Abstract
The human epidermal growth factor receptor (HER) family comprises four homologous members: EGFR, HER-2, HER-3, and HER-4. The activation of these receptors triggers a complex series of signal transduction pathways which affect pivotal tumorigenic processes. The deregulation of HER signaling is seen in several human malignancies. HER-2 is now recognized as a key oncogene in breast cancer pathogenesis. Assessment of HER-2 status is of central importance in the prognosis of breast cancer patients. In the light of clinical data suggesting that HER-2 can also be useful as a predictive marker both for trastuzumab and chemotherapy, standardized determination of the HER-2 status in tumors has become more important. Moreover, current data provide evidence for the significance of HER-3 and HER-4 alterations in breast carcinogenesis. Because of the complex interactions among the HER receptors, it is likely that the effect on cell proliferation and tumor growth depends on receptor trans-signaling and thus, the evaluation of the combined expression pattern of all family members is of particular interest. This review presents the current evidence highlighting the role of the family as a whole panel and an update on the role of HER-3 and HER-4 receptors in breast cancer. Moreover, we provide updated data regarding the prognostic value of HER family members giving emphasis to novel methods for the determination of their status, such as real-time polymerase chain reaction. In addition, we review recent therapeutic approaches aimed at targeting the HER family in breast cancer patients.
Introduction
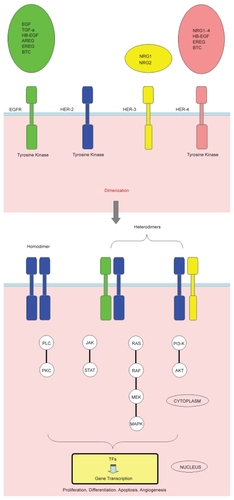
The human epidermal growth factor receptor (HER) family consists of four homologous members: ErbB-1 (epidermal growth factor [EGF] receptor [EGFR] or HER-1), ErbB-2 (HER-2) for which no ligand has been described so far, ErbB-3 (HER-3), which is characterized by its impaired kinase activity, and ErbB-4 (HER-4). All family members are transmembrane glycoproteins consisting of an extracellular ligand-binding domain, a hydrophobic transmembrane region, and a cytoplasmic section containing the tyrosine kinase domain and a carboxy-terminal region with tyrosine autophosphorylation sites. Despite their structural homology, HER receptors differ in their ligand specificities. Two main ligand classes have been recognized so far: the splice variants of neuregulins (NRGs) which bind exclusively to HER-3 and/or HER-4 and different EGF-related proteins (CitationHarris et al 2003). Binding of specific ligands to the extracellular domain allows for receptor homo- or heterodimerisation through conformational changes resulting in activation of the cytoplasmatic catalytic function, which leads to receptor autophosphorylation on tyrosine residues. This autophosphorylation triggers a complex series of signal transduction pathways such as phosphatidylinositol 3-kinase (PI3-K)-Akt, Ras-Raf-MEK-mitogen-activated protein kinase (MAPK)-dependent pathway, phospholipase C–protein kinase C (PLC–PKC), and janus kinase/signal transducer and activator of transcription (JAK/STAT). These pathways affect essential tumorigenic processes such as proliferation, differentiation, migration, inhibition of apoptosis, and enhanced survival (CitationMosesson and Yarden 2004; CitationKrause and Van Etten 2005). Signaling diversity depends not only on the presence of specific receptors, but also on the characteristics of individual ligands. The HER family is characterized by a functional interdependency among its members, in terms of activity ().
Figure 1 HER receptors and their ligands. Despite their structural homology, HER receptors differ in their ligand specificities. Some of these ligands bind exclusively to EGFR, such as EGF, TGF-α, and AREG, or bind exclusively to HER-4, such as NRG3 and NRG4. Others have a dual specificity. So far, no ligand has been described for HER-2, whereas HER-3 is characterized by impaired kinase activity. HER receptors achieve activation by forming ligand-bound homo-and/or heterodimeric receptor complexes. Ten possible dimers can be formed (Only a few examples of dimers are presented here). HER-2 is known to be the preferred heterodimerisation partner for EGFR, HER-3 and HER-4. The HER-2/HER-3 heterodimer constitutes the most mitogenic dimer in the family. The HER complexes signal from the cell surface to the nucleus through numerous downstream pathways such as phosphatidylinositol 3-kinase (PI3-K)-Akt, Ras-Raf-MEK-MAPK-dependent pathway, PLC–PKC, and JAK/STAT. These signaling cascades eventually transmit their signal to TFs, which affect the transcription of target genes, regulating critical tumorigenic processes including proliferation, differentiation, apoptosis, angiogenesis, and migration.
HER-2 and breast cancer
There is an extensive literature on the role of the HER family in breast cancer (CitationGullick and Srinivasan 1998) and particularly that of HER-2 which is considered a key oncogene in breast carcinogenesis. The extracellular domain of HER-2 is unique in that it is locked constitutively in a conformation resembling the ligand-bound states of the extracellular regions of the other HER receptors. As a ligand orphan receptor, HER-2 preferentially forms heterodimers with other family members. HER-2 is known to be the preferred heterodimerisation partner for EGFR, HER-3, and HER-4 (CitationGraus-Porta et al 1997) and plays an important role in triggering signal transduction pathways. Moreover, heterodimers containing HER-2 are more mitogenic than others (CitationCitri et al 2003). The transforming functions of HER-2 and its fundamental role in breast cancer pathogenesis are now well established (CitationMoasser 2007; CitationUrsini-Siegel et al 2007). In the majority of cases, HER-2 overexpression is a consequence of amplification at the DNA level.
Overexpression or amplification of HER-2 occurs in 15% to 30% of breast carcinomas and is considered to confer a more aggressive biology and an unfavorable impact on the course of the disease (CitationSlamon et al 1987, Citation1989; CitationRilke et al 1991; CitationRoss and Flether 1998). HER-2 overexpression is associated with estrogen receptor (ER) and progesterone receptor (PR) negativity, high histological grade, high rates of cell proliferation and lymph node involvement (CitationRilke et al 1991; CitationGusterson et al 1992; CitationLebeau et al 2003). Moreover, it is correlated with disease aggressiveness, increased rates of recurrence and poorer survival in node-positive breast cancer patients, whereas the prognostic significance in patients with node-negative tumors remains somewhat controversial (CitationBorg et al 1990; CitationWinstanley et al 1991; CitationPaterson et al 1991; CitationClark and McGuire 1991; CitationToikkanen et al 1992; CitationMarsigliante et al 1993; CitationHartmann et al 1994; CitationQuenel et al 1995; CitationMitchell and Press 1999).
HER-2 overexpression is also regarded as a predictive marker for reduced responsiveness to tamoxifen therapy (CitationTovey et al 2005; CitationKirkegaard et al 2007), although this is still an unresolved issue. The predictive value of HER-2 expression regarding response to chemotherapy is also still controversial, although numerous trials have supported an interaction between HER-2 expression and chemotherapy activity (CitationMuss et al 1994; CitationMass 2000; CitationPetit et al 2001; CitationZhang et al 2003; CitationMoliterni et al 2003). It has been suggested that HER-2 overexpression or amplification in breast cancer predicts greater sensitivity to anthracycline-containing chemotherapy (CitationDe Placido et al 1995; CitationPaik et al 1998, Citation2000; CitationRavdin et al 1998; CitationDi Leo et al 1999, Citation2001, Citation2002; CitationDe Laurentiis et al 2001; CitationMoliterni et al 2003; CitationPritchard et al 2006; CitationGennari et al 2008) and resistance to CMF regimen (CitationTLBC 1988, Citation1989; CitationMansour et al 1989; CitationAllred et al 1992; CitationGusterson et al 1992). HER-2 may also identify patients who are likely to benefit from higher doses of adjuvant chemotherapy (CitationWood et al 1994; CitationThor et al 1998; CitationArnould et al 2003; CitationBonneterre et al 2003; CitationRodenhuis et al 2003; CitationDel Mastro et al 2004; CitationDressler et al 2005). The association with response to taxane-based chemotherapy is unclear, as results have been conflicting (CitationKonecny et al 2004; CitationGonzalez-Angulo et al 2004; CitationKostopoulos et al 2006; CitationHayes et al 2007).
HER-2 status determination
Assessment of HER-2 status is of crucial importance in the management of patients with breast cancer. In view of the clinical data suggesting that HER-2 can be useful as a predictive marker both for trastuzumab and chemotherapy, standardized determination of HER-2 status in tumors has become more important. However, while the clinical benefit of assessing HER-2 status in breast carcinomas is now accepted, there is no consensus on the ideal diagnostic method to use for this purpose. HER-2 can be analyzed at the DNA-, the mRNA-or the protein level. Various techniques are available, each with benefits and disadvantages (CitationDowsett et al 2000).
