Abstract
Radioiodine (RAI) ablation following thyroidectomy is standard of care treatment for patients with intermediate or high risk differentiated thyroid cancer. Traditionally, this has been achieved by forgoing thyroid hormone replacement postoperatively, allowing endogenous thyroid stimulating hormone (TSH) levels to rise. This rise in TSH provides the stimulus for RAI uptake by the thyroid remnant, but is associated with clinical hypothyroidism and its associated morbidities. Recombinant human TSH (rhTSH, thyrotropin alfa [Thyrogen®], Genzyme Corporation, Cambridge, MA, USA) was developed to provide TSH stimulation without withdrawal of thyroid hormone and clinical hypothyroidism. Phase III studies reported equivalent detection of recurrent or residual disease when rhTSH was used compared with thyroid hormone withdrawal (THW). These trials led to its approval as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without RAI imaging in the surveillance of patients with differentiated thyroid cancer. Recently, rhTSH was given an indication for adjunctive preparation for thyroid remnant ablation after phase III studies demonstrated comparable outcomes for rhTSH preparation when compared with THW. Importantly, rhTSH stimulation has been found to be safe, well tolerated, and to result in improved quality of life. Here, we review the efficacy and tolerability studies leading to the approval for the use of rhTSH in well-differentiated thyroid cancer management.
Introduction
The use of exogenous thyroid stimulating hormone (TSH) in the evaluation and management of thyroid cancer dates back over 50 years with the use of bovine TSH to stimulate iodine uptake for radioiodine (RAI) diagnostic studies and therapy. Although use of bovine TSH was eventually abandoned because of allergic reactions, the development of recombinant human TSH (rhTSH, thyrotropin alfa [Thyrogen®], Genzyme Corporation, Cambridge, MA, USA) has dramatically changed the paradigm for the management and follow-up of patients with thyroid cancer. This review will introduce issues in the management of patients with thyroid cancer, and review the pharmacology, mode of action, and method of administration of rhTSH. We will briefly review studies of the efficacy of rhTSH in diagnostic evaluation of thyroid cancer, and then focus the bulk of the review on studies of the efficacy of rhTSH-stimulated RAI therapy for thyroid remnant ablation and treatment of metastases. Finally, we review the safety, tolerability and impact on patients’ quality of life of rhTSH preparation compared with thyroid hormone withdrawal (THW).
Management issues in thyroid cancer and use of radioiodine
Radioiodine is an important component of differentiated thyroid cancer treatment and surveillance. It has three main roles: 1) Ablation of residual normal thyroid tissue, 2) Diagnostic scanning to detect residual/recurrent disease and 3) Treatment of residual/recurrent disease. Radioiodine administration following thyroidectomy (“remnant ablation”) is performed to reduce the risk of thyroid cancer recurrence and improve the accuracy of surveillance strategies. The two goals of treatment are to destroy micrometastatic or residual disease, and ablate remaining normal thyroid tissue to facilitate RAI scanning and use of Tg as a tumor marker. No prospective studies have been done to address the question of which patients benefit from this treatment strategy. However, based on large retrospective series, the published consensus guidelines from both the European Thyroid Association (ETA) and American Thyroid Association (ATA) recommend RAI ablation for patients with higher stage disease and recommend considering ablation in lower risk patients with tumors larger than 1 or 1.5 cm (CitationCooper et al 2006; CitationPacini et al 2006b).
The use of RAI for all three purposes relies on the ability of both normal and malignant thyroid tissue to transport iodine for synthesis of thyroglobulin, triiodothyronine and thyroxine in response to TSH. An overview of the procedures for thyroid remnant ablation and diagnostic scanning are shown in and , respectively. TSH stimulation historically has been achieved by discontinuation of thyroid hormone replacement for 5 to 6 weeks, (thyroid hormone withdrawal, THW), allowing endogenous TSH levels to rise (). The optimal TSH level for ablation is felt to be >30 mU/L, based on a study that demonstrated that low TSH levels were more likely to be associated with low iodine uptake, which was more robust on reevaluation with a higher TSH (CitationEdmonds et al 1977). In addition to high TSH levels, iodine depletion, such as with a low iodine diet for 1 to 2 weeks, is important for optimal RAI uptake. If desired to assist in treatment decisions, diagnostic RAI whole body scans (WBS) are performed with a tracer dose of 74 to 185 MBq (2–5 mCi) 131I and the diagnostic scan obtained 48 to 72 hours later as shown in . Radioiodine at a dose of 1.1 to 3.7 GBq (30–100 mCi) is administered for remnant ablation, with higher doses if residual disease is known or suspected or the tumor has unfavorable histology or if treatment of metastatic disease is planned. The patient is placed back on levothyroxine suppressive therapy, as appropriate, and approximately 1 week after the treatment dose of RAI, a post-therapy WBS is performed to better assess for residual or metastatic disease. Substitution of rhTSH stimulation for TSH stimulation by THW for diagnostic evaluations (shown in and reviewed by CitationCooper et al (2006) or for thyroid remnant ablation (shown in ), has been published by a number of centers, allowing patients to remain on levothyroxine therapy and avoid the symptoms of hypothyroidism (CitationRobbins et al 2001; CitationPacini et al 2002; CitationRobbins et al 2002b; CitationBarbaro et al 2003; CitationBarbaro et al 2006; CitationPacini et al 2006a; CitationPilli et al 2007; CitationRosario et al 2008; CitationTaieb et al 2008; CitationTuttle et al 2008). Over the last 3 years, thyrotropin alfa (Thyrogen®) has been approved for RAI ablation by the United States Food and Drug Administration (FDA, 2007), in Europe (2005), and in certain Asian (2007) and South American countries (2006). Further discussion of the efficacy of rhTSH-stimulated remnant ablation follows in a subsequent section. Pertinent issues include the utility and logistics of the diagnostic preablation scan, selection of the RAI therapy dose, and the extent of iodine depletion regimens.
Figure 1 Schedules for radioiodine ablation.
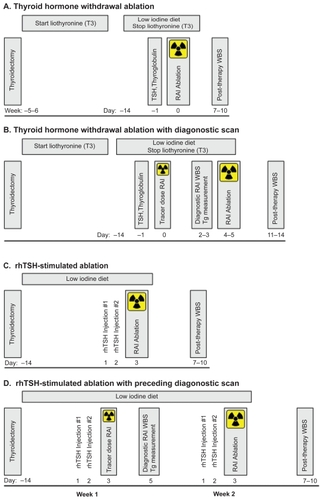
Figure 2 Schedules for diagnostic radioiodine whole body scan and thyroglobulin determonation.
