Abstract
Gastrointestinal stromal tumors (GISTs), the most common sarcoma of the GI tract, have unique kinase mutations that serve as targets for medical therapy. This article reviews the data supporting the use of the tyrosine kinase inhibitor (TKI) imatinib in GIST patients, and how this treatment should be combined with surgical resection (when possible) to optimize patient outcomes. Although surgical resection remains the mainstay of treatment for these tumors, patients with resected GISTs have high relapse rates that can be reduced by 1 year of adjuvant imatinib. Data also support the use of imatinib for patients with recurrent or unresectable GIST. In these patients the drug should be continued until progression, intolerance, or the patients are rendered resectable. Patients with advanced GIST who are successfully resected after imatinib treatment should be placed back on imatinib postoperatively. Patients who develop generalized progression (progression at 2 or more sites) on imatinib should move to other treatments, such as newer TKIs or other targeted approaches currently under study. Genotyping of the tumor should be considered in all pediatric GISTs and high risk adult GISTs, especially if there is progression on imatinib. Quality of life and the cost/benefit of new therapies are important issues for further study in patients with GIST.
Background: GI stromal tumors and tyrosine kinase inhibitors
Ramon y Cajal, a Spanish neuroanatomist and neurophysiologist, is credited with describing interstitial cells in Auerbach’s plexus which have since been found to have both neural and stromal features by electron microscopy (EM). Now known as the Interstitial Cells of Cajal (ICC), these cells are thought to give rise to gastrointestinal stromal tumors (GISTs), either directly, or, alternatively, GIST and ICC may arise from a common mesenchymal stem cell. Like the ICC, GISTs have a classic immunohistochemical (IHC) staining pattern (positive for CD34, S-100, DES, keratin, negative for desmin), and, also like the ICC, GIST cells strongly express two kinases, c-kit and platelet-derived growth factor receptor (PDGF-R). In the case of GIST, 95% express kit and approximately 7% express PDGF-R, usually in mutated forms.Citation1
A skilled and meticulous medical illustrator, Cajal was awarded the 1913 Nobel Prize (which he shared with Camillo Golgi) for his work.
Until the unique EM and IHC features of ICC and GIST were elucidated, GISTs were previously misclassified as leiomyosarcomas or other spindle cell cancers. In fact, GISTs are very different pathologically and clinically.Citation2 Importantly, GISTs are typically resistant to standard sarcoma adjuvant chemotherapy, with response rates (RRs) of ∼5%, and no impact on survival. Similarly, radiation therapy (RT) offers mostly morbidity due in part to the fact that the intrabdominal location of these tumors limits its use; RT may have some role for rectal GISTS.
Rather, as outlined in this review, the high kinase expression in these tumors has allowed for treatment of these tumors with tyrosine kinase inhibitors (TKIs),Citation3 which, when selectively combined with resection, has led to both a significant improvement in outcomes for this tumor and an explosive growth in our understanding of targeted therapy of solid tumors.
Resection of primary GISTs: pre-operative considerations
GIST may occur anywhere along the GI tract or elsewhere in the abdomen or retroperitoneum. Data from our and other institutions indicate that approximately 50% of primary GISTs are located in the stomach, 25% in the small bowel, and the rest are distributed in the colon, rectum, and esophagus.Citation4
Some GISTs are found incidentally on imaging studies such as CT or endoscopic ultrasound (EUS) or in biopsies done for other reasons. Indeed, the management of these “microGISTs” is controversial. Tumors measuring <1 cm, commonly located in the stomach, have been found in 22.5% of autopsies of German adults older than 50,Citation5 and in 35% of Japanese gastrectomy specimens removed for other reasons.Citation6 These small gastric GISTs are generally less aggressive than distal or larger tumors, and thus incidental gastric GISTs (usually found on endoscopy) can be followed with serial endoscopy and imaging as long as they remain asymptomatic. The management of incidental GISTs measuring 1 to 2 cm in size is more controversial. The mitotic rate of these tumors cannot be determined by fine needle aspiration, and therefore most surgeons would recommend resection, especially if it would involve minimal potential morbidity.