For practical reasons, immunohistochemistry (IHC) using an anti-HER-2 antibody is currently the method of choice for HER-2 testing. IHC is a rapid, simple and convenient technique, readily available as a standard method in a routine clinical service laboratory. Moreover, IHC is a relatively inexpensive assay which can be easily used on archival formalin-fixed paraffin-embedded (FFPE) tissues. Consequently, the majority of reports published on the clinical significance of HER-2 expression have used IHC to determine HER-2 status. However, the major drawbacks of IHC are that the results are not quantitative, whereas substantial inter-observer variations have been reported (CitationThomson et al 2001). The interpretation of IHC results is subjective and prone to inter-observer variability, requiring experienced pathologists. In addition, the interpretation of the findings is considerably influenced by several technical factors, such as the use of antibodies with variable sensitivities and specificities, and different fixative protocols or staining procedures (CitationPress et al 1994). While this discrepancy is improved by the use of standardized IHC tests (such as the HercepTest), it is generally recommended that (2+) HER-2 immunostaining requires further validation by fluorescence in situ hybridization (FISH) analysis (CitationBartlett et al 2003; CitationDowsett et al 2003; CitationEllis et al 2004).
FISH is a reliable, sensitive and highly specific technique for assessing HER-2 gene amplification (CitationKjeldsen et al 2002), a change that appears to be correlated with strong protein expression (CitationJacobs et al 1999). In contrast to IHC, FISH can give a more objective and reproducible estimation of HER-2 status. The result is quantitative, as it not only determines whether amplification is present, but also the degree of amplification. However, the technique is expensive and time consuming to perform. Moreover, FISH requires specialized expertise and a fluorescence microscopy facility and thus, it is currently available only in a minority of pathology laboratories. FISH is now being challenged by the chromogenic in situ hybridization (CISH) technique. CISH is similar to FISH, except that it uses a peroxidase reaction instead of a fluorescent dye, which allows evaluation in an ordinary light microscope (CitationIsola et al 2004; CitationLaakso et al 2006).
Despite efforts to standardise these methods, considerable intra-laboratory and inter-laboratory variability of the results still exist. A number of studies indicate that approximately 20% of HER-2 assays performed at the treatment site’s pathology department are incorrect when the same sample is reassessed in a high-volume central laboratory (CitationPaik et al 2002; CitationRoche et al 2002; CitationPerez et al 2006). Therefore, improvement in reproducibility of the results between different laboratories is a high priority (CitationDi Leo 2007; CitationWolff et al 2007).
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) has been recently suggested as an alternative technique for detection and quantification of HER-2 status. RT-PCR produces quantitative and reproducible results. Moreover, it can be easily standardized, reduces inter-observer variability, and does not require experienced pathologists for interpretation. However, a disadvantage of this technique is the specific requirements for handling of tissue specimens to preserve the integrity of RNA (CitationMasuda et al 1999).
HER-2 analyses using real-time PCR
A number of studies have used quantitative real-time PCR for the assessment of HER-2 status (CitationBieche et al 1999; CitationO’Malley et al 2001; CitationKim et al 2002; CitationMrhalova et al 2003; CitationKönigshoff et al 2003; CitationGinestier et al 2004; CitationSchlemmer et al 2004; CitationSuo et al 2004; CitationGjerdrum et al 2004; CitationVanden Bempt et al 2005; CitationEsteva et al 2005; CitationBenöhr et al 2005; CitationBossard et al 2005; CitationTse et al 2005; CitationVinatzer et al 2005; CitationKulka et al 2006; CitationNtoulia et al 2006; CitationLabuhn et al 2006). These studies investigated the extent of concordance of IHC, FISH (or CISH), quantitative PCR, and in some cases quantitative RT-PCR. In the majority of them, a substantial degree of agreement among different methods has been demonstrated, with respect to HER-2 status determination.
A limited number of studies have evaluated the prognostic power of HER-2 using quantitative real-time PCR. In a retrospective study, which analyzed the expression of HER-2 by real-time RT-PCR and IHC in 131 breast carcinomas, HER-2 positive patients as determined by RT-PCR had worse outcome than the HER-2 negative group. This was evident in all cases as well as in the node-positive group (CitationPotemski et al 2006). CitationVinatzer and colleagues (2005) assessed HER-2 status at the DNA, mRNA, and protein levels with IHC, FISH and quantitative real-time RT-PCR in 136 tumor samples from 85 breast cancer patients. HER-2 overexpression, as determined by quantitative RT-PCR, positively correlated with high tumor grade, positive lymph node status, ER and PR negativity, consistent with published IHC results. Regarding the prognostic significance of HER-2 status, all methods showed a significant correlation of HER-2 with disease-free survival (DFS) and overall survival (OS), except FISH alone. The authors concluded that quantitative RT-PCR seems to be clinically as useful in the assessment of HER-2 status as the current standard methods, yielding comparable prognostic information. CitationBergqvist and colleagues (2007) used quantitative real-time PCR (Q-PCR) and RNA expression profiles (RNA-EPs) to evaluate HER-2 status in relation to clinical outcome of breast cancer patients. The authors compared these techniques with IHC supplemented with FISH or CISH. Analyses of relapse-free survival (RFS) and OS on the basis of 5 and 10 years of follow-up indicated equivalent hazard ratios for all three methods. In contrast to IHC/CISH, both Q-PCR and RNA-EP analyses of HER-2 also yielded significant results regarding RFS and breast cancer-corrected survival after 10 years of follow-up. The results of this study suggested that both the Q-PCR and RNA-EP assays are associated with high-quality HER-2 status determinations, and are of similar, or even superior, prognostic value compared with the current standard techniques.
EGFR and breast cancer
EGFR is overexpressed in several human tumors and is considered to initiate a variety of important steps during the malignant transformation (CitationNicholson et al 2001). In a review of 40 studies it was found that 45% of human breast carcinomas express EGFR (range 14% to 91%) (CitationKlijn et al 1992). In contrast to HER-2, there are no widely accepted criteria for the determination of EGFR status. The prognostic significance of EGFR in breast cancer remains unclear (CitationChan et al 2006). EGFR has been correlated with ER/PR negativity (CitationFox et al 1994; CitationPawlowski et al 2000; CitationFerrero et al 2001; CitationTsutsui et al 2002; CitationBieche et al 2003; CitationBloom 2005). There may be an association between EGFR expression and high histological grade or lymph node involvement, although all studies are not in agreement (CitationFox et al 1994; CitationPawlowski et al 2000; CitationFerrero et al 2001; CitationWitton et al 2003; CitationRampaul et al 2004, Citation2005; CitationUeda et al 2005). EGFR is generally considered a negative prognostic factor in breast cancer (CitationTsutsui et al 2002; CitationWitton et al 2003) but up to now, no definitive association between EGFR expression and survival has been demonstrated.
The role of EGFR in HER-2 mediated transformation is not fully elucidated, so far. Transformation associated with the human HER-2 gene has been demonstrated, independently of the EGFR (CitationChazin et al 1992). On the other hand, experiments have provided evidence for a synergistic interaction of these receptors in cellular transformation and induction of mammary tumors (CitationKokai et al 1989; CitationMuller et al 1996; CitationDiGiovanna et al 1998). Moreover, interactions between EGFR and HER-2 with respect to the prognosis of breast cancer patients have been reported. Suo et al suggested that EGFR expression is likely to have a synergistic effect on the clinical influence of HER-2 expression (CitationSuo et al 2002). In another study, CitationDi Giovanna and colleagues (2005) showed that breast cancer patients whose tumors demonstrated co-overexpression of EGFR and HER-2 had the shortest survival.
HER-3 and breast cancer
The HER-3 gene is located on chromosome 12q13 and the encoded protein receptor binds to NRG isoforms. HER-3 overexpression has been documented in 20% to 30% of invasive breast carcinomas (CitationKaramouzis et al 2007). Moreover, HER-3 is frequently co-expressed with HER-2 (CitationNaidu et al 1998; CitationBieche et al 2003; CitationWitton et al 2003; CitationSassen et al 2008), suggesting a role of this receptor in HER-2 mediated carcinogenesis. HER-3 signaling relies on the formation of signaling-competent heterodimers with other members of the HER family. Ligand-bound or even ligand-independent HER-3 may form signaling complexes with HER-2. It has been demonstrated that the HER-2/HER-3 heterodimer constitutes the most mitogenic dimer in the HER family (CitationCitri et al 2003). It seems that these two receptors cooperate synergistically in neoplastic transformation (CitationAlimandi et al 1995). This hypothesis is supported by CitationHolbro and colleagues (2003) who showed that HER-3 couples active HER-2 to the downstream signaling PI3-K/protein kinase B pathway. In another study, the activity of HER-3 decreased dramatically when the HER-2 receptor was blocked, suggesting that HER-2/HER-3 dimers are necessary for sustained signaling (CitationNeve et al 2000). CitationLiu and colleagues (2007) indicated that downregulation of HER-3 inhibits HER-2 mediated procarcinogenic activity via inactivation of the PI3-K/Akt pathway. Furthermore, HER-3 also contributes to HER-2 associated tamoxifen resistance. HER-2/HER-3 heterodimers signal through the PI3-K/Akt pathway, which is known to be activated in a wide range of cancers. HER-2 does not directly bind PI3-K and this function is mediated through HER-3, which has multiple tyrosine containing binding sites for p85, the regulatory subunit of PI3-K (CitationPrigent and Gullick 1994; CitationSoltoff et al 1994). On the other hand, it has been demonstrated that a naturally occurring secreted form of the human HER-3 receptor, p85-soluble ErbB3 (sErbB3), is a potent negative regulator of heregulin-stimulated HER-2, HER-3 and HER-4 activation (CitationLee et al 2001).