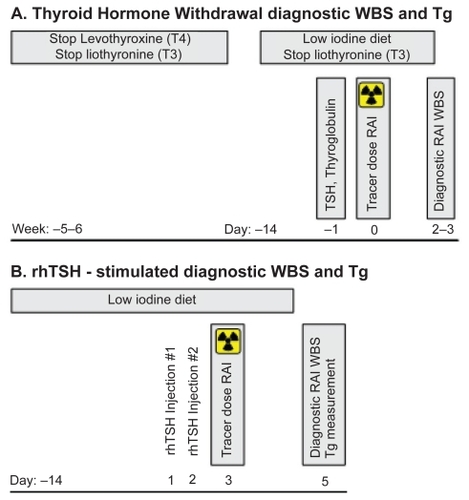
Traditionally, follow-up surveillance RAI WBS and stimulated thyroglobulin (Tg) measurements () are performed every 12 months after remnant ablation for several years and then periodically. If distant metastases are discovered during follow-up, patients may be incurable but can be retreated with various modalities (surgery, RAI) to reduce the tumor burden and provide a possible survival benefit.
Thyrotropin pharmacology and mode of action
Thyrotropin alfa is a synthetic recombinant human thyroid stimulating hormone (rhTSH) produced in Chinese hamster ovary cells. Although they have the same amino acid sequence, rhTSH differs from TSH synthesized endogenously by the human pituitary gland, in that rhTSH is sialylated but not sulfated, whereas endogenous TSH is a mixture of both glycosylation forms. Like the endogenous form, rhTSH binds to TSH receptors on normal thyroid follicular cells or well-differentiated thyroid cancer cells. RhTSH acts via the TSH receptor, a seven transmembrance G-protein coupled receptor, to activate the adenylate cyclase and phosphatidylinositol signaling pathways. TSH induces thyroid growth and thyroid hormone synthesis and secretion (CitationDumont et al 1992). It stimulates many of the steps of thyroid hormone synthesis including uptake of iodide into the thyroid follicular cell and subsequent organification of iodine at the thyroid follicular lumen. TSH also stimulates Tg gene expression and thyroid hormone secretion, involving pinocytosis of stored Tg into the thyroid follicular cell and limited lysosomal proteolysis to release T4, T3 and Tg. Because of the differences in glycosylation, rhTSH has a lower affinity for and bioactivity at the TSH receptor (CitationSzkudlinski et al 1993), but also a significantly longer elimination half-life.
Each vial of thyrotropin alfa contains 1.1 mg of rhTSH estimated by in vitro studies to contain 4 to 12 IU/mg. Immediately prior to use, it is reconstituted in 1.2 mL of sterile water producing a solution with a concentration of 0.9 mg/mL (3.6–10.8 IU). Two doses of thyrotropin alfa 0.9 mg (1.0 mL) are administered by intramuscular injection to the buttock, approximately 24 hours apart. For RAI imaging or remnant ablation, RAI is administered 24 hours after the second rhTSH dose and for diagnostic purposes, Tg levels should be measured 72 hours after the last dose (Thyrogen® package insert, Genzyme Corporation, and ). Subcutaneous injection has been studied in a limited number of patients (CitationTaieb et al 2004) and is an alternative for patients in whom intramuscular injections are contraindicated.
Pharmacokinetics parameters of rhTSH administration were evaluated in a phase I/II study of 19 patients with well-differentiated thyroid cancer on suppressive levothyroxine doses. Patients were given a variety of doses ranging from 10 to 40 IU injected IM for 1 to 3 days, and RAI uptake and Tg levels were compared with those after THW. Mean peak TSH concentrations occurred 2 to 8 hours after each injection; after the 10 IU dose, peak mean TSH was 127 ± 19 mU/L and elevated TSH persisted for at least 48 hours after the dose (CitationMeier et al 1994). The mean apparent elimination half-life was 25 ± 10 hours (Thyrogen® package insert, Genzyme Corporation). This and subsequent pharmacodynamic studies assessing the diagnostic use of rhTSH in thyroid cancer, showed that the lower dose of 10 IU was essentially as efficacious and had fewer side effects than higher doses of 30 or 40 IU and that a regimen of 0.9 mg doses two days in a row results in 3–4 days of serum TSH concentration greater than or equal to 25 mU/L (CitationLadenson et al 1997; CitationHaugen et al 1999).
Studies evaluating other factors affecting the pharmacokinetics have subsequently been done, with some showing that peak TSH levels in adults are inversely related to body weight (CitationZanotti-Fregonara et al 2007), body mass index and body surface area (CitationVitale et al 2003) but others showing no such relationship (CitationMontesano et al 2007) and one study demonstrating a positive relationship with age (CitationMontesano et al 2007). However, the clinical relevance of such variations has not been demonstrated and no dose adjustment is recommended based on body weight or age. Although not FDA approved for use in children, studies in children report similar TSH levels to adults, suggesting that dose adjustment is also not needed in this situation (CitationIorcansky et al 2005; CitationHoe et al 2006).
rhTSH in the diagnostic evaluation of thyroid cancer
Phase I/II studies demonstrated that rhTSH stimulated iodine uptake into thyroid cancer tissue and Tg production (CitationMeier et al 1994), and two phase III studies demonstrated efficacy for identification of residual/recurrent disease by RAI WBS (CitationLadenson et al 1997; CitationHaugen et al 1999). In the first phase III study of 127 patients, rhTSH resulted in equivalent or superior RAI WBS performed after 78 to 148 MBq (2–4 mCi) 131I in 86% of evaluations compared with THW. However, the rhTSH-stimulated scans were more frequently poorly visualized, thought to be due to more rapid renal clearance of iodine in the euthyroid state (CitationLadenson et al 1997). Out of 35 patients evaluated for a Tg response, 15 demonstrated a rise to >5 ng/mL after either rhTSH (13/15) or THW (14/15). Thyroglobulin levels peaked at 72 to 96 hours after the first dose of rhTSH.
The second phase III study of 229 patients compared two dosing regimens, utilized a higher RAI tracer dose (148 MBq (4 mCi) 131I in all patients) and measured Tg and thryoglobulin antibodies in all patients (CitationHaugen et al 1999). Considering rhTSH RAI scans in both arms, 93% of 220 evaluable scans after rhTSH were concordant with or superior to those done under THW (p = 0.108). Further, among patients with no interfering Tg antibodies and using a cut-off of 2 ng/mL, there was 100% sensitivity of a rhTSH-stimulated Tg to detect cervical lymph node and distant metastases and 52% sensitivity to detect residual thyroid remnant (similar to THW sensitivity of 56%). This study also demonstrated that the efficacy with a two injection regimen was equivalent to a regimen of three injections over 9 days. It was on the basis of these studies that Thyrogen® achieved FDA approval as an adjunctive diagnostic tool for Tg testing with or without RAI scanning in evaluation of patients with thyroid cancer.
