Abstract
Purpose:
The dietary supplement Mirtogenol® was previously shown to lower elevated intraocular pressure (IOP). We here present the effects of this supplement on IOP in comparison as well as in combination with latanoprost eye drops.
Methods:
Seventy-nine patients with asymptomatic ocular hypertension were randomly assigned to three groups receiving either the supplement, or latanoprost eye drops, or both in combination. Intraocular pressure and retinal blood flow were investigated in monthly intervals over 24 weeks.
Results:
Mirtogenol alone lowered IOP from baseline 38.1 to 29.0 mmHg after 16 weeks, with little further improvement during the following eight weeks. Latanoprost rapidly lowered IOP from baseline 37.7 to 27.2 mmHg within four weeks, without further effects thereafter. The combination of the supplement and latanoprost lowered IOP from 38.0 to 27.3 mmHg after four weeks, and further decreased IOP to 24.2 mmHg after six weeks. After 24 weeks IOP with the combination treatment (23.0 mmHg) was significantly lower than with latanoprost alone (27.2 mmHg). Mirtogenol and latanoprost individually showed comparable effects for gradually increasing central artery blood flow with treatment duration. Combination treatment showed higher systolic blood flow velocity throughout the trial period. The diastolic blood flow velocity gradually increased with treatment duration in all three groups. From twelve weeks onwards, the diastolic component with combination treatment was higher than with individual treatments.
Conclusions:
Mirtogenol lowered elevated IOP in patients almost as effectively as latanoprost, however, it takes much longer (24 vs 4 weeks). The combination of both was more effective for lowering IOP and the combination yielded better retinal blood flow. No serious side effects occurred during the study, apart from standard side effects in patients related to Latanoprost. These promising results warrant further research of Mirtogenol with a larger patient group.
Introduction
Dietary supplementation with Mirtogenol®: a combination of two phenolic extracts from bilberry (Mirtoselect®) (standardized to 36% anthocyanins; USP 31) and French maritime pine bark (Pycnogenol®) (standardized to 70% procyanidins; USP 31), has previously been demonstrated to improve intraocular pressure (IOP) in asymptomatic patients.Citation1 In this previous open, controlled, exploratory pilot trial a significant improvement of ocular blood flow was found after three-month intake of Mirtogenol. The lowered IOP coincided with a significant ocular blood flow improvement of patients and thus the effect on IOP was attributed to this effect. Both Mirtoselect and Pycnogenol have previously been extensively researched in ophthalmology for treatment of diabetic retinopathy.Citation2–Citation4 These studies pointed to a control of capillary leakage and decreased retinal bleedings.
Endothelial dysfunction and vascular structural changes are considered as major contributing factors to altered hemodynamics, elevated IOP, and, eventually, open-angle glaucoma.Citation5 Pycnogenol was shown in human pharmacologic studies to improve endothelial function.Citation6 Mirtoselect was shown to counteract hyperpermeability of ciliary capillaries, initiated by paracentesis, as measured by the Evans Blue concentration in the aqueous humor.Citation7 The initial study on Mirtogenol suggested a significantly increased ocular blood flow and this effect was suggested to be predominantly responsible for the decreased IOP.Citation1
The rationale for the study presented here was to find out how the intake of the dietary supplement affects IOP in patients taking standard eye drop treatment with latanoprost. Since prostaglandin F2α analogs decrease IOP by increasing the drainage of aqueous humor, while Mirtogenol is assumed to act on humor secretion, the possibility exists of an additive and/or synergistic interaction between these two principles. In this study we investigated IOP and ocular hemodynamics in asymptomatic patients presenting with ocular hypertension. They were assigned to three groups for receiving either latanoprost, Mirtogenol, or both, over an investigational period covering 24 weeks.
Methods
Participants for this study were recruited from patients with ocular hypertension diagnosed by ophthalmologists who were sent for a general cardiovascular check-up to the University Hospital of Chieti-Pescara.