The prognostic value of HER-3 expression in breast cancer is poorly documented and the available data are still controversial (CitationLemoine et al 1992; CitationGasparini et al 1994; CitationQuinn et al 1994; CitationTravis et al 1996; CitationPawlowski et al 2000; CitationKaramouzis et al 2007). Although overexpression of HER-3 has been linked to HER-2 positivity (CitationGasparini et al 1994) and lymph node involvement (CitationLemoine et al 1992; CitationBieche et al 2003), a definitive relationship with survival has not been established. In a study which evaluated HER family by IHC, patients with tumors that stained HER-3 strongly had significantly reduced survival (CitationWitton et al 2003), whereas in a recently reported study, a negative impact of HER-3 gene amplification on DFS was demonstrated (CitationSassen et al 2008). In contrast, other studies have suggested a positive prognostic value of HER-3 receptor status. CitationQuinn and colleagues (1994) showed that HER-3 overexpression was positively, but not significantly, related to negative lymph node status and survival, whereas CitationKnowlden and colleagues (1998) have demonstrated that increased HER-3 mRNA appears to be associated with the prognostically favorable ER phenotype. Moreover, CitationPawlowski and colleagues (2000) reported a univariate positive impact of HER-3 mRNA on survival. In a recent study, CitationLee and colleagues (2007) found that expression of HER-3 was correlated with positive ER and PR status and inversely correlated with histological grade. In the same study, HER-3 expression was associated with longer DFS.
HER-4 and breast cancer
The HER-4 gene is located on chromosome 2q33.3–34 and the encoded protein can be activated by both NRGs and some ligands of the EGF family. In contrast to the other HER receptors, the existing evidence suggests that HER-4 is characterized by antiproliferative activity (CitationSartor et al 2001; CitationNaresh et al 2006). Moreover, HER-4 overexpression has been reported as a favorable prognostic factor in the literature. This positive effect is most likely associated with growth controling and differentiation signaling. HER-4 is expressed in four isoforms, one of which, ErbB4 CYT-2, lacks a PI3-K binding site and thus is incapable of activating PI3-K signaling pathway (CitationKainulainen et al 2000). Other studies have indicated that NRG-activated HER-4 homodimers stimulate only the apoptosis-controling PI3-K/Akt pathway and not cell proliferation (CitationYarden and Sliwkowski 2001). In cell line experiments, when HER-2 positive cancer cells were transfected to overexpress HER-4, a reduction in proliferation and an increase in apoptosis were observed (CitationSartor et al 2001), suggesting that HER-4 antagonizes HER-2 signaling activity (CitationBarnes et al 2005). More recent studies have increased our knowledge regarding the HER-4 associated apoptosis (CitationNaresh et al 2006). On the other hand, contrasting results of the prognostic significance of HER-4 have also been reported (CitationBieche et al 2003). CitationTang and colleagues (1999) have shown that ribozyme-mediated down-regulation of HER-4 in breast cancer cells inhibits their proliferation both in vitro and in vivo. These differences may be explained by the variable responses of HER-4 to its ligands, resulting in either proliferation or differentiation, and perhaps influenced by dimerisation with other HER-family members.
HER-4 expression has been associated with favorable prognostic factors (ER positivity, low histological grade) (CitationKnowlden et al 1998; CitationTang et al 1999; CitationKew et al 2000; CitationSuo et al 2002; CitationWitton et al 2003; CitationZaczek et al 2008) and a more favorable outcome in breast cancer patients (CitationPawlowski et al 2000; CitationSuo et al 2002; CitationWitton et al 2003; CitationAubele et al 2007). Suo et al (CitationSuo et al 2002) suggested that HER-4 antagonizes the HER-2 effect on the patient clinical course and thus, integrating HER-4 status analysis into the diagnosis of breast cancer may also be of importance (CitationBarnes et al 2005). In contrast, CitationBieche and colleagues (2003) suggested that HER-4 mRNA status might be a molecular marker of poor outcome in subsets of breast cancer patients.
Studies evaluating the HER family as a whole panel
Most clinicopathological studies have focused on the expression and/or gene amplification of individual HER family members. Consequently, the clinical outcome of breast cancer patients with regard to HER family as a whole panel remains largely unidentified. Because of the complex interactions among the HER receptors, it is likely that the effect on cell proliferation and tumor growth depends on receptor trans-signaling and thus, the evaluation of the combined expression pattern of all family members is of particular interest.
Few data are available on the expression pattern of all four HER receptors in large series of breast tumors. In a study (CitationWitton et al 2003) which investigated the HER family by IHC in 220 breast carcinomas, patients whose tumors overexpressed EGFR, HER-2, or HER-3 had reduced survival (P ≤ 0.001), whereas those whose tumors overexpressed HER-4 had increased survival (P = 0.013). In Cox’s multiple regression analysis, EGFR, HER-2, HER-3 and HER-4 positivity, independently affected the survival. A recent study (CitationSassen et al 2008) evaluated the four members (EGFR, HER-2, HER-3, HER-4), both at the DNA and protein levels using FISH and IHC, in 278 patients. In this study, the negative impact of HER-2 amplification on patient DFS and OS was verified. Moreover, a univariate negative impact of HER-3 gene amplification on DFS was demonstrated (P = 0.031).
A number of studies have demonstrated strong correlations between HER mRNA copy numbers and HER protein levels, suggesting that HER family expression can reliably be assessed at the mRNA level (CitationKnowlden et al 1998; CitationSrinivasan et al 1998; CitationWalker and Dearing 1999; CitationSuo et al 2002). Data regarding the evaluation of all HER family members using RT-PCR are limited. Suo et al evaluated the HER family members using IHC and RT-PCR in 100 breast cancer patients. In this study, all the immunoreactive tumors were confirmed positive by RT-PCR. Statistical analysis revealed a significant association between HER-2 expression and reduced DFS (P = 0.033) and cancer-specific survival (P = 0.042). HER-4 expression was correlated with a longer DFS (P = 0.049) and cancer-specific survival (P = 0.044). Co-expression of HER-2 and EGFR was associated with a worse prognosis (CitationSuo et al 2002). CitationPawlowski and colleagues (2000) assessed the expression of the family with real-time RT-PCR, in a series of 365 breast cancers. HER-3 and HER-4 were positively correlated to each other and negatively correlated to EGFR. In RFS studies, Cox univariate analyses revealed prognostic value of HER-4 (P = 0.015; risk ratio [RR], 0.65) which was retained in multivariate analyses (P = 0.035; RR, 0.67). Regarding OS studies, univariate analyses demonstrated prognostic significance of EGFR (P = 0.026; RR, 1.6), HER-3 (P = 0.0093; RR, 0.58), and HER-4 (P = 0.0024; RR, 0.52), whereas the expression of HER-2 was not a prognostic factor. In the multivariate analyses, none of these receptors maintained their prognostic value on OS. HER-4 was found to be an independent prognostic factor on RFS (CitationPawlowski et al 2000). CitationBieche and colleagues (2003) used a real-time quantitative RT-PCR assay to quantify HER family mRNA copy numbers in 130 breast tumors from patients with known long-term outcome. In this study, a positive correlation between HER-3 and HER-4 mRNA levels was found, together with a negative correlation between the expression of these two genes and that of EGFR. RFS was shorter among patients with HER-3-overexpressing tumors (P = 0.0092) and longer among those with HER-4-underexpressing tumors (P = 0.0085), relative to patients with normal expression of the respective genes. In contrast, RFS was not significantly influenced by EGFR or HER-2 mRNA status. Only HER-4 retained its prognostic significance in Cox multivariate regression analysis (P = 0.015).