While all GISTs should be considered potentially malignant, nongastric GISTs are never considered “benign”, and therefore most would favor resection of these lesions irrespective of size.Citation7 Regardless, most GISTS are discovered on work-up for GI symptoms, and therefore are typically >2 cm and/or symptomatic at the time of discovery. These lesions clearly require therapy.
The 2007 National Comprehensive Center Network (NCCN) GIST Guidelines state that the first step in the management of a potentially respectable GIST is to determine resectability with tests such as computed tomography (CT) and/or magnetic resonance imaging (MRI), chest imaging, endoscopic ultrasound (EUS), and endoscopy. We favor triple phase (porto-venous, early arterial, and late arterial) CT scanning because it best elucidates and rules out liver metastases, the most frequent site of metastases. Fluorodeoxyglucose-positron emission tomography (FDG-PET) is an excellent functional test that can be helpful in demonstrating tumor response to imatinib, but its role in determining resectability of primary GISTS is limited and it is usually not indicated. Importantly, the NCCN guidelines stress the role of a multidisciplinary GI program in assessing possible resection for primary GIST. If metastatic disease is ruled out by these tests preoperative biopsy of suspected GISTs is usually not indicated; the NCCN recommends biopsy only if the tumor is unresectable, if the diagnosis in doubt, or if neoadjuvant therapy is planned.Citation8 If a biopsy is done EUS is usually the best method if the tumor is assessable endoscpically.Citation3
Resectable GISTs should be resected – surgery remains the principal and only potentially curative therapy for localized, resectable primary GIST.Citation7 After successful resection, the 2007 NCCN guidelines recommended postoperative imatinib if the surgeon removed all gross disease but the operative specimen reveals positive microscopic margins (R1 resection) or if gross disease was left behind (R2 resection): observation was recommended if an R0 resection (negative microscopic margins) was achieved.Citation8 With the recent (December 2008) approval by the FDA for imatinib as adjuvant therapy for all GISTs, the issues have become somewhat more complex and will be discussed at the end of this review in light of the information that follows regarding the available data to date.
Conduct and importance of surgical resection for primary GISTs
Once in the operating room with a patient with an apparently resectable GIST, the surgeon should perform complete gross resection of the tumor with its pseudocapsule, using a “no-touch technique” as much as possible. This often requires segmental resection rather than peritumoral resection in order to achieve the best margins, within the limits of any retroperitoneal/intraperitoneal sarcoma resection (ie, margins are sometimes limited by adherence of the tumor to major vascular structures or nerves). Optimal resection is often assisted by the fact that like other sarcomas, GISTS are “pushers” rather than “invaders” and can often be lifted off surrounding organs; even lesions that appeared to be invading adjacent structures on preoperative scans are often found to be respectable at operation. That is, preoperative imaging can sometimes underestimate the resectability of tumors. Conversely, images may have missed small tumor implants that may render the tumor unresectable. The surgeon should look for such metastases by examining liver and peritoneal surfaces carefully and resecting them whenever possible.Citation3,Citation7
Adult GISTs do behave like other sarcomas in that they almost never spread to nodes; therefore routine lymph node dissection is not required. Similarly, extra-abdominal metastases and ascites are rare, facts that also assist with surgical resection. As alluded to above, it is essential to avoid tumor rupture and bleeding; GISTs are fragile tumors, and such events promote tumor dissemination and recurrence. Thus, the conduct of the resection of primary GISTs is critical to determining patient outcomes.