Subjects presenting with any cardiovascular diseases requiring medical treatment, and those who had had surgery, radiotherapy or chemotherapy in the last three months were excluded. None of the patients was hypertensive. Subjects who were pregnant, breastfeeding, or planning conception were excluded as well.
Seventy-nine subjects presenting with diagnosed intraocular hypertension (≥35 and ≤40 mmHg) were recruited for this investigation. All subjects had complete eye exams, showing no signs of primary open-angle glaucoma. Their cup-to-disk ratio was lower than 0.5, they had a central corneal thickness greater than 555 μm, and no visual field defects. Subjects with any degenerative eye disorder were excluded. The general cardiovascular examination ensured that patients had no systemic diseases.
All subjects were informed about the aim of the investigation and treatment procedure, according to the Declaration of Helsinki, and gave their written informed consent for participation in this investigation.
Patients were randomly divided into three groups to receive latanoprost, Mirtogenol, or both simultaneously, as detailed in . Patients in this study had ocular hypertension in absence of symptoms. The dietary supplement was previously demonstrated to significantly lower elevated IOP. Nonetheless, it was considered a necessity that a patient’s IOP should improve substantially in response to treatment with the dietary supplement within a given time period of two months. In any case where their IOP did not respond, they were to be given latanoprost in addition. Under these conditions the ethical committee of the University of Chieti Pescara approved the study.
Table 1 Details of patients in the three investigational groups
Mirtogenol was taken as one tablet in the morning. The tablet contained, as active ingredient, 80 mg Mirtoselect® standardized bilberry extract (Indena, Milan, Italy).Citation8 This Vaccinium myrtillus L extract is composed of flavonoids, and standardized to contain 36% anthocyanins, with conformance to the USP 31 on ‘Powdered Bilberry Extract’. Mirtogenol tablets further contain 40 mg French maritime pine bark extract, Pycnogenol (Horphag Research, London, UK),Citation9 which consists of flavan-3-ols standardized to 70% ± 5% procyanidins with conformance to the USP 31 on “Maritime Pine Extract”.Citation6,Citation7 Xalatan® (Pharmacia, Pfizer) was taken one drop per eye daily, equivalent to 1.5 μg latanoprost, in the evening.
The intraocular pressure was always measured in the morning between 9 and 10 a.m. The patient was seated before a slit lamp and Goldmann’s contact applanation tonometer was used. The IOP measurements of a given patient were always performed by the same person to rule out variations from one investigator to another. No drugs were used within two hours before measurements. The patient had been resting, sitting for at least 20 minutes, avoiding ‘rush’ measurements made as soon as the patients arrived into the clinic. The patients had been briefed with the procedures and were familiar with the measurement environment. At each visit the IOP was measured in triplicate, with 10-minute intermissions between measurements, and mean values were recorded.
High resolution color Doppler imaging (Esaote, Genoa, Italy) was used to measure the peak systolic flow velocity, and the end diastolic flow velocity of the central retinal artery, as previously described.Citation10
Data are presented as mean values with standard deviation. Since the distribution of the IOP and the central retinal artery blood flow were not normally distributed, and no standard data was available for these patients, a group of at least 15 subjects in each group was considered a minimal requirement. One-way analysis of the variance (ANOVA) for repeated measurements followed by post hoc Bonferroni tests was used for the intragroup comparisons. A value of P < 0.05 was used as the criterion for statistical significance.
Results
The three groups of subjects were comparable for age:details are presented in . The baseline IOP values were comparable with 37.7 ± 2.0 mmHg in the latanoprost group, 38.1 ± 2.0 mmHg in the Mirtogenol group and 38.0 ± 3.1 mmHg in the group receiving both treatments. None of the patients had been taking latanoprost, or other eye drops for IOP, directly prior to this investigation. The vast majority of the patients had taken latanoprost in the past, but had discontinued at least one month prior to participation in this trial (wash-out period). None of the recruited patients had cataract and there were no cases of pseudophakic eyes present.