The existing data with respect to the expression of HER family members, particularly that of EGFR, HER-3, and HER-4, are extremely variable and thus, a comparison of the results from different studies is difficult. Most of those studies have evaluated the expression at the protein level, whereas the majority of them have not investigated the expression of all HER members simultaneously. Up to now, it is not clear whether the assessment of the prognostic value of the HER family at the DNA-, the mRNA- or the protein level yields comparable results. In a study which investigated the HER receptors using both IHC and RT-PCR, the authors used protein expression for the evaluation of relationships to clinicopathological parameters, considering that the biological influence of these factors is reflected by protein level (CitationSuo et al 2002). Studies evaluating the HER receptors as a whole panel at the protein level have confirmed the value of HER-2 as a negative prognostic factor (CitationSuo et al 2002; CitationWitton et al 2003; CitationSassen et al 2008). A similar finding was not demonstrated in studies which assessed the HER family at the mRNA level (CitationPawlowski et al 2000; CitationBieche et al 2003), although those which investigated the prognostic power of HER-2 only, using real-time RT-PCR, showed that this technique seems to be as useful as the current standard methods, yielding comparable correlations of HER-2 status with the patient outcome (CitationVinatzer et al 2005; CitationPotemski et al 2006; CitationBergqvist et al 2007). Regarding the EGFR receptor, although a number of studies suggest a negative prognostic value (CitationPawlowski et al 2000; CitationWitton et al 2003), others have failed to demonstrate its prognostic significance (CitationBieche et al 2003; CitationSassen et al 2008). Furthermore, most of the studies evaluating the HER family are in agreement regarding the negative prognostic value of HER-3 in breast cancer patients (CitationBieche et al 2003; CitationWitton et al 2003; CitationSassen et al 2008). Likewise, the favorable impact of HER-4 on patient outcome has been demonstrated through the majority of the studies which assessed all family members simultaneously (CitationPawlowski et al 2000; CitationSuo et al 2002; CitationWitton et al 2003). Moreover, co-expression of HER receptors (EGFR/HER-2, HER-2/HER-3, HER-2/HER-4) is likely to have clinical importance, due to the possible synergistic or antagonistic effect among HER family members.
The results of studies evaluating the HER family demonstrate a complex expression pattern of HER receptors in breast cancer patients. Moreover, the available data provide evidence of an implication of HER-3 and HER-4 alterations in breast carcinogenesis. Thus, it is likely that HER-3 and HER-4 could have a role as prognostic markers and that their integration into the routine management of the disease would provide useful additional information. Taken together, the findings of the relevant studies indicate that the combined expression profile of the HER family, and not the isolated expression of individual members, is likely to be more important when assessing the prognosis of the patients. Therefore, it is possible that studies evaluating the HER receptors as a whole panel may shed light on the role of the HER family in breast carcinogenesis and open new directions in patient management.
Targeting the HER family
Based on the evidence implicating the HER family in breast cancer pathogenesis, numerous approaches aimed at targeting these receptors have been developed (CitationPetrelli et al 2008). The dependency of HER-2 overexpressing breast tumors on HER-2 activity has rendered this receptor an attractive target. A humanized monoclonal antibody directed against the HER-2 protein, trastuzumab (Herceptin), has demonstrated substantial efficacy in breast cancer and has been considered as a “therapeutic revolution” in the management of the disease. Clinical trials evaluating trastuzumab monotherapy in HER-2 positive metastatic breast cancer have indicated overall response rates ranging from 15% to 30% (CitationVogel et al 2002; CitationBaselga et al 2005a). The pivotal phase III study showed that the addition of trastuzumab to first-line chemotherapy [either doxorubicin (or epirubicin) and cyclophosphamide or paclitaxel] was associated with a longer survival (median survival, 25.1 vs 20.3 months; P = 0.01) in patients with metastatic breast cancer and HER-2 overexpression (CitationSlamon et al 2001). Furthermore, a recent phase II randomized trial which compared first-line trastuzumab plus docetaxel versus docetaxel alone in patients with HER-2 positive metastatic breast cancer, showed a survival advantage (median survival, 31.2 vs 22.7 months; P = 0.0325) from the addition of trastuzumab to chemotherapy (CitationMarty et al 2005). Various nonrandomized studies have demonstrated the activity of trastuzumab in combination with the majority of chemotherapeutic agents used in the management of breast cancer.
The efficacy of trastuzumab in patients with advanced disease prompted the evaluation of this monoclonal antibody in patients with HER-2 positive early breast cancer. Four randomized trials have been recently reported, showing that the addition of trastuzumab to adjuvant chemotherapy halves the risk of relapse (CitationRomond et al 2005; CitationPiccart-Gebhart et al 2005; CitationSlamon et al 2005; CitationJoensuu et al 2006). Moreover, in the joint analysis of two North-American trials, treatment with trastuzumab for 52 weeks, combined with paclitaxel after doxorubicin and cyclophosphamide, was associated with a 33 percent reduction in the risk of death (P = 0.015) among women with surgically removed HER-2 positive breast cancer (CitationRomond et al 2005). Likewise, after a median follow-up of 2 years in the Herceptin Adjuvant (HERA) study which compared 1 or 2 years of trastuzumab treatment with observation alone after standard neoadjuvant or adjuvant chemotherapy, 1 year of treatment with trastuzumab was associated with a significant reduction in the risk of death (P = 0.0115) (CitationSmith et al 2007). However, only interim analyses with relatively short follow-up have been reported so far and thus, important issues with respect to the cumulative toxicity and the optimal duration of use of trastuzumab in the adjuvant treatment of early breast cancer remain unclear.
The mechanisms of trastuzumab action have not been fully elucidated yet (CitationValabrega et al 2007). Accumulating data indicate that the effect of trastuzumab on cancer cells may be due to the activation of antibody-dependent cellular cytotoxicity (ADCC) (CitationLewis et al 1993; CitationCooley et al 1999; CitationClynes et al 2000; CitationGennari et al 2004). Other possible mechanisms of action include inhibition of shedding of the extracellular HER-2 domain (CitationMolina et al 2001), induction of HER-2 downregulation and degradation (CitationAustin et al 2004; CitationValabrega et al 2005), inhibition of the PI3-K pathway (CitationDelord et al 2005), inhibition of angiogenesis (CitationIzumi et al 2002; CitationKlos et al 2003) and G1 cell cycle arrest (CitationLane et al 2001).
Resistance to trastuzumab treatment may be either primary or secondary. It has been shown that only 15% to 30% of HER-2 overexpressing metastatic breast cancers responded to trastuzumab monotherapy (CitationVogel et al 2002; CitationBaselga et al 2005a). In the majority of these cases the disease will progress, usually within one year (CitationSlamon et al 2001). Even in the adjuvant setting, approximately 15% of patients eventually develop metastatic disease. Thus, both de novo and acquired resistance are significant problems in patients treated with trastuzumab (CitationBender and Nahta 2008). Although the development of resistance remains unclear, several hypotheses have been suggested. These include loss of the tumor-suppressor phosphatase with tensin homologue (PTEN) (CitationNagata et al 2004), activation of alternative signaling pathways such as insulin-like growth factor-I receptor (IGF-IR) pathway (CitationLu et al 2001), increased expression of ligands of the HER family receptors such as transforming growth factor-α (TGF-α) (CitationValabrega et al 2005) and receptor masking or epitope inaccessibility (CitationNagy et al 2005).
Another potential mechanism of resistance is the presence of multiple truncated forms of HER-2 and the effects of these forms on trastuzumab response (CitationNahta and Esteva 2007). HER-2 targeted monoclonal antibodies have been shown to bind to circulating HER-2 ECD, decreasing the level of antibodies available to bind to membrane-bound HER-2 (CitationZabrecky et al 1991). Moreover, the accumulation of truncated forms of the HER-2 receptor that lack the extracellular trastuzumab-binding domain represents another possible mechanism of resistance. Amino terminally truncated carboxyl terminal fragments of HER-2, collectively known as p95HER2 or C-terminal fragments, are frequently found in HER-2 overexpressing breast cancer cell lines and tumors (CitationMolina et al 2002). These fragments result either from alternative translation start sites (CitationAnido et al 2006) or through the proteolytic shedding of the extracellular domain of HER-2 (CitationChristianson et al 1998). In a recent study (CitationScaltriti et al 2007), breast cancer cells stably expressing p95HER2 were resistant to trastuzumab but remained sensitive to the antiproliferative effects of the tyrosine kinase inhibitor (TKI) lapatinib, both in vitro and in vivo. Furthermore, in a series of patients with HER-2 positive metastatic breast cancer treated with trastuzumab, the presence of p95HER2 was correlated with clinical resistance, whereas tumors expressing only the full-length receptor exhibited a high response rate (CitationScaltriti et al 2007).
Trastuzumab engages both activatory (fragment C receptor [Fc gamma R] IIIa; Fc gamma RIIa) and inhibitory (Fc gamma RIIb) antibody receptors. Fc gamma R polymorphisms may affect the ADCC of natural-killer cells/monocytes. Recently, CitationMusolino and colleagues (2008) evaluated the role of Fc gamma RIIIa, Fc gamma RIIa, and Fc gamma RIIb polymorphisms in predicting activity of trastuzumab in patients with HER-2 positive metastatic breast cancer. In this study, the Fc gamma RIIIa 158 valine/valine (V/V) genotype, alone and in combination with the Fc gamma RIIa 131 histidine/histidine (H/H) genotype, was significantly associated with better response rate and progression-free survival to trastuzumab compared with other Fc gamma R genotypes. Moreover, ADCC analysis showed that 158 V/V and/or 131 H/H peripheral blood mononuclear cells (PBMCs) had a significantly higher trastuzumab-mediated cytotoxicity than PBMCs harboring other genotypes. This study supports the hypothesis that Fc gamma R polymorphisms play a role in trastuzumab-mediated ADCC and have predictive ability in patients with breast cancer treated with trastuzumab-based therapy.