Several special issues regarding GIST resection deserve mention. First, it is unclear whether the surgeon should immediately return the patient to the operating room for a re-operation if the final microscopic margins are positive (R1 resection) when the surgeon expected an R0 report. This appears to vary by center, but is clearly also dependent on the findings at operation, patient factors, and surgeon and patient preference. There are no data that directly address this issue, although many centers now recommend starting imatinib instead of reoperation, with clinical follow-up. Secondly, esophageal GISTs may require either esophagectomyCitation9 or enucleation (segmental resection is not an option in the esophagus). Which of these very different operative approaches is chosen depends on tumor, patient, and institutional factors.Citation3 Thirdly, because half of all GISTS occur in the stomach, the surgeon frequently needs to decide on the extent of gastric resection. Most commonly, this can be segmental, with major gastrectomies reserved for GE junction or pylorus tumors.Citation10
Finally, the use of laparoscopy deserves special mention. Although the 2004 NCCN guidelines listed GIST as a contraindication to laparoscopic resection, our and other institutions have been resecting GISTs safely using laparoscopic or laparoscopic-assisted techniques for some time. In 2006 Novitsky et al reported on 50 selected cases of primary GIST resected laparoscopically using careful techniques, including the use of specimen extraction bags and intra-operative ultrasound/endoscopy. Forty-seven patients had local resections and 3 had segmental resection. The mean operative time and average blood loss were very favorable (135 min and 85 mL, respectively). At 36 months average follow-up, 92% of patients remained free of disease, and 4 had recurred, all with liver metastases (ie, recurrences were nature of disease and not local recurrences).Citation11 This and other reportsCitation3,Citation12,Citation13 emphasized that, as with any laparoscopic cancer resection, laparoscopic resection of GIST is safe as long as the surgeon adheres to oncologic principles, especially by avoiding direct instrumentation of tumor. Based on such reports, the 2007 NCCN guidelines listed laparoscopic resection as an option for the treatment of primary GIST tumors.Citation8
The complete resection of a primary GIST is an important prognostic marker. The surgeon’s goal should always be an R0 resection, as complete resection of GIST remains the best treatment even in the era of imatinib.Citation3 Many tumors can be completely resected at presentation. After R0 resection, the most important prognostic factors are tumor factors, and include tumor size (<2 cm, vs 2 to 5 cm, vs 5 to 10 cm, vs >10 cm), location (gastric best, small bowel worst), and tumor mitotic rate (<5, vs 5 to 10, vs >10 mitoses [M]/50 high powered fields [hpf]).Citation14,Citation15 Using these criteria, certain subgroups of GISTs (eg, larger tumors with higher numbers of mitoses) can be considered as “high risk”, although-as will be stressed in the sections that follow-the definition of “high risk” can vary in the literature, among various institutions, and between different clinical trials. For example, at our institution the definition of “high risk” GISTs actually depends on the tumor site, which takes into account the importance of tumor location.Citation4 Specifically, we define “high risk” for gastric GISTS as tumors >10 cm and having >5 M/50 hpf, and for GISTS distal to the stomach as those that are >5 cm and having at least 5 M/50 hpf.
Resected GIST: rationale for adjuvant imatinib
While GISTs are usually treated up front with resection, R0 resection, as noted above, is clearly not the only predictor of patient outcome. Resected GISTs can have high recurrence rates, and prior to the availability of imatinib GIST patients had particularly high failure rates. Tumors >10 cm in size were associated with 5-year disease-free survival (DFS) of only 20% and median times to progression (TTP) of 7 months to 2 years. Only 10% of patients remained disease-free after extended follow-up.Citation16 Given this high recurrence rate and the existence of an effective oral drug with a low toxicity profile which targets a tyrosine kinase (TK) expressed in over 95% of tumors, it was only natural that clinical investigators turned research endeavors towards combining resection with systemic approaches using Imatinib.
Imatinib (Gleevec®; Novartis) is a TKI which inhibits the Philadelphia chromosomal Bcr-Abl and the TK c-kit. It is also thought to induce apoptosis (see below). The most encouraging rationale for the use of this inhibitor in GIST were data from the Multicenter (“Pivotal”) trial,Citation17 Sarcoma Intergroup Trial,Citation18 The European Organization for Research and Treatment of Cancer (EORTC)-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group,Citation19 and other trialsCitation3 of imatinib in patients with advanced (unresectable and metastatic) GIST. These studies demonstrated clinical benefit in over 80% of patients, with survival dramatically better than historical controls from the Southwest Oncology Group (SWOG) 8616 and 9627 trials.Citation16
Pharmacology of imatinib
Imatinib, a 2-phenylaminopyrimidine derivative, is a specific inhibitor of a number of TK enzymes. It binds to the TK active site, leading to a decrease in kinase activity (). Imatinib is specific for the TK domain in abl (the Abelson proto-oncogene), c-kit and PDGF-R. Imatinib is rapidly absorbed orally and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system, including CYP3A4 and, to a lesser extent, CYP1A2, CYP2D6, CYP2C9, and CYP2C19. The main metabolite, N-demethylated piperazine derivative, is also active. The major routes of elimination are billiary and enteral; only a small portion of the drug is excreted in the urine. Most of imatinib is eliminated as metabolites: only 25% is eliminated unchanged. The half-lives of imatinib and its main metabolite are 18 and 40 hours, respectively.Citation20
Figure 1 Mechanism of action of imatinib (source: http:\\wikipedia/imatnib [accessed March 2, 2009]).