Reproducibility of rhTSH testing has been evaluated in only one study of 23 patients with no evidence of disease by RAI scanning, Tg measurements or clinical evaluation. (CitationNiederkohr and McDougall 2007) The time interval between the two rhTSH-stimulated evaluations was 41 months. Peak serum TSH was >50 mU/L in all evaluations and >100 mU/L in 73% of studies. There was good correlation of the 48 hour RAI uptake between the two studies (r = 0.85, p = 0.0001), peak serum TSH (r = 0.69, p = 0.0003) and Tg (r = 0.81, p < 0.0001). However, since most evaluations showed very low RAI uptake and low Tg levels (mean 2.05 ng/mL), it is difficult to extrapolate these results to patients with positive evaluations.
Recognition of the ability of rhTSH-stimulated Tg measurement to identify residual/recurrent thyroid cancer with high accuracy and the potential for avoiding the morbidity of hypothyroidism associated with THW has led to a shift in the paradigm for the follow-up evaluation of patients with thyroid cancer. It is now clear that stimulated TG levels (usually combined with a neck ultrasound) represent the most sensitive means of detecting residual or recurrent thyroid cancer. Specifically, in patients where Tg measurement was possible (eg, those lacking antithyroglobulin antibodies), the accuracy of rhTSH-stimulated Tg evaluation met or exceeded that of rhTSH-stimulated or even THW RAI WBS (CitationCailleux et al 2000; CitationMazzaferri et al 2003; CitationPacini et al 2003; CitationTorlontano et al 2003; CitationSchlumberger et al 2004). Additionally, several studies demonstrated that in low risk patients, if the first rhTSH-stimulated Tg was undetectable, the likelihood of thyroid cancer recurrence over 2 to 4 years was exceedingly small (CitationCailleux et al 2000; CitationBaudin et al 2003; CitationCastagna et al 2008; CitationCrocetti et al 2008). Based on this, the ATA and ETA guidelines recommend against RAI WBS in the routine follow-up of low-risk patients without evidence of disease (clinically and based on unstimulated Tg or ultrasound). Rather, unstimulated Tg and neck ultrasonography are routine in follow-up, with further stimulated Tg measurements and additional evaluation if indicated. Recently, highly sensitive (eg, functional sensitivity of 0.1 ng/mL) unstimulated Tg measurements have been reported to be equally predictive of disease (CitationIervasi et al 2007; CitationSmallridge et al 2007), albeit in small cohorts and with only short follow-up. As the reliability and long-term predictive value of highly sensitive Tg measurements improves, this may eventually supplant stimulated Tg measurements for thyroid cancer surveillance.
One additional aspect to be considered in the evaluation of patients with thyroid cancer is the utility of rhTSH in patients undergoing 18-fluorodeoxyglucose (18-FDG) positron emission tomography (PET) scanning. Detection of lesions by PET scanning is often complementary to that of RAI scanning, with more aggressive lesions having lost iodine avidity but demonstrating significant glucose uptake. In addition, PET scanning has a role in predicting the biologic behavior of lesions, with mortality closely related to volume of PET+ disease (CitationWang et al 2000; CitationRobbins et al 2006b) which may influence therapeutic decisions. Several studies have demonstrated enhanced lesion detection, SUV levels and reduced background in PET scans performed after rhTSH stimulation, reviewed recently by CitationLeboulleux et al (2007), presumably due to increased GLUT1 synthesis and glucose transport stimulated by TSH action. However, the utility of the incremental improvement in PET performance with rhTSH stimulation for affecting clinical decision making is not clear.
rhTSH for thyroid remnant ablation
The literature reflects a growing experience with rhTSH for thyroid remnant ablation, summarized in , which details efficacy results from studies that included 10 or more patients. Studies evaluating rhTSH for thyroid remnant ablation include retrospective series (with historical or nonrandomized THW controls), prospective consecutively enrolling studies (CitationRobbins et al 2001; CitationPacini et al 2002; CitationRobbins et al 2002b; CitationBarbaro et al 2003; CitationBarbaro et al 2006; CitationPilli et al 2007; CitationRosario et al 2008; CitationTuttle et al 2008) and two randomized trials which compared the efficacy and safety of rhTSH stimulation during levothyroxine treatment with conventional THW remnant ablation (CitationPacini et al 2006a; CitationTaieb et al 2008). Only one of the randomized studies and one uncontrolled study have reported clinical follow-up beyond one year (CitationElisei et al 2007; CitationTuttle et al 2008). Most studies utilized the schedule of rhTSH administration illustrated in .
Table 1 Studies evaluating the effectiveness of rhTSH or thyroid hormone withdrawal preparation for radioiodine ablation of thyroid remants
One of the randomized trials (CitationTaieb et al 2008) compared subjects who all received 3.7 GBq (100 mCi) 131I after rhTSH preparation or THW. Subjects received levothyroxine therapy for 1 week and then were randomized to either rhTSH (n = 36) or THW (n = 35) preparation for RAI ablation. For patients randomized to rhTSH preparation, levothyroxine was continued and rhTSH 0.9 mg was administered intramuscularly on 2 consecutive days. Twenty-four hours after the final rhTSH injection, the ablative dose of RAI was administered. Patients randomized to THW discontinued levothyroxine for 5 weeks, then received the RAI dose. Ablation outcome was evaluated at 9 months post ablation with a rhTSH-stimulated RAI scan and Tg measurement and neck ultrasonography. No uptake was visible on scans in 72% of patients prepared by rhTSH stimulation and 91% of those prepared by THW (p = 0.04). However, most of the patients had a trivial amount of uptake, with 95% of all rhTSH stimulated and 100% of THW prepared patients having a RAI scan with <0.1% uptake (p = 0.49). A third criteria for ablation success was the rhTSH-stimulated Tg measurement, which was <0.8 ng/mL in 92% of rhTSH-stimulated ablations and 97% of hypothyroid ablations (p = 0.61).
The other randomized trial (CitationPacini et al 2006a) also compared subjects who all received 3.7 GBq (100 mCi) 131I after TSH stimulation preparation by rhTSH or THW. In this study, 33 patients were randomized to the euthyroid (rhTSH) group and received levothyroxine therapy for 4 to 6 weeks until their TSH was 5 mU/L or less. RhTSH 0.9 mg was then given on 2 consecutive days, and 24 hours after the final injection, the ablative dose of RAI was administered. Thirty patients were randomized to the hypothyroid group and had levothyroxine replacement withheld, and after 4 to 6 weeks when the TSH was least 25 mU/L, they then received the ablative RAI dose. On a rhTSH-stimulated RAI scan 8 months after treatment, 24 of 32 evaluable euthyroid patients (75%) and 24 of 28 hypothyroid evaluable patients (86%) had no visible uptake (p = 0.3). Using a level of rhTSH-stimulated serum Tg less than 2 ng/mL at the 8-month point as a measure of successful ablation, 23 of 24 evaluable euthyroid patients (96%) and 18 of 21 evaluable hypothyroid patients (85%) were successfully ablated (p = 0.2).