Since a considerable proportion of patients do not respond to trastuzumab, the evaluation of additional molecular parameters such as alternate HER family members or the co-expression profile of HER receptors, is an ongoing challenge. Cetuximab is a chimeric monoclonal antibody that competitively binds to the extracellular domain of the EGFR. A randomized phase II study evaluated the combination of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer (CitationO’Shaughnessy et al 2007). The preliminary assessment showed that the addition of cetuximab to chemotherapy was associated with a higher response rate but also with greater toxicity. Clinical studies evaluating EGFR TKIs failed to demonstrate activity in metastatic cancer patients with disease refractory to chemotherapy (CitationRoy and Perez 2006). Available data from phase II trials which investigated the EGFR TKI gefitinib in pretreated patients have shown limited efficacy (CitationAlbain et al 2002; CitationBaselga et al 2005b; Citationvon Minckwitz et al 2005). Furthermore, when gefitinib was combined with first-line chemotherapy, an additional benefit was not found (CitationFountzilas et al 2005). A combination of erlotinib with docetaxel as first-line treatment resulted in a response rate of 55% (CitationKaur et al 2006). However, the nonrandomized nature of this trial does not clarify the added benefit with respect to the efficacy of erlotinib. A number of studies have demonstrated underexpression of the EGFR receptor in breast tumors (CitationDittadi et al 1993; CitationRobertson et al 1996; CitationDeFazio et al 2000; CitationBieche et al 2003). Moreover, in another study a marked reduction of EGFR expression with breast cancer progression was found, and such a decrease of expression of the receptor was associated with resistance to gefitinib in vitro (CitationChoong et al 2007). These findings might be an explanation for the low activity of EGFR TKIs in breast cancer and are likely to have implications in the design of further clinical trials targeting the HER family.
It has been suggested that the form of EGFR/HER-2 dimers might be important for breast cancer cell growth and thus, the inhibition of these receptors could possibly block cell proliferation (CitationJannot et al 1996). Recently, it has been found that the growth inhibitory activity of trastuzumab on HER-2 overexpressing breast cancer cells is significantly modulated by EGFR co-expression (CitationDiermeier et al 2005). Therefore, it is likely that the optimization of treatments targeting the HER family requires to account for EGFR co-expression. Lapatinib is an oral dual TKI selective for inhibition of EGFR and HER-2. It shows synergy with trastuzumab, and has demonstrated clinical activity in trastuzumab-resistant tumors (CitationBlackwell et al 2004). Recent data provide encouraging evidence of the effectiveness of lapatinib in advanced breast cancer and for its potential in patients with brain metastases (CitationGomez et al 2005; CitationGeyer et al 2006; CitationCameron et al 2008). Several clinical studies exploring the activity of lapatinib in combination with chemotherapeutic agents, hormonal therapy and other targeted treatments are ongoing in advanced or in neo-adjuvant and adjuvant settings (CitationBilancia et al 2007). In contrast, dual targeting of EGFR and HER-2 using concomitant gefitinib and trastuzumab might be detrimental in breast cancer patients, due to a possible antagonistic effect between these agents (CitationECOG E1100 2003).
The relatively limited activity of TKIs in HER-2 overexpressing breast tumors is likely to be associated with a failure to inhibit HER-3 efficiently. Even though these agents block EGFR and HER-2 autophosphorylation, the transphosphorylation of HER-3 is only transiently inhibited, leading to PI3-K/Akt pathway resistance (CitationSergina et al 2007). Therefore, the HER-3 receptor might also present a challenging target that could potentially overcome TKI resistance. However, the inhibition of HER-3 using current therapeutic approaches would be difficult since this receptor is catalytic kinase deficient and thus, not a direct target of TKIs (CitationHsieh and Moasser 2007). Moreover, another therapeutic approach is to inhibit simultaneously all members of the HER family using TKIs such as canertinib. Nevertheless, a number of studies have indicated that HER-4 antagonizes the effect of HER-2 on the clinical course of breast cancer (CitationSuo et al 2002) and thus, the use of pan-HER targeted treatments could possibly attenuate the favorable effect of HER-4 on patient outcome.
Accumulating data suggest that ER and HER-2 have a bidirectional cross talk which leads to tamoxifen resistance or conversion of tamoxifen to an ER agonist (CitationPietras et al 1995; CitationShou et al 2004; CitationYang et al 2004). Increased expression of EGFR and HER-2 receptors might be associated with tamoxifen resistance (CitationSchiff et al 2005; CitationMassarweh et al 2008). In a recent study (CitationKirkegaard et al 2007), high amplified in breast cancer 1 (AIB1) expression in patients with HER-2 and HER-3 overexpressing tumors or tumors expressing one or more of EGFR, HER-2, or HER-3 was associated with an increased risk of relapse on tamoxifen. These findings indicate a cross-talk between ER-alpha and growth factor receptor pathways through changes in expression of specific coactivator proteins, such as AIB1. A number of studies have investigated the use of drugs against EGFR, in tamoxifen-resistant breast cancer (CitationNicholson et al 2002; CitationKnowlden et al 2003; CitationNicholson et al 2004). Moreover, clinical trials have examined the inhibition of growth factor signaling as a therapeutic strategy in endocrine-resistant breast cancer patients (CitationRobertson et al 2003; CitationMarcom et al 2007).
In conclusion, the HER family represents an attractive area for the application of targeted therapies in breast cancer and considerable treatment advances have been made so far. However, the incorporation of targeted agents into the treatment of the disease has been associated with variable and in some cases unexpected results. HER-2 overexpression alone is probably inadequate to predict the impact of targeted agents on cell proliferation. Since trans-signaling is now considered an essential feature of HER family function, the role of lateral signaling partners such as HER-3 is increasingly recognized. Studies including a more comprehensive evaluation of all HER receptors and their ligands are required to elucidate how these different signaling pathways interact in breast carcinogenesis, providing a basis for the development of targeted treatments with respect to individualized patient management.
Disclosure
The authors report no conflicts of interest in this work.
