Abstract
Herpetic keratitis is caused by herpes simplex virus (HSV) and is a common cause of corneal blindness. Following a primary ocular herpetic infection, latency of the virus occurs, followed by subsequent recurrences of herpetic keratitis. Such recurrences may lead to structural damage of the cornea. Recurrent herpetic keratitis is a common indication for corneal transplantation. Recurrences of herpetic keratitis in the corneal graft may lead to corneal graft rejection. Several antiviral agents for HSV are available, including the thymidine analogs. Prolonged use of thymidine analogs may lead to toxicity of the ocular surface, including epithelial keratitis, corneal ulcers, follicular conjunctivitis, and punctal occlusions. Availability of topical antiviral agents that are safe and effective in the treatment and prophylaxis of herpetic keratitis is highly desirable. Ganciclovir is a potent inhibitor of members of the herpes virus family. The drug has been used systemically for the treatment of cytomegalovirus (CMV) retinitis. Its hematologic toxicity secondary to systemic administration led to its limited use in herpetic infections. On the other hand, topical ganciclovir has been shown to be as safe and effective as acyclovir in the treatment of herpetic epithelial keratitis. Furthermore, topical ganciclovir can reach therapeutic levels in the cornea and aqueous humor following topical application. Several clinical trials have shown that topical ganciclovir 0.15% ophthalmic gel is safe and effective in the treatment and prophylaxis of herpetic epithelial disease. Long-term use of ganciclovir ophthalmic gel in patients with penetrating keratoplasty following herpetic keratitis has prevented recurrences of the disease. Topical ganciclovir ophthalmic gel is well tolerated, does not cause toxic effects on the ocular surface, and does not cause hematologic abnormalities. Clinical studies have underscored the potential role of ganciclovir ophthalmic gel in the treatment and prophylaxis of herpetic epithelial keratitis. Future randomized, controlled, multicenter, prospective clinical trials are needed to assess the long-term safety and efficacy of topical ganciclovir in the treatment and prevention of herpetic keratitis and uveitis.
Introduction
The ocular and adnexal structures are frequently the target of infection by herpes simplex virus (HSV). HSV is involved in the pathogenesis of several ocular disorders and may lead to blepharitis, vesicular dermatitis of the eyelids, conjunctivitis, keratitis, trabeculitis, anterior uveitis, acute retinal necrosis syndrome, retinitis, and optic neuritis (). Herpetic corneal epithelial infections are by far the most common ocular manifestation. Herpetic ocular disease may be primary, latent, or recurrent (secondary).Citation1–Citation3 Ocular infection in a patient who is not immune to the virus and not previously infected is referred to as primary disease. Patient may develop viral latency after a primary infection and the virus stays dormant in the nerve ganglia. It may later cause recurrent clinical manifestations. Ocular infections in adults are predominantly due to HSV Type 1. Genito-ocular spread can occur and may be caused by HSV Type 2, but is less common. The disease may also be related to postnatal infection with HSV Type 1. An intrauterine- or neonatal birth canal-acquired infection with HSV Type 2 may occur. In herpes-infected neonates and infants without central nervous system involvement, keratitis occurs in 80% of cases and conjunctivitis occurs in approximately 10% of cases. Visual loss in ocular herpetic infection may be due to keratitis, uveitis, cataract, or glaucoma, while in neonatal herpetic infections the visual loss is commonly due to optic atrophy and chorioretinitis. Following a primary infection, the disease may recur in the cornea and may result in epithelial keratitis. Recurrent epithelial keratitis may stay in the epithelial layer or may involve the deeper stromal layer and endothelium.
Table 1 Ocular manifestations of herpes simplex of the eye and adnexa
Risk factors for HSV reactivation are well known and include stress, exposure to ultraviolet radiation, menstruation, fever, use and abuse of corticosteroids, and trauma including surgical trauma.Citation4,Citation5 The disease is usually unilateral. Bilateral herpetic keratitis may occur in patients with systemic diseases, such as atopic disorders, malignancy, debilitating illnesses, or in patients on immunosuppressive agents.Citation2
Herpetic keratitis is a common cause of ocular morbidity and represents the most common cause of corneal blindness.Citation1 Recurrent herpetic keratitis is also a common indication for corneal transplantation. Recurrences of herpetic epithelial keratitis may occur in the corneal graft and result in structural damage of the cornea, leading to corneal graft rejection and failure. The ocular disease could take one of two forms. The first is the primary ocular disease occurring in a patient who has no previous exposure to herpes virus, and the second is latent disease in patients who have dormant HSV in the nerve ganglia. Primary herpetic disease can be clinical or subclinical. Viral reactivation in latency may lead to involvement of the lids, conjunctiva, cornea, uvea, retina, and/or optic nerve. Recurrent disease of the cornea is the most common, occurs in the latent phase, and may be epithelial, stromal, endothelial, or disciform.