For the safety of the patients taking the supplement only, it was planned to give them latanoprost in addition, should their IOP not show satisfactory signs of improvement during the two months. After four weeks of treatment with Mirtogenol a nonsignificant decrease to 34.1 ± 4.0 mmHg was found. After six weeks’ intake of the dietary supplement the IOP decreased significantly compared to baseline, to value 33.3 ± 5.0 mmHg (P < 0.05). As there were no nonresponders, none of the patients treated with the supplement only were transferred to the group taking latanoprost in combination treatment.
Latanoprost showed a significantly faster and more pronounced lowering of IOP than in the group taking Mirtogenol (). Latanoprost had already significantly lowered IOP after four weeks to 27.2 ± 0.9 mmHg (P < 0.05 compared to baseline values). At all time points after trial start the IOP in the latanoprost group was significantly lower than in the group taking the dietary supplement (P <0.05).
Figure 1 The development of intraocular pressure (IOP) in the three groups receiving Mirtogenol, latanoprost or both, respectively, over the investigational period of 24 weeks. Mirtogenol significantly decreased IOP compared to baseline after six weeks and all later time points during the study (P < 0.05). Latanoprost alone, as well as in combination with Mirtogenol, lowered IOP after four weeks, and onwards (P < 0.05).
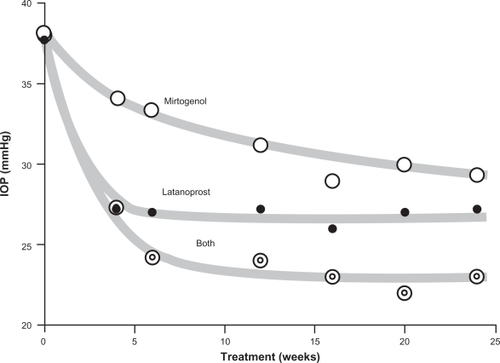
After six weeks of treatment, the combination of latanoprost eye drops together with the dietary supplement decreased the IOP values better than latanoprost alone, or the supplement alone. The IOP values of patients on combination treatment were significantly lower than those of the two groups having individual treatment, at time points 16, 20 and 24 weeks, respectively (P < 0.05).
At baseline the blood flow velocity of the central retinal artery in the latanoprost group was slightly, but nonsignificantly, higher than in the two other groups. In all groups the diastolic and systolic components of the blood flow velocities increased gradually with treatment duration as detailed in and . The supplement and latanoprost contribute in a comparable fashion to improve the central retinal artery blood flow. Only after six weeks was the systolic blood flow velocity in the Mirtogenol group higher than in the latanoprost group. However, the combination of Mirtogenol and latanoprost yielded a better blood flow than both medications taken individually. The diastolic blood flow with the combination treatment was higher than with individual treatments from 16 weeks onwards (P <0.05).
Table 2 The development of the diastolic blood flow velocity in response to treatment, measured using high resolution color duplex ultrasonography
Table 3 The development of the systolic blood flow velocity in response to treatment as established employing high resolution color duplex ultrasonography
Both treatments were well-tolerated with minor transient side-effects resulting from latanoprost. In the latanoprost group, three subjects reported temporary blurred vision, and one patient presented with eyelid redness. In the group treated with latanoprost eye drops in addition to Mirtogenol, two subjects reported blurred vision, and another two suffered conjunctival hyperemia. It is impossible to identify which treatment regimen accounted for these minor side effects, but they are typical for latanoprost.Citation11 In the group taking Mirtogenol alone no side effects occurred. None of the patients in this trial discontinued participation before completion.
Discussion
This study has confirmed the IOP-lowering activity of Mirtogenol, finding a significant activity in dosages lower than those previously reported.Citation1 A vis-à-vis response evaluation was not possible because of differences in the baseline IOP values of patients in the two studies (39 mmHg versus 25 mmHg as mean values), but the higher Mirtogenol dose in the previous study had a faster effect.Citation1 As expected latanoprost relieved ocular hypertension much more rapidly than the dietary supplement. The activity of latanoprost had already reached maximum effect by the time of the four week measurement, and thereafter no further decrease of IOP could be observed. Latanoprost is well described for the rapidity of its effect on IOP, with significant activity detectable within as little as eight hours after a single dose.Citation12 The combined treatment with latanoprost and the supplement significantly decreased IOP after four weeks, an effect predominantly attributed to latanoprost. Interestingly, after six weeks, and at all later time points, the IOP was lower in subjects receiving the combination treatment than in the group taking the eye drops exclusively. After 16 weeks, the IOP values were significantly lower with the combination treatment than with latanoprost alone (P < 0.05).