References
- AlbainKElledgeRGradisharWJ2002Open-label, phase II, multicenter trial of ZD1839 (‘Iressa’) in patients with advanced breast cancerBreast Cancer Res Treat76Suppl 1A20
- AlimandiMRomanoACuriaMC1995Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomasOncogene101813217538656
- AllredDCClarkGMTandonAK1992Her-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinomaJ Clin Oncol105996051548522
- AnidoJScaltritiMBech SerraJJ2006Biosynthesis of tumorigenic HER-2 C-terminal fragments by alternative initiation of translationEMBO J2532344416794579
- ArnouldLFargeotPBonneterreJ2003Epirubicin dose response effect in node positive breast cancer patients is independent of HER2 overexpression: 10-year retrospective analysis of French Adjuvant Study Group 05 trialBreast Cancer Res Treat76A538
- AubeleMAuerGWalchAK2007PTK (protein tyrosine kinase)- 6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomasBr J Cancer96801717299391
- AustinCDDe MaziereAMPisacanePI2004Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycinMol Biol Cell1552688215385631
- BarnesNLKhavariSBolandGP2005Absence of HER-4 expression predicts recurrence of ductal carcinoma in situ of the breastClin Cancer Res112163815788662
- BartlettJMallonECookeT2003The clinical evaluation of HER-2 status: which test to use?J Pathol1994111712635130
- BaselgaJAlbanellJRuizA2005bPhase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancerJ Clin Oncol2353233315939921
- BaselgaJCarbonellXCastaneda-SotoNJ2005aPhase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly scheduleJ Clin Oncol2321627115800309
- BenderLMNahtaR2008Her2 cross talk and therapeutic resistance in breast cancerFront Biosci1339061218508484
- BenöhrPHenkelVSpeerR2005Her-2/neu expression in breast cancer – A comparison of different diagnostic methodsAnticancer Res25189590016158923
- BergqvistJOhdJFSmedsJ2007Quantitative real-time PCR analysis and microarray-based RNA expression of HER2 in relation to outcomeAnn Oncol188455017351254
- BiecheIOnodyPLaurendeauI1999Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applicationsClin Chem4511485610430778
- BiecheIOnodyPTozluS2003Prognostic value of ERBB family mRNA expression in breast carcinomasInt J Cancer1067586512866037
- BilanciaDRosatiGDinotaA2007Lapatinib in breast cancerAnn Oncol182630
- BlackwellKKaplanEFrancoS2004A phase II, open-label, multicenter study of GW572016 in patients with trastuzumab-refractory metastatic breast cancer [abstract]Proc Am Soc Clin Oncol223006
- BloomK2005The distribution of EGFR mRNA expression in 247 breast carcinomas and its relationship to ER mRNA expression [abstract]San Antonio Breast Cancer Symposium3039
- BonneterreJRocheHKerbratP200310-Year update of benefit/risk ratio after adjuvant chemotherapy (CT) in node positive (N+), early breast cancer (EBC) patients (pts) [abstract]Proc Am Soc Clin Oncol2293
- BorgATandonAKSigurdssonH1990HER-2/neu amplification predicts poor survival in node-positive breast cancerCancer Res50432272114213
- BossardCBiecheILe DoussalV2005Real-time RT-PCR: a complementary method to detect HER-2 status in breast carcinomaAnticancer Res2546798316334160
- CameronDCaseyMPressM2008A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analysesBreast Cancer Res Treat Epub ahead of print
- ChanSKHillMEGullickWJ2006The role of the epidermal growth factor receptor in breast cancerJ Mammary Gland Biol Neoplasia1131116947082
- ChazinVRKalekoMMillerAD1992Transformation mediated by the human HER-2 gene independent of the epidermal growth factor receptorOncogene71859661354348
- ChoongLYLimSLohMC2007Progressive loss of epidermal growth factor receptor in a subpopulation of breast cancers: implications in target-directed therapeuticsMol Cancer Ther628284217989321
- ChristiansonTADohertyJKLinYJ1998NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancerCancer Res58512399823322
- CitriASkariaKBYardenY2003The deaf and the dumb: The biology of ErbB-2 and ErbB-3Exp Cell Res284546512648465
- ClarkGMMcGuireWL1991Follow-up study of HER-2/neu amplification in primary breast cancerCancer Res5194481988136
- ClynesRATowersTLPrestaLG2000Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targetsNat Med6443610742152
- CooleySBurnsLJRepkaT1999Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neuExp Hematol2715334110517495
- De LaurentiisMCaputoFMassarelliE2001HER2 expression and anthracycline effect: results from the Naples GUN 3 randomized trial [abstract]Proc Am Soc Clin Oncol20133
- De PlacidoSPerroneFCarlomagnoC1995CMF vs alternating CMF/EV in the adjuvant treatment of operable breast cancer: a single centre randomised clinical trial (Naples GUN-3 study)Br J Cancer71128377779724
- DeFazioAChiewY-ESiniRL2000Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell linesInt J Cancer874879810918187
- Del MastroLBruzziPVenturiniM2004HER2 expression and efficacy of dose-dense anthracycline containing adjuvant chemotherapy in early breast cancer patients [abstract]Proc Am Soc Clin Oncol22571
- DelordJPAllalCCanalM2005Selective inhibition of HER2 inhibits AKT signal transduction and prolongs disease-free survival in a micrometastasis model of ovarian carcinomaAnn Oncol1618899716219625
- Di LeoAGancbergDLarsimontD2002HER-2 amplification and topoisomerase II alpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracilClin Cancer Res811071612006526
- Di LeoALarsimontDBeauduinM1999CMF or anthracycline-based adjuvant chemotherapy for node-positive breast cancer patients: 4-year results of a Belgian randomized clinical trial with predictive markers analysis [abstract]Proc Am Soc Clin Oncol1869
- Di LeoALarsimontDGancbergD2001Her-2 and topoisomerase IIalpha as predictive markers in a population of node-positive breast cancer patients randomly treated with adjuvant CMF or epirubicin plus cyclophosphamideAnn Oncol121081911583189
- Di LeoA2007The state of HER-2 statusAnn Oncol188131517488732
- DiermeierSHorvathGKnuechel-ClarkeR2005Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activationExp Cell Res3046041915748904
- DiGiovannaMPLermanMACoffeyRJ1998Active signaling by Neu in transgenic miceOncogene171877849778054
- DiGiovannaMPSternDFEdgertonSM2005Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patientsJ Clin Oncol2311526015718311
- DittadiRDonisiPMBrazzaleA1993Epidermal growth factor receptor in breast cancer. Comparison with non malignant breast tissueBr J Cancer67798427782
- DowsettMBartlettJEllisIO2003Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centresJ Pathol1994182312635131
- DowsettMCookeTEllisI2000Assessment of HER2 status in breast cancer: why, when, and how?Eur J Cancer36170610741274
- DresslerLGBerryDABroadwaterG2005Comparison of HER2 status by fluorescence in situ hybridisation and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patientsJ Clin Oncol2342879715994142
- ECOG E11002003A phase II trial of trastuzumab and gefitinib in patients with metastatic breast cancer that overexpress HER2/neu (erbB-2)Clin Adv Hematol Oncol123716224413
- EllisIOBartlettJDowsettM2004Best practice No 176: updated recommendations for HER2 testing in the UKJ Clin Pathol57233714990588
- EstevaFJSahinAACristofanilliM2005Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapyClin Cancer Res1133151915867229
- FerreroJMRamaioliALargillierR2001Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-upAnn Oncol12841611484962
- FountzilasGPectasidesDKalogera-FountzilaA2005Paclitaxel and carboplatin as first-line chemotherapy combined with gefitinib (IRESSA) in patients with advanced breast cancer: a phase I/II study conducted by the Hellenic Cooperative Oncology GroupBreast Cancer Res Treat921915980985
- FoxSBSmithKHollyerJ1994The epidermal growth factor receptor as a prognostic marker: results of 370 patients and review of 3009 patientsBreast Cancer Res Treat294198018963
- GaspariniGGullickWJMalutaS1994C-erbB-3 and c-erbB-2 protein expression in node-negative breast carcinoma. An immunocytochemical studyEur J Cancer30A16227908213
- GennariASormaniMPPronzatoP2008HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trialsJ Natl Cancer Inst100142018159072
- GennariRMenardSFagnoniF2004Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2Clin Cancer Res105650515355889
- GeyerCEForsterJLindquistD2006Lapatinib plus capecitabine for HER2-positive advanced breast cancerN Engl J Med35527334317192538
- GinestierCCharafe-JauffretEPenault-LlorcaF2004Comparative multi-methodological measurement of ERBB2 status in breast cancerJ Pathol2022869814991893
- GjerdrumLMSorensenBSKjeldsenE2004Real-time quantitative PCR of microdissected paraffin-embedded breast carcinoma: an alternative method for HER-2/neu analysisJ Mol Diagn6425114736826
- GomezHChavezMDovalD2005A phase II, randomized trial using the small molecule tyrosine kinase inhibitor lapatinib as a first-line treatment in patients with FISH positive advanced or metastatic breast cancer [abstract]Proc Am Soc Clin Oncol233046
- Gonzalez-AnguloAMKrishnamurthySYamamuraY2004Lack of association between amplification of her-2 and response to preoperative taxanes in patients with breast carcinomaCancer1012586315241821
- Graus-PortaDBeerliRRDalyJM1997ErbB-2, the preferred heterodimerization partner of all HER receptors, is a mediator of lateral signalingEMBO J161647559130710
- GullickWJSrinivasanR1998The type I growth factor receptor family: new ligands and receptors and their role in breast cancerBreast Cancer Res Treat52435310066071
- GustersonBAGelberRDGoldhirschA1992Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study GroupJ Clin Oncol101049561351538
- HarrisRCChungECoffeyRJ2003EGF receptor ligandsExp Cell Res28421312648462
- HartmannLCIngleJNWoldLE1994Prognostic value of c-erbB2 overexpression in axillary lymph node positive breast cancer. Results from a randomized adjuvant treatment protocolCancer742956637954259