There are several antiviral agents that are effective against HSV, and these include idoxuridine, vidarabine, trifluridine, acyclovir, famciclovir, valganciclovir, valacyclovir, and cidofovir. Idoxuridine and trifluridine act as thymidine analogs by formation of defective viral DNA molecules due to inhibition of DNA polymerase.Citation3 Vidarabine, a purine analog, inhibits viral DNA polymerase and is incorporated by the viral DNA molecule. Idoxuridine, trifluridine, and vidarabine cause corneal epithelial toxicity because of their non-selective action on infected and noninfected host cell DNA molecules. Prolonged use of thymidine analogs, however, may cause ocular surface toxicity. Acyclovir is activated by HSV thymidine kinase to the monophosphate form and by cellular enzymes to acyclovir triphosphate, causing inhibition of Herpes virus DNA polymerase. Topical acyclovir is available as a 3% ophthalmic ointment for the treatment of HSV corneal epithelial.
Several studies have shown that ganciclovir is highly effective in the inhibition of HSV Type 1 and Type 2. Systemic ganciclovir is indicated for sight-threatening CMV retinitis in severely immunocompromised people, CMV pneumonitis in bone marrow transplant recipients, prevention of CMV disease in bone marrow and solid organ transplant recipients, and confirmed CMV retinitis in people with acquired immunodeficiency syndrome (intravitreal implant).
The purpose of this paper is to provide a critical review of topical ganciclovir in the treatment of acute herpetic epithelial keratitis.
Pharmacology and pharmacokinetics
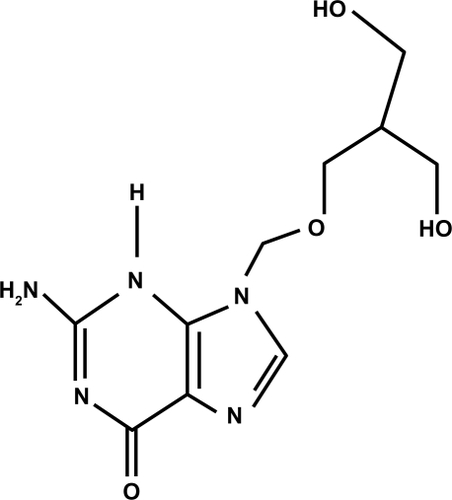
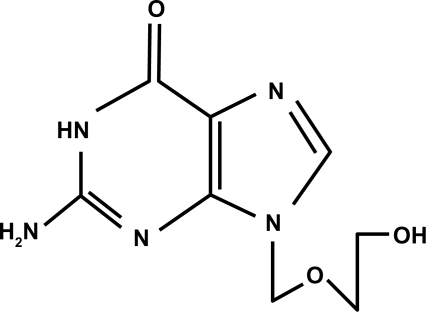
Ganciclovir () is an antiviral agent that is similar to acyclovir (). Acyclovir is used for herpetic keratitis, whereas ganciclovir is used to treat or prevent cytomegalovirus (CMV) infections. Ganciclovir sodium is marketed under the trade names Cytovene® and Cymevene® (Roche). Ganciclovir for intraocular inserts is marketed under the trade name Vitrasert® (Bausch and Lomb). The device is approved for the treatment of CMV retinitis. A prodrug form with improved oral bioavailability (valganciclovir) is also available. Topical ganciclovir is available from Laboratoires Théa (Louis-Bleriot, Clermont-Ferrand, France) and is marketed under the names Virgan® and Zirgan® as 1.5 mg/g eye gel in tubes of 5 g each. The other ingredients in ganciclovir are carbomer (Carbopol® 974P), sorbitol, sodium chloride, and benzalkonium chloride.