In this same study, 61 of these patients were followed for a median of 3.7 years after their ablation. During that time, 7 patients had additional therapy (surgery or RAI), 3 in the rhTSH ablated and 4 in the hypothyroid group. Follow-up evaluations revealed equivalent numbers of patients with “visible” (but <0.1%) uptake on RAI WBS and positive (>2 ng/mL) stimulated Tg measurements (1 in each group) (CitationElisei et al 2007).
The only other study reporting outcome data beyond one year is that of a large series of 394 patients treated with either rhTSH (n = 320) or THW (n = 74) preparation at Memorial Sloan Kettering Cancer Center (CitationTuttle et al 2008). In this series, the choice of preparation was uncontrolled, and patients underwent ablation with the RAI dose determined according to clinical features, histologic findings, intraoperative findings, risk of recurrence and results of a diagnostic I-123 WBS, regardless of method of preparation (rhTSH or THW). Ablative RAI doses ranged from 2.7–3.7 GBq (75–100 mCi) 131I for intrathyroidal papillary thyroid carcinoma, 3.7 to 5.5 GBq (100–150 mCi) for patients with cervical lymph node metastases and >5.5 GBq (150 mCi) if locally aggressive disease or known distant metastases were present. Patients who underwent rhTSH-stimulated ablation received a median of 109 mCi compared with THW patients who received a median of 103 mCi 131I (p < 0.01), median age was slightly higher in the rhTSH group (46.5 years vs 44.0 years, p = 0.03) and median follow-up duration was shorter (27 months vs 45 months, p < 0.001); other characteristics of the patients were not different between the groups. Ablation success was evaluated in 291 of the subjects who underwent a follow-up rhTSH-stimulated RAI WBS at 12 to 18 months. Successful ablation defined as no visible uptake on the RAI WBS was achieved in 83% of rhTSH stimulated ablations and 76% of THW prepared patients. Using a definition of <0.1% uptake, successful ablation was achieved in 95% of those undergoing rhTSH ablation and in 90% of THW prepared patients (p = 0.35). Suppressed and stimulated Tg levels were not different between the groups at the 12 to 18 month follow-up; 68.6% of rhTSH-prepared patients had a stimulated Tg < 2 ng/mL compared with 61.9% of THW prepared patients (p-value not significant, Tuttle, personal communication).
Follow-up of this cohort was for a median of 2.5 years and the authors reported four clinical outcomes: no clinical evidence of disease (NCED: negative rhTSH WBS, no clinical recurrence, suppressed Tg < 2 ng/mL and stimulated <10 ng/mL), clinical recurrence (new disease after a period of NCED), persistent disease (suppressed Tg > 2 ng/mL or stimulated >10 ng/mL 1 year after ablation, persistent anatomic disease, new metastasis within six months of ablation or known distant metastases at diagnosis) and thyroid bed uptake only (persistent at 12- to 18-month follow-up with no other Tg or ultrasonographic evidence of disease). There were more treatment failures in patients prepared by THW (p = 0.02), but equivalent numbers of patients in the two groups when considering only patients without distant metastases. This lends reassurance that the initial favorable response to rhTSH-stimulated remnant ablation is durable.
In summary, data on almost 600 patients have been reported in several uncontrolled studies and two relatively small randomized controlled trials, demonstrating that rhTSH stimulation for RAI thyroid remnant ablation is successful in about 80% to 90% of patients, comparable to rates seen with THW preparation. In December of 2007, the FDA approved Thyrogen® for use “as an adjunctive treatment for RAI ablation of thyroid tissue remnants in patients who have undergone a near-total or total thyroidectomy for well-differentiated thyroid cancer and who do not have evidence of metastatic thyroid cancer.” It should be noted, that the patients who participated in these studies were generally patients with low stage disease, and because of concern about lower RAI uptake from early studies with rhTSH, care should be taken when evaluating patients for selection for rhTSH stimulated ablation.
Optimal radioiodine dose and other factors important for successful thyroid remnant ablation under rhTSH stimulation
The issue of the minimum adequate dose required for remnant ablation has been a controversial one even prior to the use of rhTSH preparation. The efficacy of doses lower than 3.7 GBq (100 mCi) 131I for ablation of thyroid remnants with rhTSH preparation has been examined in four studies and is compared to that reported in studies using greater than 3.7 GBq (100 mCi) 131I, all of which are summarized in . One of these was a randomized trial that compared 1.9 GBq (50 mCi) with 3.7 GBq (100 mCi) (CitationPilli et al 2007). This study as well as 2 of the 3 uncontrolled studies utilizing 1.1 GBq (30 mCi) (CitationPacini et al 2002; CitationBarbaro et al 2003; CitationBarbaro et al 2006) reported 77% to 89% ablation rates, defined as no visible uptake on a WBS, similar to the 6 studies administering 3.7 GBq (100 mCi) or more which had ablation rates from 72% to 89% (CitationRobbins et al 2002b; CitationPacini et al 2006a; CitationRosario et al 2008; CitationTaieb et al 2008; CitationTuttle et al 2008). The third uncontrolled study of 1.1 GBq (30 mCi) found a lower ablation rate (54%); however, the investigators utilized a different schedule of rhTSH administration, which may have compromised iodine uptake.
Table 2 Studies evaluating the effective dose of 131I for rhTSH-stimulated thyroid remnant ablation
Compared with THW, renal clearance of RAI is not reduced when rhTSH is used. For this reason, the use of a low iodine diet to deplete iodine stores and/or evaluation of urinary iodine excretion has been routinely done in these studies. An additional source of iodine is that contained in thyroid hormone replacement preparations. Therefore, the impact of continuing thyroid hormone preparations on the efficacy of rhTSH ablation has also been studied (CitationBarbaro et al 2003). This study demonstrated that omission of levothyroxine for 4 days reduced urinary iodine, but urinary iodine was not different in another study between hypothyroid and euthyroid subjects where levothyroxine was continued (CitationPacini et al 2006a). Further, in these studies, it did not appear that discontinuing thyroid hormone improved ablation rates compared to studies that allowed patients to continue their thyroid hormone replacement. However, no study has evaluated this question in a single study in a randomized fashion.
In summary, the limited data available suggests that doses of RAI less than 3.7 GBq (100 mCi) 131I may indeed be as efficacious as higher doses. Short term discontinuation of thyroid hormone preparations does not appears to improve ablation rates, but may warrant evaluation in additional patient populations in a randomized study. Finally, the timing of RAI administration in relation to rhTSH stimulation may be a critical factor.