- HayesDFThorADDresslerLG2007Cancer and Leukemia Group B (CALGB) Investigators. HER2 and response to paclitaxel in node-positive breast cancerN Engl J Med357149650617928597
- HolbroTBeerliRRMaurerF2003The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferationProc Natl Acad Sci U S A1008933812853564
- HsiehACMoasserMM2007Targeting HER proteins in cancer therapy and the role of the non-target HER3Br J Cancer97453717667926
- IsolaJTannerMForsythA2004Interlaboratory comparison of HER-2 oncogene amplification as detected by chromogenic and fluorescence in situ hybridizationClin Cancer Res104793815269154
- IzumiYXuLDiTE2002Tumour biology: herceptin acts as an anti-angiogenic cocktailNature4162798011907566
- JacobsTWGownAMYazijiH1999Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancerJ Clin Oncol1719748210561247
- JannotCBBeerliRRMasonS1996Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cellsOncogene13275828710366
- JoensuuHKellokumpu-LehtinenPLBonoP2006Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancerN Engl J Med3548092016495393
- KainulainenVSundvallMMäättäJA2000A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxisJ Biol Chem2758641910722704
- KaramouzisMVBadraFAPapavassiliouAG2007Breast cancer: The upgraded role of HER-3 and HER-4Int J Biochem Cell Biol39851617254832
- KaurHSilvermanPSinghD2006Toxicity and outcome data in a phase II study of weekly docetaxel in combination with erlotinib in recurrent and/or metastatic breast cancer (MBC)J Clin Oncol24Suppl 1810623
- KewTYBellJAPinderSE2000C-erbB-4 protein expression in human breast cancerBr J Cancer8211637010735500
- KimYRChoiJRSongKS2002Evaluation of HER2/neu status by real-time quantitative PCR in breast cancerYonsei Med J433354012089741
- KirkegaardTMcGlynnLMCampbellFM2007Amplified in breast cancer 1 in human epidermal growth factor receptor – positive tumours of tamoxifen-treated breast cancer patientsClin Cancer Res1314051117332282
- KjeldsenEK⊘lvraaSRautenstraussBLiehrT2002FISH techniques, FISH probes and their applications in medicine and biology: an overviewFISH TechnologyBerlinSpringer350
- KlijnJGBernsPMSchmitzPI1992The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patientsEndocr Rev133171313356
- KlosKSZhouXLeeS2003Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment aloneCancer9813778514508823
- KnowldenJMGeeJMWSeeryLT1998c-erbB-3 and c-erbB-4 expression is a feature of the endocrine responsive phenotype in clinical breast cancerOncogene171949579788438
- KnowldenJMHutchesonIRJonesHE2003Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen resistant MCF-7 cellsEndocrinology14410324412586780
- KokaiYMyersJNWadaT1989Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblastsCell58287922568888
- KonecnyGEThomssenCLuckHJ2004Her-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancerJ Natl Cancer Inst9611415115292386
- KönigshoffMWilhelmJBohleRM2003HER-2/neu gene copy number quantified by real-time PCR: comparison of gene amplification, heterozygosity, and immunohistochemical status in breast cancer tissueClin Chem492192912560343
- KostopoulosIArapantoni-DadiotiPGogasH2006Evaluation of the prognostic value of HER-2 and VEGF in breast cancer patients participating in a randomized study with dose–dense sequential adjuvant chemotherapyBreast Cancer Res Treat962516116538542
- KrauseDSVan EttenRA2005Tyrosine kinases as targets for cancer therapyN Engl J Med3531728716014887
- KulkaJTôkésAMKaposi-NovákP2006Detection of HER-2/neu gene amplification in breast carcinomas using quantitative real-time PCR – A comparison with immunohistochemical and FISH resultsPathol Oncol Res1219720417189981
- LaaksoMTannerMIsolaJ2006Dual-colour chromogenic in situ hybridization for testing of HER-2 oncogene amplification in archival breast tumoursJ Pathol2103916823892
- LabuhnMVuaroqueauxVFinaF2006Simultaneous quantitative detection of relevant biomarkers in breast cancer by quantitative real-time PCRInt J Biol Markers2130916711511
- LaneHAMotoyamaABBeuvinkI2001Modulation of p27/Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signalingAnn Oncol12Suppl 1S21S2211521716
- LebeauAUnholzerAAmannG2003EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breastBreast Cancer Res Treat791879812825853
- LeeHAkitaRWSliwkowskiMX2001A naturally occurring secreted human ErbB3 receptor isoform inhibits heregulin-stimulated activation of ErbB2, ErbB3, and ErbB4Cancer Res6144677311389077
- LeeYChoSSeoJH2007Correlated expression of erbB-3 with hormone receptor expression and favourable clinical outcome in invasive ductal carcinomas of the breastAm J Clin Pathol1281041918024331
- LemoineNRBarnesDMHollywoodDP1992Expression of the ERBB3 gene product in breast cancerBr J Cancer661116211333787
- LewisGDFigariIFendlyB1993Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodiesCancer Immunol Immunother37255638102322
- LiuBOrdonez-ErcanDFanZ2007Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cellsInt J Cancer12018748217266042
- LuYZiXZhaoY2001Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin)J Natl Cancer Inst931852711752009
- MansourEGGrayRShatilaAH1989Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer: an intergroup studyN Engl J Med320485902915651
- MarcomPKIsaacsCHarrisL2007The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancersBreast Cancer Res Treat10243916897431
- MarsiglianteSMuscellaACiardoV1993Enzyme-linked immunosorbent assay of HER-2/neu gene product (p185) in breast cancer: its correlation with sex steroid receptors, cathepsin D and histologic gradesCancer Lett751952067906196
- MartyMCognettiFMaraninchiD2005Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study groupJ Clin Oncol2342657415911866
- MassR2000The role of HER-2 expression in predicting response to therapy in breast cancerSemin Oncol27465211236028
- MassarwehSOsborneCKCreightonCJ2008Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic functionCancer Res688263318245484
- MasudaNOhnishiTKawamotoS1999Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samplesNucleic Acids Res2744364310536153
- MitchellMSPressMF1999The role of immunohistochemistry and fluorescence in situ hybridization for HER2/neu in assessing the prognosis of breast cancerSemin Oncol261081610482202
- MoasserMM2007The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesisOncogene2664698717471238
- MolinaMACodony-ServatJAlbanellJ2001Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cellsCancer Res614744911406546
- MolinaMASaezRRamseyEE2002NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancerClin Cancer Res83475311839648
- MoliterniAMenardSValagussaP2003HER2 overexpression and doxorubicin in adjuvant chemotherapy for resectable breast cancerJ Clin Oncol214586212560435
- MosessonYYardenY2004Oncogenic growth factor receptors: Implications for signal transduction therapySemin Cancer Biol142627015219619
- MrhalovaMKodetRKalinovaM2003Relative quantification of ERBB2 mRNA in invasive duct carcinoma of the breast: correlation with ERBB2 protein expression and ERBB2 gene copy numberPathol Res Pract1994536114521261
- MullerWJArteagaCLMuthuswamySK1996Synergistic interaction of the Neu proto-oncogene product and transforming growth factor alpha in the mammary epithelium of transgenic miceMol Cell Biol165726368816486
- MusolinoANaldiNBortesiB2008Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancerJ Clin Oncol2617899618347005
- MussHBThorADBerryDA1994c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancerN Engl J Med330126067908410
- NagataYLanKHZhouX2004PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patientsCancer Cell61172715324695
- NagyPFriedlanderETannerM2005Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell lineCancer Res654738215695389
- NahtaREstevaFJ2007Trastuzumab: triumphs and tribulationsOncogene2636374317530017
- NaiduRYadavMNairS1998Expression of c-erbB3 protein in primary breast carcinomasBr J Cancer781385909823984
- NareshALongWVidalGA2006The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cellsCancer Res6664122016778220
- NeveRMSutterlutyHPullenN2000Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cellsOncogene1916475610763821
- NicholsonRIGeeJMHarperME2001EGFR and breast cancer prognosisEur J Cancer3791511165124
- NicholsonRIHutchesonIRHarperME2002Modulation of epidermal growth factor receptor in endocrine-resistant, estrogen-receptor-positive breast cancerAnn N Y Acad Sci96310415
- NicholsonRIHutchesonIRKnowldenJM2004Nonendocrine pathways and endocrine resistance: observations with antiestrogens and signal transduction inhibitors in combinationClin Cancer Res101 Pt 2346S54S14734490
- NtouliaMKaklamanisLValavanisC2006HER-2 DNA quantification of paraffin-embedded breast carcinomas with Light Cycler real-time PCR in comparison to immunohistochemistry and chromogenic in situ hybridizationClin Biochem39942616916505
- O’MalleyFPParkesRLattaE2001Comparison of HER2/neu status assessed by quantitative polymerase chain reaction and immunohistochemistryAm J Clin Pathol1155041111293897
- O’ShaughnessyJWecksteinDJVukeljaSJ2007Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancerBreast Cancer Res Treat106Suppl 1S32
- PaikSBryantJParkC1998erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancerJ Natl Cancer Inst901361709747867
- PaikSBryantJTan-ChiuE2000HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-15J Natl Cancer Inst921991811121461
- PaikSBryantJTan-ChiuE2002Real-world performance of HER2 testing-National Surgical Adjuvant Breast and Bowel Project experienceJ Natl Cancer Inst94852412048273
- PatersonMCDietrichKDDanylukJ1991Correlation between c-erb-2 amplification and risk of recurrent disease in node-negative breast cancerCancer Res515667
- PawlowskiVRevillionFHebbarM2000Prognostic value of the type I growth factor receptors in a large series of human primary breast cancers quantified with a real-time reverse transcription-polymerase chain reaction assayClin Cancer Res642172511106235