Ganciclovir is a nucleoside analog that is selectively phosphorylated by virus-encoded thymidine kinase, and is subsequently phosphorylated by cellular enzymes.Citation1 Ganciclovir is converted into ganciclovir monophosphate and then to ganciclovir diphosphate and finally, to ganciclovir triphosphate. Ganciclovir triphosphate inhibits Herpes virus DNA polymerase and arrests HSV replication.Citation6–Citation9 Following topical application of ganciclovir, it has been shown that the drug can penetrate the corneal stroma and can reach the aqueous humor in therapeutic levels.Citation9
In a study by Castela et al,Citation9 ganciclovir ophthalmic gel was prepared in Carbomer 934P (NF XVII, Transphyto SA, Clermont-Ferrand, France). The drug-free vehicle was used as a placebo and the ganciclovir concentration levels of the tested gels were 0.0125%, 0.05%, and 0.2%. The investigators studied the effects of ganciclovir ophthalmic gel on HSV keratitis in rabbits. The efficacy of the three ganciclovir gel strengths were compared with placebo and 3% acyclovir ophthalmic ointment using an HSV Type 1 rabbit keratitis animal model. All three ganciclovir concentrations showed clinical efficacy and significant reduction of corneal ulcer area, clouding, and vascularization (P < 0.05) and rapid inhibition of HSV shedding in tear films. The distribution of ganciclovir and acyclovir in the rabbit eyes were determined by high performance liquid chromatography. Both ganciclovir and acyclovir were found to reach higher levels in the cornea than in the aqueous humor. No ocular toxicity was noted in this study. Corneal penetration of ganciclovir was due to the small size of the ganciclovir molecule, its high lipophilicity, and its high cellular affinity resulting from its structural analogy with the endogenous nucleosides. This may explain the high diffusion of ganciclovir in the corneal epithelium (pKa 2.2 and 9.4 at pH 6.5–7.4). The destruction of the corneal epithelium by the virus allows penetration of the drug into the cornea so that it can reach the anterior chamber. Ganciclovir was found in the aqueous humor of healthy rabbits three hours after a series of six administrations of the gel formulation.Citation9 The level of concentration was dependent on the ganciclovir dosage and frequency of application. Ganciclovir has poor water solubility, and its affinity for melanin in cells explains its preferential distribution in solid ocular tissues, such as the iris, compared with the aqueous humor. Ganciclovir was recovered from the aqueous humor four hours after the last topical administration of ganciclovir 0.2%, and this could be due to the slow diffusion of the drug from the cornea to the aqueous humor.Citation9
The physiochemical properties of acyclovir and ganciclovir are similar, and this explains the similarity in the ocular tissue distribution. The tissue concentrations are of the same order of magnitude when comparing ganciclovir ophthalmic gel 0.15% with 3% acyclovir ointment despite the difference in dosage strength. Furthermore, the plasma concentration four hours after the administration of ganciclovir gel was very low. This illustrates the limited plasma diffusion of the drug and its lack of systemic toxicity. With regard to the experimental design, one has to keep in mind that the rabbit model of herpetic keratitis is different from that of human ocular herpes. Extrapolation of the findings from the animal model to humans should be done with caution. The intraocular diffusion of ganciclovir may permit its use in the treatment of herpetic keratouveitis. The low plasma ganciclovir concentration after chronic gel application limits the risk of systemic toxicity. The efficacy of ganciclovir was observed in vitro and in vivo against HSV strains resistant to both idoxuridine and acyclovir.Citation10
Therapeutic interventions for HSV epithelial keratitis
WilhelmusCitation11 performed a systematic review of the literature reported in the Cochrane Database. Surveys of ocular antiviral drugs for HSV eye disease were evaluated in a systematic review of all comparative clinical studies. The review revealed that antiviral agents are effective and nearly equivalent in their efficacy. The combination of a nucleoside antiviral agent with either debridement of the epithelium or with interferon seems to speed up healing of the epithelium. The studies that were reviewed did not consider the size of the lesion patients had prior to treatment. Debridement in combination with antiviral therapy appears to be more effective than antiviral chemotherapy alone, but this issue remains inconclusive. Removal of infected epithelial cells by debridement appears to be effective, but antiviral agents against HSV are needed to prevent recrudescence of epithelial keratitis.Citation1 Antiviral agents did not increase the speed of healing when compared with debridement, but reduced the risks of recurrence of epithelial keratitis. Ganciclovir appear to be as effective as other antiviral agents in the treatment of herpetic keratitis.Citation1