Logistic issues with rhTSH stimulated thyroid remnant ablation
The studies summarized above generally used a standard schedule of administering rhTSH on days 1 and 2, then giving the ablative RAI on day 3 when TSH levels are at their peak, which should be optimal for RAI uptake into the thyroid gland. However, this schedule is problematic if one needs to obtain a diagnostic scan before administering the therapeutic RAI dose. When a diagnostic scan is indicated to assist in ablative dosage selection or as a baseline for comparison to future scans, various schedules have been proposed to circumvent this problem. These include administering a tracer dose of 123I (which has a shorter half-life) on day 3, which allows an 123I diagnostic scan to be performed prior to an 131I ablation dose given the same day. Alternatively some clinicians perform either an 123I or 131I scan on days 3 and 4, and delay administration of the ablative 131I dose until day 4, 2 days after the last rhTSH dose. This has the disadvantage of delaying RAI therapy until day 4 when TSH levels have decreased significantly, thereby potentially lowering treatment efficacy. Only one study has specifically reported ablation rates with this schedule and found that only 54% of patients were ablated when given 1.1 GBq (30 mCi) 131I on day 4 after rhTSH (CitationPacini et al 2002). Whether this lower rate was related to the lower RAI dose or the later RAI administration is not clear, however, other studies with 1.1 GBq (30 mCi) 131I (including studies from the same group) have significantly higher ablation rates (see previous section and for details). The value of the diagnostic scan prior to ablation has been questioned and 1 study has demonstrated that the diagnostic scan rarely assists in managing the patient (CitationMandac et al 2008). Most providers feel the value of the diagnostic scan is minimal and perform only a post-therapy scan approximately 1 week after the ablative dose. Alternatively, if the diagnostic scan is felt to be critical to decision-making, a second course of rhTSH stimulation can be administered the week following the diagnostic scan, ensuring adequate TSH stimulation for remnant ablation (as show in ).
A second logistic issue with rhTSH stimulated ablation is the timing of the measurement of the rhTSH-stimulated Tg levels. Several studies have shown the predictive value of the post-thyroidectomy, preablative stimulated Tg level (CitationGrunwald et al 1996; CitationToubeau et al 2004; CitationKim et al 2005). However, pharmacodynamic studies of rhTSH for diagnostic uses demonstrated that the peak Tg level in response to rhTSH occurred on day 5 after the 2 rhTSH injections (CitationLadenson et al 1997; CitationHaugen et al 1999). This is after the ablative dose of RAI, which could impact Tg levels on the basis of radiation-induced tumor lysis. One study examined the effect of this intervening ablative dose of RAI on Tg levels and found that Tg levels increased after RAI therapy due to acute radiation effects (CitationTaieb et al 2006). Because of the two simultaneous stimuli to Tg levels, rhTSH and RAI tumor destruction, this paradigm would not result in Tg levels that would be comparable to values obtained in diagnostic settings. Other clinicians draw a Tg on day 3, just before the ablative RAI dose, but this is less predictive because of the difference in timing of the Tg level in relationship to rhTSH. Further studies will be needed to determine if this measurement has any clinical utility.
Utility of rhTSH for radioiodine treatment of metastatic thyroid cancer
The first report of RAI treatment of metastatic thyroid cancer using rhTSH stimulation dates back to 1997 (CitationRudavsky and Freeman 1997). Concern about the efficacy of this approach stems from observations of lower apparent RAI uptake observed in the early phase diagnostic studies, thought to be due to more rapid iodine clearance in euthyroid patients (CitationPark et al 1996; CitationLadenson et al 1997). However, the impact of this biologic measure on the ultimate clinical outcome, effective treatment of the metastatic deposits, is less clear. Although lower iodine uptake has been shown to lack clinical significance for thyroid remnant ablation, described in the previous section, it may be more relevant in treating metastatic disease due to the impaired iodine uptake and organification observed in carcinomatous tissue compared with normal thyroid tissue (CitationSchlesinger et al 1989). Thyrogen® is FDA approved as an adjunct to RAI treatment of metastatic thyroid cancer only in patients who are either unable to mount an adequate endogenous TSH response to THW or in whom THW is medically contraindicated, as options are otherwise limited in that patient population. Although there are no randomized trials comparing rhTSH stimulated RAI treatment to THW prepration, data from this “compassionate use” population and other case series (CitationLuster et al 2000; CitationMariani et al 2000; CitationLippi et al 2001; CitationPellegriti et al 2001; CitationBerg et al 2002; Citationde Keizer et al 2003; CitationJarzab et al 2003; CitationRobbins et al 2006a), summarized in , provide some information about the effectiveness of this approach. Unfortunately, the literature is heterogeneous and limited in particular by the lack of outcome data. Further, the interpretation of this literature is difficult given the relative lack of comparable data for the efficacy of RAI therapy prepared by THW, with reports ranging from 20% to 90% (CitationMaxon et al 1992; CitationPacini et al 1994; CitationAlzahrani et al 2002; CitationRobbins et al 2002a; CitationDam et al 2004; CitationRosario et al 2004; CitationKloos and Mazzaferri 2005).
Table 3 Studies evaluating the effectiveness of rhTSH for 131I treatment of metastatic disease after prior thyroidectomy
Despite these limitations, almost all of the listed studies report uptake on post-therapy scans in a majority of patients, providing reasonable evidence for rhTSH stimulation of iodine uptake in metastatic deposits. Further, two studies compare the rhTSH scans to prior hypothyroid scans favorably, with concordant or superior rhTSH scans in 82% to 90% of patients (CitationLippi et al 2001; CitationJarzab et al 2003). However, even though some uptake is observed after rhTSH, the concern remains that since lower RAI uptake has fairly consistently been observed in several studies (CitationPark et al 1996; CitationLadenson et al 1997; CitationPacini et al 2002; CitationLuster et al 2003; CitationHanscheid et al 2006; CitationPotzi et al 2006; CitationVaiano et al 2007), the radiation dose to the tumor may be reduced in patients treated with rhTSH. This has been examined in several dosimetric studies, summarized in . The methods and reported parameters (eg, initial dose rate, effective half-life, residence time, cumulative activity) across the studies are quite heterogeneous. In addition, the conclusions in regard to effective radiation dose are mixed, with 3 of 6 studies showing a reduced radiation dose to the thyroid remnant or tumor (CitationPacini et al 2002; CitationPotzi et al 2006; CitationVaiano et al 2007), 2 showing an increased radiation dose (CitationLuster et al 2003; CitationHanscheid et al 2006) and 1 estimating the dose to be the same as historical hypothyroid-prepared patients (Citationde Keizer et al 2003). Also potentially concerning is that the studies summarized in include few reports of complete remission with rhTSH-stimulated treatment, even by surrogate markers such as Tg or follow-up RAI scans, and there is even a low rate of partial responses. However, in many of the series, the patient populations had very advanced disease, such that complete or partial responses might not be expected, and stable disease would be considered to represent a clinical benefit to the patient.
