumanJSKatzGJLewisRAEfficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertensionAm J Ophthalmol2005140224225016086946
- HughesBABacharachJCravenERA three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertensionJ Glaucoma200514539239916148589
- BarnebeyHSOrengo-NaniaSFlowersBEThe safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solutionAm J Ophthalmol200514011715990081
- BoyleJEGhoshKGieserDKAdamsonsIAA randomized trial comparing the dorzolamide-timolol combination given twice daily to monotherapy with timolol and dorzolamide. Dorzolamide-Timolol Study GroupOphthalmology199810510194519519787368
- AsraniSZeimerRWilenskyJGieserDVitaleSLindenmuthKLarge diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucomaJ Glaucoma20009213414210782622
- BergeaBBodinLSvedberghBImpact of intraocular pressure regulation on visual fields in open-angle glaucomaOphthalmology199910659971004discussion 1004–100510328403
- SuzukiERJrFranklinLMda SilvaLJFigueiredoCRNettoJABatistaWDComparison of the efficacy and safety of travoprost with a fixed-combination of dorzolamide and timolol in patients with open-angle glaucoma or ocular hypertensionCurr Med Res Opin20062291799180516968583
- ChiselițăDAntohiIMedvichiRDanielescuCComparative analysis of the efficacy and safety of latanoprost, travoprost and the fixed combination timolol-dorzolamide; a prospective, randomized, masked, cross-over design studyOftalmologia2005493394516408674
- LafumaABerdeauxGCosts and effectiveness of travoprost versus a dorzolamide + timolol fixed combination in first-line treatment of glaucoma: analysis conducted on the United Kingdom General Practitioner Research DatabaseCurr Med Res Opin200723123009301617958945
- LeskeMCHeijlAHymanLBengtssonBDongLYangZPredictors of long-term progression in the early manifest glaucoma trialOphthalmology2007114111965197217628686
- LippaEACarlsonLEEhingerBDose response and duration of action of dorzolamide, a topical carbonic anhydrase inhibitorArch Ophthalmol199211044954991562255
- GrossRLPeaceJHSmithSEDuration of IOP reduction with travoprost BAK-free solutionJ Glaucoma200817321722218414108
- PatelSCSpaethGLCompliance in patients prescribed eyedrops for glaucomaOphthalmic Surg19952632332367651690