Ganciclovir ophthalmic gel in herpetic keratitis
Topical ganciclovir has been studied in animal models of herpetic keratitis and healthy volunteers, and in several prospective, randomized clinical trials.Citation12–Citation17 Topical ganciclovir has been found to be safe and effective in the treatment of herpetic epithelial keratitis. A randomized prospective clinical trial was carried out in Africa and Europe. Sixty-seven patients were included in the first clinical trial in Africa, and 37 patients were included in the second clinical trial in Europe.Citation12 The results of these two clinical trials showed no statistically significant difference between ganciclovir 0.15% gel and acyclovir 3% ophthalmic ointment in patients with herpetic epithelial keratitis. Healing was achieved within one week in 85% of patients treated with ganciclovir 0.15% gel compared with 32% of patients treated with acyclovir ointment. On the other hand, 83% of patients achieved healing within one week following ganciclovir 0.15% gel compared with 72% in patients treated with acyclovir ointment. There were fewer complaints (stinging, burning sensation, blurring of vision) in the ganciclovir group than in the acyclovir group. Patients treated with ophthalmic acyclovir ointment complained of irritation and blurring of vision. The findings of these two clinical trials support the fact that ganciclovir gel is safe and effective in the treatment of HSV epithelial keratitis, and that in this regard it is superior to acyclovir.Citation12
Hoh et alCitation18 studied the effects of topical ganciclovir gel and acyclovir ointment in the treatment of HSV dendritic keratitis in a multicenter, prospective, randomized clinical trial. Their findings demonstrated that topical ganciclovir ophthalmic gel was as safe and effective as acyclovir in the treatment of herpetic epithelial keratitis. There was no significant difference in adverse effects between the two treatment modalities.
Following topical application, ganciclovir has been shown to penetrate the corneal stroma and to reach the aqueous humor in therapeutic levels.Citation18 It has been shown to be as effective as acyclovir in the treatment of herpetic keratitis in humans.Citation10,Citation18 The effects of topical ganciclovir 0.15% gel were studied in 16 consecutive patients with corneal grafts and previous recurrences of herpetic keratitis.Citation19 Topical ganciclovir was found to be safe and effective in this open, non-randomized study. Furthermore, long-term topical ganciclovir afforded protection against recurrence of the disease in patients who had undergone penetrating keratoplasty.Citation19
Patients undergoing corneal transplantation for herpetic corneal disease may be treated with topical prophylactic antiviral agents. Such patients have latent HSV in the trigeminal ganglion and may develop recurrence of the disease in the graft, with subsequent corneal graft failure.Citation15,Citation16,Citation20,Citation21 These patients may be given either acyclovir 3% ophthalmic ointment or ganciclovir 0.15% gel four times daily in conjunction with topical prednisolone 1% eye drops. Ganciclovir 0.15% gel may help in the prevention of recurrences of herpetic keratitis in patients undergoing corneal transplantation. Epithelial disease improvement and healing of the ulcer can occur within one week of treatment with topical ganciclovir.Citation10,Citation18,Citation19 Ganciclovir given in a gel form is easy to apply and does not cause blurring of vision, unlike acyclovir petrolatum-based ophthalmic ointment (). The drug is well tolerated and has no toxic effects on the ocular surface. Corneal tissue absorption of ganciclovir can reach therapeutic levels and be found in the aqueous humor following topical application.Citation9,Citation12 and show herpetic epithelial keratitis in two patients before and one week after treatment with ganciclovir 0.15% ophthalmic gel.
Figure 3 A) Shows the right cornea of a 12-year-old boy with herpetic epithelial keratitis before treatment, and B) five days after treatment with topical ganciclovir 0.15% ophthalmic gel.
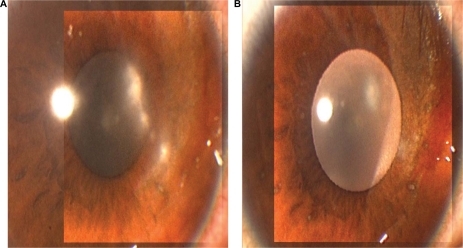
Figure 4 A) An 80-year-old male with geographic epithelial keratitis stained with fluorescein, and B) one week after treatment with ganciclovir 0.15% ophthalmic gel showing healing and re-epithelialization of the cornea.