Table 4 Studies reporting dosimetric evaluation of thyroid remnant, metastatic tumor, blood and whole body radiation exposure after rhTSH compared to thyroid hormone withdrawal preparation
Safety and tolerability
In clinical trials rhTSH for both diagnostic and therapeutic purposes, the incidence of side effects was low. Combined data from clinical trials leading to FDA approval reveal the most common adverse events to be nausea (11.9%) and headache (7.3%) (Thyrogen® package insert, Genzyme Corporation). According to the Genzyme package insert, post-marketing experience has shown that administration of rhTSH can cause transient flu-like symptoms for up to 48 hours. Hypersensitivity has also been reported in patients with advanced disease, as manifested by urticaria, rash, pruritis, flushing, and respiratory symptoms. Similar to THW, there are case reports of tumor enlargement, edema, and hemorrhage resulting in paresthesias, hemiplegia, pathologic vertebral fractures, neck edema as well as exacerbation of bone pain seen within 12 to 48 hours of rhTSH administration (CitationVargas et al 1999; CitationRobbins et al 2000; CitationBraga et al 2001; CitationLippi et al 2001; CitationBerg et al 2002; CitationGoffman et al 2003; CitationJarzab et al 2003). For this reason, it is recommended that pretreatment with glucocorticoids be considered in patients with tumors located where transient expansion may compromise vital anatomic structures (eg, CNS and spinal metastases, bulky neck metastases). It is also recommended that patients who have extensive functional thyroid tissue or cardiac conditions (for whom rhTSH-induced stimulation of thyroid hormone production causing hyperthyroidism could have serious consequences) be hospitalized for administration and observation.
One potential advantage to rhTSH stimulation may be lower radiation exposure. Since there is reduced renal clearance of iodine and longer retention of the RAI in hypothyroid patients, preparation with rhTSH allows patients to remain euthyroid and potentially clear their radiation dose faster. Several recent studies have examined this issue and are summarized in . The methods across the studies are quite heterogeneous and not all studies reported both blood and whole body exposure. However, all studies that report either blood or red marrow doses, whole body retention times or residence times show the radiation exposure to be reduced by 10%– 35% when prepared with rhTSH preparation rather than THW (CitationPacini et al 2002; Citationde Keizer et al 2003; CitationLuster et al 2003; CitationMenzel et al 2003; Citationde Keizer et al 2004; CitationHanscheid et al 2006; CitationPotzi et al 2006; CitationVaiano et al 2007).
Quality of life
As mentioned previously, rhTSH was developed to avoid the consequences of prolonged hypothyroidism, and the negative impact of symptoms of hypothyroidism on patient quality of life. The impact of treatment on quality of life has been assessed in several of the randomized prospective efficacy trials comparing rhTSH with THW. In all studies that report it, quality of life by a variety of measures (hypothyroid symptoms and signs by the self-reported Billewicz scale or a validated hypothyroid clinical questionnaire; global quality of life by SF-36 health survey or a cancer specific survey; mood by various inventories) reveal increased symptoms and decrements in quality of life with THW preparation that is prevented by preparation with rhTSH instead (CitationBotella-Carretero et al 2003; CitationGiusti et al 2005; CitationMernagh et al 2006; CitationPacini et al 2006a; CitationSchroeder et al 2006; CitationBorget et al 2007; CitationTaieb et al 2008).
Other groups have sought to correlate these improvements in morbidity with increased productivity and economic outcomes. In one study, THW resulted in a median of 11 days of missed work (CitationLuster et al 2005), which was equivalent to the cost of administering rhTSH. A recently published study from Europe looked at sick leave as an indirect measure of morbidity. From the 306 patients included, 292 (95%) completed a questionnaire detailing their treatment, economic and sick leave data. They found there were 194 actively working patients among this group. Those who were treated with rhTSH, when compared to those treated by THW, were less likely to require sick leave (11% vs 33%, p = 0.001). Among those who did require sick leave, the mean duration was shorter (3.1 vs 11.2 days, p = 0.002) (CitationBorget et al 2007).
Because of the faster clearance of RAI when patients are euthyroid receiving rhTSH and anecdotal reports of shorter hospital admissions for RAI therapy, Borget also evaluated a potential impact on length of hospital stay with a case-control study. Thirty-five rhTSH-prepared patients were matched to 64 THW patients on factors affecting RAI clearance. The modeled simulations predicted a length of stay of 2.4 days for patients who received rhTSH preparation compared to 3.5 days for THW preparation (p < 0.001). This resulted in a savings of 338, which would defray approximately half the cost of thyrotropin alfa administration (CitationBorget et al 2008).
An economic analysis from a societal perspective evaluated quality of life improvements from avoiding hypothyroidism, increased work productivity, earlier discharge from a radiation safety perspective and theoretical reduction in risk of secondary malignancy. Utilizing a lifetime Markov model expressing benefits in terms of quality adjusted lifeyears (QALY), this analysis found the additional benefits of rhTSH to patients and society at relatively modest net cost (CitationMernagh et al 2006).
In summary, there is no question that rhTSH-stimulated preparation for diagnostic and therapeutic procedures in thyroid cancer improves patient quality of life, reduces sick time and potentially reduces some direct costs such as hospitalization for radioprotection. Further, quality of life and productivity end-points are particularly important for thyroid cancer patients, since thyroid cancer often strikes young adults, with a median age of presentation of 48 years and about 80% of patients presenting between the ages of 21 and 65 (CitationSEER 2008).
Summary
Clearly, development of rhTSH has dramatically changed the initial management and follow-up of patients with well-differentiated thyroid cancer. There is clear evidence that preparation of patients for RAI ablation of thyroid remnants is as efficacious as THW in low risk patients, at least for 2 to 3 years of follow-up. In addition, diagnostic follow-up evaluations with rhTSH-stimulated Tg are as informative as THW WBS and Tg measurements. Evaluation of this follow-up paradigm has been accompanied by a reconsideration of the role of RAI WBS and a shift to recommendations of rhTSH-stimulated Tg and neck ultrasonography as the primary modalities of follow-up. The use of rhTSH preparation in these situations and avoidance of hypothyroid symptoms and complications is cost-effective, or even cost-saving (in some macroeconomic models of health care and medical leave coverage). Further, limited studies suggest that whole body and blood radiation exposure from RAI may be lower after rhTSH preparation due to faster renal clearance of iodine, which, theoretically, may reduce long-term complications such as permanent sialoadenitis (CitationMandel and Mandel 2003) and secondary malignancies (CitationRubino et al 2003; CitationSandeep et al 2006; CitationBrown et al 2008).
Unresolved issues remain in regard to the definitive minimal efficacious dose of RAI for remnant ablation, confirmation of the long-term durability of the ablation success with rhTSH, determining optimal paradigms for initial diagnostic WBS and Tg determinations, and determining the required frequency of follow-up stimulated Tg measurements in low risk patients.
Disclosures
Dr. Schuff is on the Speaker’s Bureau for Genzyme Corporation. Dr. Gramza has no conflicts to disclose.