Comparison of the individual IOP-lowering effects of the supplement and latanoprost with the combination treatment suggests additive affects, not synergistic activities. The additive effects of the supplement and latanoprost point to different pharmacologic activities involved for lowering IOP. Latanoprost has been extensively investigated for its pharmacologic activities.Citation13 The prostaglandin F2α analogues (PGF2α) enhance drainage of aqueous humor, predominantly via the uveoscleral outflow pathway, though significant effects on the trabecular outflow facility have also been reported. The PGF2α are suggested to stimulate remodeling of the extracellular matrix of the ciliary muscle and sclera.
We speculate that the effect of the combination of Mirtoselect and Pycnogenol predominantly affects vascular responses involved in ocular hypertension by normalising capillary filtration of the ciliary body. Pharmacologic studies have demonstrated that Mirtoselect counteracts the hyperpermeability of ciliary capillaries, initiated by paracentesis, as measured by the Evans Blue concentration in the aqueous humor.Citation7,Citation14 Pycnogenol was shown to improve endothelial functionCitation6 and to lower blood pressure in asymptomatic hypertension.Citation15 The improved ocular blood flow shown in this study supports the assumption that the dietary supplement may exert an action on lowering IOP by decreasing humor inflow. Yet, it will remain difficult to identify whether the supplement affects outflow pathways, or aqueous humor inflow, or both.
There is growing evidence that decreased endothelial function is the primary cause of age-related deterioration of ocular hemodynamics leading to glaucoma.Citation5 In this study, an improved blood flow velocity of the central retinal artery was again shown for the supplement, but also found in the latanoprost group. Interestingly, this effect could be demonstrated earlier in the supplement group than in the latanoprost group. After six weeks’ treatment, the systolic blood flow rate was significantly higher with the supplement than with the eye drops. A measurable, yet nonsignificant, increase of central retinal blood flow was described for latanoprost in normotensive glaucoma patients after one month of treatment.Citation16 However, the authors argue that the improved ocular hemodynamics may be secondary to the reduction of intraocular pressure. This might explain the increased blood flow velocity in this study, for patients treated with latanoprost. Indeed, the same explanation might underlie the improved hemodynamics observed with the supplement, and with its combination with latanoprost. Our study has a limitation resulting from the use of color Doppler imaging (CDI). As it is impossible to determine the diameter of orbital vessels in vivo, CDI cannot reflect the blood volume. However, other groups have described a correlation between blood flow velocity and vascular blood volume.Citation17 Further research will be required to draw conclusions about the pharmacologic activities of Mirtogenol as a single agent, as well as in combination with latanoprost.
A major advantage of the investigated dietary supplement may be the safe, nutritional approach for preventing the development of ocular hypertension. This, in turn, would decrease the risk of having primary open angle glaucoma later in life.Citation18 Latanoprost and related prostaglandin F2α represent a valuable tool to treat intraocular hypertension, and inhibit its progression to glaucoma, but are unsuitable as preventative agents, as latanoprost was shown to decrease IOP below physiological levels.Citation19 Furthermore, apart from irreversible iris pigmentation and abnormal growth and darkening of eyelashes, latanoprost seems to have also less-common but more-serious side-effects, like the induction of iris cysts, cystoid macular edema and anterior uveitis.Citation10 Conversely, serious side-effects have never been reported for Mirtoselect® and Pycnogenol®, despite their decades-old use in ophthalmology, predominantly for diabetic retinopathy.Citation2–Citation4 While the supplement does not represent a replacement for prostaglandin F2α analogs, taking the supplement in addition, appears to be safe, and may further contribute to the attainment of healthier IOP values.