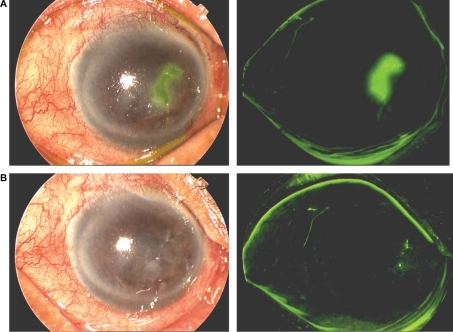
Table 2 Topical ganciclovir in herpetic keratitis
Ganciclovir is an important inducer of cell death. The efficacy of ganciclovir and acyclovir in inducing apoptosis in the HSV thymidine kinase system was compared by Shaw et al.Citation13 Treatment with ganciclovir induced rapid apoptosis of HSV-infected cells. In contrast with up to seven days’ exposure, acyclovir did not induce apoptosis. Ganciclovir given topically did not cause systemic or hematologic adverse effects.
Long-term prophylaxis with ganciclovir in patients who had recurrent herpetic keratitis has not been studied. Future prospective, long-term clinical trials are needed to assess the safety and efficacy of topical ganciclovir prophylactic strategies in the prevention of recurrent herpetic corneal disease.Citation19 Corneal transplantation for corneal scarring induced by HSV can restore vision and induce rapid recovery. Recurrence of herpetic keratitis in the transplanted graft, however, may lead to corneal graft rejection and decreased vision due to recurrence of HSV in the transplanted cornea.Citation14–Citation21 Therefore, it is recommended that long-term prophylaxis with topical ganciclovir gel or acyclovir ointment might help to prevent recurrence of the disease and subsequent graft rejection. It is generally agreed that corneal graft rejection and failure following corneal transplantation in patients with herpetic keratitis remains high and that the survival rate of the regraft is around 50%.Citation21 The use of topical antiviral prophylaxis may help in the prevention of herpetic keratitis.Citation15–Citation17,Citation19–Citation23 Long-term application of ganciclovir ophthalmic gel is an effective prophylaxis against herpetic keratitis in this group of patients and appears to be a good therapeutic strategy.Citation21,Citation24–Citation26 Recurrence of herpetic keratitis in the corneal graft following penetrating keratoplasty poses a therapeutic dilemma. Herpetic keratitis may lead to corneal graft rejection which requires increased use of topical corticosteroids which may aggravate the herpetic infection.
Low-dose oral acyclovir has been shown to be effective in preventing recurrent genital herpes and appears to be a safe long-term prophylactic measure.Citation27–Citation29 Recurrences of genital herpes are prevented by long-term, low-dose oral acyclovir. This therapeutic strategy may also be applied to recurrences of ocular herpes. Prophylaxis in patients who have undergone penetrating keratoplasty for herpetic disease may prevent corneal graft failure. Oral acyclovir does not appear to be cost-effective for the prevention of HSV eye disease.Citation22 Topical use of ganciclovir or acyclovir ointment as prophylaxis in patients with corneal grafts may spare the use of systemic oral antiviral agents such as acyclovir.Citation21 The good levels of ganciclovir in aqueous humor following topical application make this drug a potential therapeutic modality for the treatment and prophylaxis of herpetic keratouveitis. However, the long-term safety and efficacy of ganciclovir in the treatment of stromal keratitis and herpetic keratouveitis remain to be determined. Prospective clinical trials to assess the safety and efficacy of long-term topical ganciclovir ophthalmic gel in the prophylaxis of herpetic keratitis are needed.
Chee and JapCitation30 studied the effects of ganciclovir in patients with cytomegalovirus (CMV) uveitis. The CMV uveitis was diagnosed by the clinical findings and by aqueous polymerase chain reaction. The recurrence rate of anterior uveitis was significantly lower in patients who received topical ganciclovir gel than patients who were treated with systemic ganciclovir. Systemic and intravitreal ganciclovir and ganciclovir implant had good response rates but also had high recurrence rates. Ganciclovir 0.15% gel had moderate response rates, but its recurrence rates were also lower than those of the other treatment modalities. Topical ganciclovir 0.15% gel, therefore, may be considered for the treatment of CMV anterior uveitis.