References
- AlzahraniASRaefHSultanA2002Impact of cervical lymph node dissection on serum TG and the course of disease in TG-positive, radioactive iodine whole body scan-negative recurrent/persistent papillary thyroid cancerJ Endocrinol Invest255263112109624
- BarbaroDBoniGMeucciG2003Radioiodine treatment with 30 mCi after recombinant human thyrotropin stimulation in thyroid cancer: effectiveness for postsurgical remnants ablation and possible role of iodine content in L-thyroxine in the outcome of ablationJ Clin Endocrinol Metab884110512970272
- BarbaroDBoniGMeucciG2006Recombinant human thyroid-stimulating hormone is effective for radioiodine ablation of post-surgical thyroid remnantsNucl Med Commun276273216829763
- BaudinEDo CaoCCailleuxAF2003Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patientsJ Clin Endocrinol Metab8811071112629092
- BergGLindstedtGSuurkulaM2002Radioiodine ablation and therapy in differentiated thyroid cancer under stimulation with recombinant human thyroid-stimulating hormoneJ Endocrinol Invest25445211883865
- BorgetICoroneCNocaudieM2007Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with Thyrogen and thyroid hormone withdrawalEur J Endocrinol156531817468188
- BorgetIRemyHChevalierJ2008Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and thyrogenEur J Nucl Med Mol Imaging3514576318385999
- Botella-CarreteroJIGalanJMCaballeroC2003Quality of life and psychometric functionality in patients with differentiated thyroid carcinomaEndocr Relat Cancer106011014713270
- BragaMRingelMDCooperDS2001Sudden enlargement of local recurrent thyroid tumor after recombinant human TSH administrationJ Clin Endocrinol Metab8651485111701668
- BrownAPChenJHitchcockYJ2008The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancerJ Clin Endocrinol Metab935041518029468
- CailleuxAFBaudinETravagliJP2000Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer?J Clin Endocrinol Metab85175810634383
- CastagnaMGBrilliLPilliT2008Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levelsJ Clin Endocrinol Metab93768117971424
- CooperDSDohertyGMHaugenBR2006Management guidelines for patients with thyroid nodules and differentiated thyroid cancerThyroid161094216420177
- CrocettiUDuranteCAttardM2008Predictive value of recombinant human TSH stimulation and neck ultrasonography in differentiated thyroid cancer patientsThyroid1810495318816184
- DamHQKimSMLinHC2004131I therapeutic efficacy is not influenced by stunning after diagnostic whole-body scanningRadiology2325273315286323
- de KeizerBBransBHoekstraA2003Tumour dosimetry and response in patients with metastatic differentiated thyroid cancer using recombinant human thyrotropin before radioiodine therapyEur J Nucl Med Mol Imaging303677312634964
- de KeizerBHoekstraAKonijnenbergMW2004Bone marrow dosimetry and safety of high 131I activities given after recombinant human thyroid-stimulating hormone to treat metastatic differentiated thyroid cancerJ Nucl Med4515495415347723
- DumontJELamyFRogerP1992Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factorsPhysiol Rev72667971320763
- EdmondsCJHayesSKermodeJC1977Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodineBr J Radiol50799807588901
- EliseiRCoroneCDriedgerA2007Follow-up of differentiated thyroid (DTC) cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after recombinant human thyrotropin (rhTSH) or thyroid hormone withdrawalHorm Res6833
- GiustiMSibillaFCappiC2005A case-controlled study on the quality of life in a cohort of patients with history of differentiated thyroid carcinomaJ Endocrinol Invest2859960816218042
- GoffmanTIoffeVTuttleM2003Near-lethal respiratory failure after recombinant human thyroid-stimulating hormone use in a patient with metastatic thyroid carcinomaThyroid138273014558927
- GrunwaldFMenzelCFimmersR1996Prognostic value of thyroglobulin after thyroidectomy before ablative radioiodine therapy in thyroid cancerJ Nucl Med37196248970514
- HanscheidHLassmannMLusterM2006Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawalJ Nucl Med476485416595499
- HaugenBRPaciniFReinersC1999A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancerJ Clin Endocrinol Metab8438778510566623
- HoeFMCharronMMoshangTJr2006Use of the recombinant human TSH stimulated thyroglobulin level and diagnostic whole body scan in children with differentiated thyroid carcinomaJ Pediatr Endocrinol Metab19253016509525
- IervasiAIervasiGFerdeghiniM2007Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancerClin Endocrinol (Oxf)674344117555505
- IorcanskySHerzovichVQualeyRR2005Serum thyrotropin (TSH) levels after recombinant human TSH injections in children and teenagers with papillary thyroid cancerJ Clin Endocrinol Metab906553516174712
- JarzabBHandkiewicz-JunakDRoskoszJ2003Recombinant human TSH-aided radioiodine treatment of advanced differentiated thyroid carcinoma: a single-centre study of 54 patientsEur J Nucl Med Mol Imaging3010778612783219
- KimTYKimWBKimES2005Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinomaJ Clin Endocrinol Metab901440515613412
- KloosRTMazzaferriEL2005A single recombinant human thyrotropin- stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years laterJ Clin Endocrinol Metab9050475715972576
- LadensonPWBravermanLEMazzaferriEL1997Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinomaN Engl J Med337888969302303
- LeboulleuxSSchroederPRSchlumbergerM2007The role of PET in follow-up of patients treated for differentiated epithelial thyroid cancersNat Clin Pract Endocrinol Metab31122117237838
- LippiFCapezzoneMAngeliniF2001Radioiodine treatment of metastatic differentiated thyroid cancer in patients on L-thyroxine, using recombinant human TSHEur J Endocrinol14451111174831
- LusterMFelbingerRDietleinM2005Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administrationThyroid1511475516279848
- LusterMLassmannMHaenscheidH2000Use of recombinant human thyrotropin before radioiodine therapy in patients with advanced differentiated thyroid carcinomaJ Clin Endocrinol Metab853640511061516
- LusterMShermanSISkarulisMC2003Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinomaEur J Nucl Med Mol Imaging301371712856155
- MandacJCLeboeufRBrokhinM2008In properly selected patients, the results of a diagnostic whole-body RAI scan prior to RAI remnant ablation (RRA) seldom alters either the decision to proceed with RRA or the choice of administered activityThyroid18S36
- MandelSJMandelL2003Radioactive iodine and the salivary glandsThyroid132657112729475
- MarianiGFerdeghiniMAugeriC2000Clinical experience with recombinant human thyrotrophin (rhTSH) in the management of patients with differentiated thyroid cancerCancer Biother Radiopharm15211710803328
- MaxonHREnglaroEEThomasSR1992Radioiodine-131 therapy for well-differentiated thyroid cancer – a quantitative radiation dosimetric approach: outcome and validation in 85 patientsJ Nucl Med33113261597728
- MazzaferriELRobbinsRJSpencerCA2003A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinomaJ Clin Endocrinol Metab8814334112679418