- PerezEASumanVJDavidsonNE2006HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trialJ Clin Oncol243032816809727
- PetitTBorelCGhnassiaJP2001Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant settingClin Cancer Res715778111410493
- PetrelliFCabidduMCazzanigaME2008Targeted therapies for the treatment of breast cancer in the post-trastuzumab eraOncologist133738118448551
- Piccart-GebhartMJProcterMLeyland-JonesB2005Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancerN Engl J Med35316597216236737
- PietrasRJArboledaJReeseDM1995HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cellsOncogene102435467784095
- PotemskiPPluciennikEBednarekAK2006A comparative assessment of HER2 status in operable breast cancer by real-time RT-PCR and by immunohistochemistryMed Sci Monit125761
- PressMFHungGGodolphinW1994Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expressionCancer Res54277177909495
- PrigentSAGullickWJ1994Identification of c-erbB-3 binding sites for phosphatidylinositol 3’-kinase and SHC using an EGF receptor/c-erbB-3 chimeraEMBO J132831418026468
- PritchardKIShepherdLEO’MalleyFP2006HER2 and responsiveness of breast cancer to adjuvant chemotherapyN Engl J Med35421031116707747
- QuenelNWafflartJBonichonF1995The prognostic value of c-erbB-2 in primary breast carcinomas: a study on 942 casesBreast Cancer Res Treat35283917579499
- QuinnCMOstrowskiJLLaneSA1994C-erbB-3 protein expression in human breast cancer: comparison with other tumour variables and survivalHistopathology25247527821892
- RampaulRSPinderSENicholsonRI2005Clinical value of epidermal growth factor receptor expression in primary breast cancerAdv Anat Pathol12271316210923
- RampaulRSPinderSEWencykPM2004Epidermal growth factor receptor status in operable invasive breast cancer: is it of any prognostic value?Clin Cancer Res10257815073139
- RavdinPMGreenSAlbainK1998Initial report of the SWOG biological correlative study of c-erbB-2 expression as a predictor of outcome in a trial comparing adjuvant CAF T with tamoxifen (T) alone [abstract]Proc Am Soc Clin Oncol1797
- RilkeFColnaghiMICascinelliN1991Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factorsInt J Cancer494491678734
- RobertsonJGutteridgeECheungK2003Gefitinib (ZD1839) is active in acquired tamoxifen (TAM)-resistant oestrogen receptor (ER)-positive and ER-negative breast cancer: results from a phase II study [abstract]Proc Am Soc Clin Oncol2223
- RobertsonKWReevesJRSmithG1996Quantitative estimation of epidermal growth factor receptor and c-erbB-2 in human breast cancerCancer Res563823308706030
- RochePCSumanVJJenkinsRB2002Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831J Natl Cancer Inst94855712048274
- RodenhuisSBontenbalMBeexL2003High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancerN Engl J Med34971612840087
- RomondEHPerezEABryantJ2005Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancerN Engl J Med35316738416236738
- RossJSFletcherJA1998The Her-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapyStem Cells16413289831867
- RoyVPerezEA2006New therapies in the treatment of breast cancerSemin Oncol3338
- SartorCIZhouHKozlowskaE2001Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cellsMol Cell Biol2142657511390655
- SassenARochonJWildPJ2008Cytogenetic analysis of HER1/EGFR, HER2, HER3, AND HER4 in 278 breast cancer patientsBreast Cancer Res10R218182100
- ScaltritiMRojoFOcañaA2007Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancerJ Natl Cancer Inst996283817440164
- SchiffRMassarwehSAShouJ2005Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulatorsCancer Chemother Pharmacol56102016273359
- SchlemmerBOSorensenBSOvergaardJ2004Quantitative PCR-new diagnostic tool for quantifying specific mRNA and DNA molecules: HER2/neu DNA quantification with Light Cycler real-time PCR in comparison with immunohistochemistry and fluorescence in situ hybridizationScand J Clin Lab Invest645112215276916
- SerginaNVRauschMWangD2007Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3Nature4454374117206155
- ShouJMassarwehSOsborneCK2004Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancerJ Natl Cancer Inst969263515199112
- SlamonDEiermannWRobertN2005Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 studyBreast Cancer Res Treat94Suppl 1S5
- SlamonDJClarkGMWongSG1987Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogeneScience235177823798106
- SlamonDJGodolphinWJonesLA1989Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience244707122470152
- SlamonDJLeyland-JonesBShakS2001Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med3447839211248153
- SmithIProcterMGelberRD20072-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trialLancet3699555293617208639
- SoltoffSPCarrawayKL3rdPrigentSA1994ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factorMol Cell Biol14355087515147
- SrinivasanRPoulsomRHurstHC1998Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour typesJ Pathol185236459771476
- SuoZDaehliKULindboeCF2004Real-time PCR quantification of c-erbB-2 gene is an alternative for FISH in the clinical management of breast carcinoma patientsInt J Surg Pathol123111815494857
- SuoZRisbergBKalssonMG2002EGFR family expression in breast carcinomas. C-erbB-2 and c-erbB-4 receptors have different effects on survivalJ Pathol196172511748637
- TangCKConcepcionXZMilanM1999Ribozyme-mediated downregulation of ErbB-4 in estrogen receptor-positive breast cancer cells inhibits proliferation both in vitro and in vivoCancer Res5953152210537315
- [TLBC] The Ludwig Breast Cancer Study Group1988Combination adjuvant chemotherapy for node-positive breast cancer: inadequacy of a single perioperative cycleN Engl J Med319677832901037
- [TLBC] The Ludwig Breast Cancer Study Group1989Prolonged disease-free survival after one course of perioperative adjuvant chemotherapy for node-negative breast cancerN Engl J Med32049162644533
- ThomsonTAHayesMMSpinelliJJ2001HER-2/neu in breast cancer: inter-observer variability and performance of immunohistochemistry with four antibodies compared with fluorescent in situ hybridizationMod Pathol1410798611706067
- ThorADBerryDABudmanDR1998erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancerJ Natl Cancer Inst901346609747866
- ToikkanenSHelinHIsolaJ1992Prognostic significance of Her-2 oncoprotein expression in breast cancer: a 30-year follow-upJ Clin Oncol10104481351537
- ToveySDunneBWittonCJ2005Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer?Clin Cancer Res1148354216000581
- TravisAPinderSERobertsonJFR1996C-erbB-3 in human breast carcinoma : expression and relation to prognosis and established prognostic indicatorsBr J Cancer74229338688326
- TseCBraultDGligorovJ2005Evaluation of the quantitative analytical methods real-time PCR for HER-2 gene quantification and ELISA of serum HER-2 protein and comparison with fluorescence in situ hybridization and immunohistochemistry for determining HER-2 status in breast cancer patientsClin Chem51109310115976096
- TsutsuiSOhnoSMurakamiS2002Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancerBreast Cancer Res Treat71677511859875
- UedaSTsudaHSatoK2005Differential overexpressions of EGFR, c-erbB-2, and IGF1R in histological types, nuclear grades, and hormone receptors status of breast carcinoma [abstract]San Antonio Breast Cancer Symposium5114
- Ursini-SiegelJSchadeBCardiffRD2007Insights from transgenic mouse models of ERBB2-induced breast cancerNat Rev Cancer73899717446858
- ValabregaGMontemurroFAgliettaM2007Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancerAnn Oncol189778417229773
- ValabregaGMontemurroFSarottoI2005TGFalpha expression impairs trastuzumab-induced HER2 downregulationOncogene2430021015735715
- Vanden BemptIVanhentenrijkVDrijkoningenM2005Real-time reverse transcription-PCR and fluorescence in-situ hybridization are complementary to understand the mechanisms involved in HER-2/neu overexpression in human breast carcinomasHistopathology464314115810955
- VinatzerUDampierBStreubelB2005Expression of HER2 and the coamplified genes GRB7 and MLN64 in human breast cancer: quantitative real-time reverse transcription-PCR as a diagnostic alternative to immunohistochemistry and fluorescence in situ hybridizationClin Cancer Res1183485716322295
- VogelCLCobleighMATripathyD2002Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancerJ Clin Oncol207192611821453
- von MinckwitzGJonatWFaschingP2005A multicentre phase II study on gefitinib in taxane-and anthracycline-pretreated metastatic breast cancerBreast Cancer Res Treat891657215692759
- WalkerRADearingSJ1999Expression of epidermal growth factor receptor mRNA and protein in primary breast carcinomasBreast Cancer Res Treat531677610326794
- WinstanleyJCookeTMurrayGD1991The long term prognostic significance of c-erb-2 in primary breast cancerBr J Cancer63447501672256
- WittonCJReevesJRGoingJJ2003Expression of the HER1–4 family of receptor tyrosine kinases in breast cancerJ Pathol200290712845624
- WolffACHammondMESchwartzJN2007American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancerJ Clin Oncol251184517159189
- WoodWCBudmanDRKorzunAH1994Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinomaN Engl J Med330125398080512
- YangZBarnesCJKumarR2004Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cellsClin Cancer Res103621815173068
- YardenYSliwkowskiMX2001Untangling the ErbB signalling networkNat Rev Mol Cell Biol21273711252954
- ZabreckyJRLamTMcKenzieSJ1991The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, sk-br-3J Biol Chem2661716201671042
- ZaczekAWełnicka-JaśekiewiczMBielawskiKP2008Gene copy numbers of HER family in breast cancerJ Cancer Res Clin Oncol134271917661082
- ZhangFYangYSmithT2003Correlation between HER-2 expression and response to neoadjuvant chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide in patients with breast carcinomaCancer9717586512655533