Recent studies have focused on the ocular bioavailability of ganciclovir. In a study by Majumdar et al,Citation31 a series of dipeptide monoester ganciclovir (GCV) prodrugs were evaluated with the goal of improving the ocular bioavailability of ganciclovir topical ophthalmic solution. Val-Val-GCV, Tyr-Val-GCV, and Gly-Val-GCV were more stable in aqueous solution than Val-GCV. All three prodrugs had much higher aqueous solubility than the current drug. Transcorneal permeability of Val-GCV and Val-Val-GCV was seven-fold to eight-fold greater than that of GCV in the presence of a proton gradient and was significantly decreased in the presence of Gly-Pro. Val-Val-GCV 1% provided significantly better therapeutic activity than trifluorothymidine against HSV-1 epithelial keratitis and was equivalent in its therapeutic activity against stromal keratitis in the rabbit eye model of herpetic keratitis. Val-Val-GCV demonstrated excellent corneal permeability and chemical stability, high aqueous solubility, and substantial in vivo antiviral activity against HSV-1.
Topical ganciclovir 0.15% gel has been shown to be as effective as acyclovir in the treatment of active herpetic epithelial keratitis.Citation32,Citation33 Prospective, randomized clinical trials are needed to assess the long-term safety and efficacy of topical ganciclovir in the prevention of recurrence of herpetic keratitis. Recurrent herpetic keratitis may lead to structural damage of the cornea and loss of vision. Herpes keratitis in a graft leads to rejection and graft failure. Prophylactic use of topical ganciclovir may prove to be safe in the prevention of recurrence of the disease. The intraocular penetration of topical ganciclovir in the aqueous humor may prove to be effective in the treatment of HSV keratouveitis, HSV, and CMV uveitis.
Prophylaxis may require a lower dose frequency, such as once or twice daily. The efficacy of such a therapeutic approach remains to be determined. In patients who had undergone penetrating keratoplasty for herpetic corneal scars, topical daily application of ganciclovir 0.15% gel may prove to be an effective prophylactic measure for the prevention of recurrences in the corneal graft. The dose and frequency remain to be determined. Most recurrences of HSV keratitis occur in the first year after the graft. Our patients with penetrating keratoplasty following herpetic keratitis are treated with ganciclovir 0.15% gel four times daily in the first week followed by two times daily for a period of one year. Future clinical trials are needed to assess the safety and efficacy of topical ganciclovir in the treatment and prevention of herpetic keratouveitis, and herpetic and CMV anterior uveitis.
Disclosure
The authors report no conflict of interest in this work.
References
- WilhelmusKRThe treatment of herpes simplex virus epithelial keratitisTrans Am Ophthalmol Soc20009850553211190039
- HyndiukRGlasserDHerpes simplex keratitisTabbaraKFHyndiukRInfections of the Eye2nd edBoston, MALittle Brown & Co1996
- O’BrienWAntiviral agentsTabbaraKHyndiukRInfections of the Eye2nd edBoston, MALittle Brown & Co1996
- ElftmanMDHunzekerJTMellingerJCBonneauRHNorburyCCTruckenmillerMEStress-induced glucocorticoids at the earliest stage of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell functionJ Immunol201018441867187520089700
- El HayderiLRatyLFaillaVCaucanasMPauroballyDNikkelsAFSevere herpes simplex virus type-1 infections after dental proceduresMed Oral Patol Oral Cir Bucal612010 [Epub ahead of print]
- DaviesMEBondiJVGrabowskiLSchofieldTLFieldAK2’-nor-2’deoxyguanosine is an effective therapeutic agent for treatment of experimental herpes keratitisAntiviral Res1987721191253495237
- GordonYJCaponeASheppardJGordonARomanowskiEAraullo-CruzT2’-nor-cGMP, a new cyclic derivative of 2’NDG, inhibits HSV-1 replication in vitro and the mouse keratitis modelCurr Eye Res1987612472533030646