- MeierCABravermanLEEbnerSA1994Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study)J Clin Endocrinol Metab78188968288703
- MenzelCKranertWTDöbertN2003rhTSH stimulation before radioiodine therapy in thyroid cancer reduces the effective half-life of (131)IJ Nucl Med441065812843221
- MernaghPCampbellSDietleinM2006Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspectiveEur J Endocrinol1554051416914594
- MontesanoTDuranteCAttardM2007Age influences TSH serum levels after withdrawal of l-thyroxine or rhTSH stimulation in patients affected by differentiated thyroid cancerBiomed Pharmacother614687117553654
- NiederkohrRDMcDougallIR2007Reproducibility of whole-body 131I scan and serum thyrotropin and stimulated thyroglobulin values in patients studied twice after injection of recombinant human thyrotropinEur J Nucl Med Mol Imaging34363717021814
- PaciniFCapezzoneMEliseiR2002Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatmentJ Clin Endocrinol Metab87149950111932271
- PaciniFCetaniFMiccoliP1994Outcome of 309 patients with metastatic differentiated thyroid carcinoma treated with radioiodineWorld J Surg1860047725751
- PaciniFLadensonPWSchlumbergerM2006aRadioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled studyJ Clin Endocrinol Metab919263216384850
- PaciniFMolinaroECastagnaMG2002Ablation of thyroid residues with 30 mCi (131)I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawalJ Clin Endocrinol Metab874063812213846
- PaciniFMolinaroECastagnaMG2003Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinomaJ Clin Endocrinol Metab8836687312915653
- PaciniFSchlumbergerMDralleH2006bEuropean consensus for the management of patients with differentiated thyroid carcinoma of the follicular epitheliumEur J Endocrinol15478780316728537
- ParkSGReynoldsJCBrucker-DavisF1996Iodine kinetics during I-131 scanning in patients with thyroid cancer: comparison of studies with recombinant human TSH (rhTSH) vs hypothyroidismJ Nucl Med3715
- PellegritiGScolloCGiuffridaD2001Usefulness of recombinant human thyrotropin in the radiometabolic treatment of selected patients with thyroid cancerThyroid1110253011762711
- PilliTBrianzoniECapoccettiF2007A comparison of 1850 (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancerJ Clin Endocrinol Metab923542617609306
- PotziCMoameniAKaranikasG2006Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patientsClin Endocrinol (Oxf)655192316984246
- RobbinsRJDriedgerAMagnerJ2006aRecombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxineThyroid1611213017123339
- RobbinsRJLarsonSMPentlowKS2002aEffectiveness of I-131 in destroying metastatic thyroid cancer lesionsThyroid12199
- RobbinsRJLarsonSMSinhaN2002bA retrospective review of the effectiveness of recombinant human TSH as a preparation for radioiodine thyroid remnant ablationJ Nucl Med431482812411552
- RobbinsRJTuttleRMSonenbergM2001Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropinThyroid11865911575856
- RobbinsRJVoelkerEWangW2000Compassionate use of recombinant human thyrotropin to facilitate radioiodine therapy: case report and review of literatureEndocr Pract6460411155220
- RobbinsRJWanQGrewalRK2006bReal-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-Dglucose- positron emission tomography scanningJ Clin Endocrinol Metab9149850516303836
- RosarioPWBorgesMAPurischS2008Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicityJ Nucl Med4917768218927337
- RosarioPWMaiaFFCardosoLD2004Correlation between cervical uptake and results of postsurgical radioiodine ablation in patients with thyroid carcinomaClin Nucl Med293586115166882
- RubinoCde VathaireFDottoriniME2003Second primary malignancies in thyroid cancer patientsBr J Cancer8916384414583762
- RudavskyAZFreemanLM1997Treatment of scan-negative, thyroglobulin-positive metastatic thyroid cancer using radioiodine 131I and recombinant human thyroid stimulating hormoneJ Clin Endocrinol Metab821148989223
- SandeepTCStrachanMWReynoldsRM2006Second primary cancers in thyroid cancer patients: a multinational record linkage studyJ Clin Endocrinol Metab9118192516478820
- SchlesingerTFlowerMAMcCreadyVR1989Radiation dose assessments in radioiodine (131I) therapy. 1. The necessity for in vivo quantitation and dosimetry in the treatment of carcinoma of the thyroidRadiother Oncol1435412928556
- SchlumbergerMBergGCohenO2004Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspectiveEur J Endocrinol1501051214763906
- SchroederPRHaugenBRPaciniF2006A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawalJ Clin Endocrinol Metab918788416394083
- SEER2008SEER Stat Fact Sheets – ThyroidSEER Cancer Statistics Review, 1975–2005Accessed 26 November 2008 URL: http://seer.cancer.gov/csr/1975_2005/
- SmallridgeRCMeekSEMorganMA2007Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patientsJ Clin Endocrinol Metab9282717077133
- SzkudlinskiMWThotakuraNRBucciI1993Purification and characterization of recombinant human thyrotropin (TSH) isoforms produced by Chinese hamster ovary cells: the role of sialylation and sulfation in TSH bioactivityEndocrinology13314905038404588
- TaiebDLussatoDGuedjE2006Early sequential changes in serum thyroglobulin after radioiodine ablation for thyroid cancer: possible clinical implications for recombinant human thyrotropin-aided therapyThyroid16177916676403
- TaiebDLussatoDMundlerO2004Subcutaneous administration of recombinant human thyrotropin as an alternative to thyroid hormone withdrawal in patients with anticoagulated thyroid cancer: preliminary resultsThyroid14463415242575
- TaiebDSebagFCherenkoM2008Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation with recombinant human thyrotropin: a randomized controlled studyClin Endocrinol (Oxf)in press
- TorlontanoMCrocettiUD’AloisoL2003Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancerEur J Endocrinol148192412534353
- ToubeauMTouzeryCArveuxP2004Predictive value for disease progression of serum thyroglobulin levels measured in the postoperative period and after (131)I ablation therapy in patients with differentiated thyroid cancerJ Nucl Med459889415181134
- TuttleRMBrokhinMOmryG2008Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawalJ Nucl Med497647018413378
- VaianoAClaudio TrainoABoniG2007Comparison between remnant and red-marrow absorbed dose in thyroid cancer patients submitted to 131I ablative therapy after rh-TSH stimulation versus hypothyroidism induced by L-thyroxine withdrawalNucl Med Commun282152317264781
- VargasGEUyHBazanC1999Hemiplegia after thyrotropin alfa in a hypothyroid patient with thyroid carcinoma metastatic to the brainJ Clin Endocrinol Metab8438677110566621
- VitaleGLupoliGACiccarelliA2003Influence of body surface area on serum peak thyrotropin (TSH) levels after recombinant human TSH administrationJ Clin Endocrinol Metab8813192212629125
- WangWLarsonSMFazzariM2000Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancerJ Clin Endocrinol Metab8511071310720047
- Zanotti-FregonaraPDuronFKellerI2007Stimulation test in the follow-up of thyroid cancer: plasma rhTSH levels are dependent on body weight, not endogenously stimulated TSH valuesNucl Med Commun28257917325587