The results obtained from this, and the previous pilot trial, with the dietary supplement Mirtogenol on IOP, appear to be very promising. A much larger study with a significant number of patients should further assess the benefits of the supplement for controlling IOP.
Acknowledgments/Disclosures
The authors report no conflicts of interest in this work. All subjects were informed about the aim of the investigation and treatment procedure, according to the Declaration of Helsinki, and gave their written informed consent for participation in this investigation. The study was approved by the ethical committee of the University of Chieti-Pescara. This study was supported by a grant from Indena S.p.A. Italy and Horphag Research UK Ltd.
References
- SteigerwaltRDBelcaroGPaoloMBombardelliEBurkiCSchönlauFEffects of Mirtogenol on ocular blood flow and intraocular hypertension in asymptomatic subjectsMol Vis2008141288129218618008
- SchönlauFRohdewaldPPycnogenol® for diabetic retinopathy. A reviewInt Ophthal200224161171
- PerossiniMGuidiGChielliniSSiravoDDiabetic and hypertensive retinopathy therapy with Vaccinium myrtillus anthocyanosides (Tegens®): Double blind placebo-controlled clinical trialAnn Ottal Clin Ocul198711311731190
- RepossiPMalagolaRDe CadilhacCThe role of anthocyanosides on vascular permeability in diabetic retinopathyAnn Ottal Clin Ocul1987113357361
- EhrlichRKheradiyaNSWinstonDMMooreDBWirostkoBHarrisAAge-related ocular vascular changesGraefes Arch Clin Exp Ophthalmol200924758359119084984
- NishiokaKHidakaTTakemotoHPycnogenol®, French maritime pine bark extract, augments endothelium-dependent vasodilation in humansHypertens Res20073077578018037769
- VirnoMPecori GiraldiJAuriemmaLAntocianosidi di mirtillo e permeabilità dei vasi del corpo ciliareBoll Ocul198665789795
- CassineseCde CombarieuEFalzoniMFuzzatiNPaceRSardoneNNew liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparationsJ AOAC Int20079091191917760327
- RohdewaldPA review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacologyInt J Clin Pharmacol Ther20024015816811996210
- SteigerwaltRDJrLauroraGBelcaroGVOcular and retrobulbar blood flow in ocular hypertensives treated with topical timolol, betaxolol and carteololJ Ocul Pharmacol Ther20011753754411777177
- AlmAGriersonIShieldsMBSide-effects associated with prostaglandin analog therapySurv Ophthalmol200853Suppl 1S9310519038628
- AkarsuCBilgiliYKTanerPUnalBErginAShort-term effect of latanoprost on ocular circulation in ocular hypertensionClin Experiment Ophthalmol20043237337715281970
- TorisCBGabeltBTKaufmanPLUpdate on the mechanism of action of topical prostaglandins for intraocular pressure reductionSurv Ophthalmol200853Suppl 1S10712019038618
- MorazzoniPBombardelliEVaccinium myrtillus LFitoterapia199667329
- HosseiniSLeeJSepulvedaRTFaganTRohdewaldPWatsonRRA randomized, double blind, placebo controlled, prospective, 16 week crossover study to determine the role of Pycnogenol® in modifying blood pressure in mildly hypertensive patientsNutr Res2001216776
- ZeitzOMatthiessenETReussJEffects of glaucoma drugs on ocular hemodynamics in normal tension glaucoma: a randomized trial comparing bimatoprost and latanoprost with dorzolamideBMC Ophthalmol20055615811188
- TaylorGAShortBLWalkerLKIntracranial blood flow: quantification with duplex Doppler and color Doppler flow USRadiology19901762312112768
- ColemanALMigliorSRisk factors for glaucoma onset and progressionSurv Ophthalmol200853Suppl 1S3S1019038621
- KjellgrenDDouglasGMikelbergFSDranceSMAlmAThe short-time effect of latanoprost on the intraocular pressure in normal pressure glaucomaActa Ophthalmol Scand1995732332367493234