- ShiotaHNaitoTMimuraYAnti-herpes simplex virus (HSV) effect of 9-(1,3-dihydroxy-2-propoxymethyl) guanine (DHPG) in rabbit corneaCurr Eye Res1987612412453030645
- CastelaNVermerieNChastFGanciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: Intraocular penetration and efficacyJ Ocul Pharmacol19941024394518083563
- ColinJHohHBEastyDLGanciclovir ophthalmic gel (Virgan 0.15%) in the treatment of herpes simplex keratitisCornea19971643933999220235
- WilhelmusKRTherapeutic interventions for herpes simplex virus epithelial keratitisCochrane Database Syst Rev20071CD00289817253484
- PouliquenPElenaPPMalecazeFAssessment of the safety and local pharamacokinetics of a 0.15% gel of ganciclovir (Virgan®) in healthy volunteersInvest Ophthalmol Vis Sci199637Suppl 3S313
- ShawMMGürrWKWattsPALittlerEFieldHJGanciclovir and penciclovir, but not acyclovir, induce apoptosis in herpes simplex virus thymidine kinase-transformed baby hamster kidney cellsAntivir Chem Chemother200112317518612959326
- EpsteinRJSeedorJADreizenNGPenetrating keratoplasty for herpes simplex keratitis and keratoconus: Allograft rejection and survivalOphthalmology19879489359443309775
- FickerLAKirknessCMRiceNSSteeleADThe changing management and improved prognosis for corneal grafting in herpes simplex keratitisOphthalmology19899611158715962694048
- SonkinPLBaratzKHFrothinghamRCoboLMAcyclovir-resistant herpes simplex virus keratouveitis after penetrating keratoplastyOphthalmology19929912180518081336155
- RapuanoCJCohenEJBradySEArentsenJJLaibsonPRIndications for and outcomes of repeat penetrating keratoplastyAm J Ophthalmol199010966896952346198
- HohHBHurleyCClaoueCRandomized trial of ganciclovir and acyclovir in the ttreatment of herpes simplex dendritic keratitis: A multicentre studyBr J Ophthalmol19968021401438814744
- TabbaraKFTreatment of herpetic keratitisOphthalmology20051129164016139674
- De KeselRJKoppenCIevenMZeyenTPrimary graft failure caused by herpes simplex virus type 1Cornea200120218719011248827
- TabbaraKFPharmacologic strategies in the prevention and treatment of corneal transplant rejectionInt Ophthalmol200828322323217634865
- LairsonDRBegleyCEReynoldsTFWilhelmusKRPrevention of herpes simplex virus eye disease: A cost effectiveness analysisArch Ophthalmol2003121110811212523894
- AkovaYAOnatMDaumanSEfficacy of low dose and long term oral acyclovir therapy after penetrating keratoplasty for herpes simplex keratitisOcul Immunol Inflamm199971516010410875
- BarronBAGeeLHauckWWHerpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitisOphthalmology199410112187118827997323
- Herpetic Eye Disease Study GroupAcyclovir for the prevention of recurrent herpes simplex virus eye diseaseN Engl J Med199833953003069696640
- Herpetic Eye Disease Study GroupOral acyclovir for herpes simplex virus eye disease: Effect on prevention of epithelial keratitis and stromal keratitisArch Ophthalmol200011881030103610922194
- GoldbergLHKaufmanRHKurtzTOContinuous five-year treatment of patients with frequently recurring genital herpes simplex virus infection with acyclovirJ Med Virol1993Suppl 145508245892
- MertzGJEronLKaufmanRProlonged continuous versus intermittent oral acyclovir treatment in normal adults with frequently recurring genital herpes simplex virus infectionAm J Med1988852A14193044076
- FifeKHCrumpackerCSMertzGJHillELBooneGSRecurrence and resistance patterns of herpes simplex virus following cessation of more than or equals to 6 years of chronic suppression with acyclovir. Acyclovir Study GroupJ Infect Dis19941696133813418195614
- CheeSPJapACytomegalovirus anterior uveitis: outcome of treatmentBr J Ophthalmol2010624 [Epub ahead of print]
- MajumdarSNashedYEPatelKDipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: In vitro and in vivo evaluationsJ Ocul Pharmacol Ther200521646347416386088
- ColinJGanciclovir ophthalmic gel, 0.15%: A valuable tool for treating ocular herpesClin Ophthalmol20071444145319668521
- LabetoulleMThe latest in herpes simplex keratitis therapyJ Fr Ophtalmol2004275547557 [in French]15179314