![Figure 1 Mechanism of action of imatinib (source: http:\\wikipedia/imatnib [accessed March 2, 2009]).](/cms/asset/ed986e34-b60c-4a67-ae5d-fb9825f5246e/dott_a_4740_f0001_c.jpg)
Resected GIST: adjuvant trials/efficacy studies
Based on the above mentioned encouraging data on imatinib in advanced GIST, the American College of Surgeons Oncology Group (ACoSOG) embarked on 2 adjuvant trials of imatinib after resection of primary GIST – a nonrandomized phase II administration of imatinib to patients with high risk GIST (ACoSOG Z9000), and a placebo-controlled, randomized study of imatinib for more intermediate-risk patients (ACoSOG Z9001).Citation7
The primary objective of the Z9000 trial was overall survival (OS) after administration of 400 mg/day for 1 year of the drug to patients after resection of high risk GIST; the secondary objectives were 2- and 5-year recurrence and toxicity. To be included in the trial patients needed to be imatinib-naïve, have tumors that were c-KIT positive, received no prior adjuvant therapy, and undergo at least an R1 resection with no residual disease on post-operative imaging. “High risk” for this trial was defined as any of the following: tumors at least 10 cm in greatest dimension, the presence of tumor rupture before or during operation, intraperitoneal hemorrhage, or the finding at operation of multifocal intraperitoneal tumors (all resected); ie, number of mitoses was not included in the definition of high risk.
The 4-year results of this trial were presented by DeMatteo et al in 2008. Among the 107 eligible patients, imatinib was started at a median of 59 (range 25 to 84) days after operation. Patients had a median age of 58 years (range 19 to 79) and a median tumor size of 13 cm (range 3 to 42). Fifty percent of the tumors were gastric and 42% were small intestine. Imatinib therapy was well tolerated (previously reported in 2005)Citation21. At a the median follow-up of 4 years the 1-, 2-, and 3-year OS were 99%, 97%, and 97%, respectively, and the 1-, 2-, and 3-year relapse-free survivals (RFS) were 94%, 73%, and 61%, respectively. The authors concluded that imatinib 400 mg for 1 year after resection of high-risk primary GIST prolonged RFS and OS compared with historical controls.Citation22
The primary objective of the Z9001 trial was to determine the OS of patients with GIST treated with imatinib (again, 400 mg/day for 1 year) in an adjuvant setting relative to placebo. The secondary objectives were RFS and safety/efficacy in an adjuvant setting. This trial included all patients with GISTS ≥ 3 cm, resected within 70 days prior to registration, who had kit-positive tumors, were imatinib-naïve, and had no prior adjuvant therapy.
The preliminary results of this trial were presented by DeMatteo et al in 2007. At a median follow-up of 1.2 years, the 1-year RFS among the 708 completely resected patients was 97% for the imatinib arm, and 83% for placebo. This difference reached statistical significance for tumors >6 cm. No effect on OS has yet been seen; 10-year survival follow-up is ongoing.Citation23
Based on these and other trials, imatinib was FDA approved in December 2008 for the adjuvant treatment of all resected primary GISTs. Ongoing trials continue to address the question of adjuvant imatinib, including a study from the EORTC (for GIST >3 cm, randomizing 2 years of imatinib vs placebo), and a trial from the Scandinavian Sarcoma Group (randomizing between 12 vs 36 months of treatment in high risk GIST patients).Citation7
Overtreatment of GIST patients with adjuvant imatinib, an expensive drug with known toxicity, is an obvious concern for a number of reasons. First, as noted above, the randomized trials leading to FDA approval included only those GISTs >3 cm, with a statistically significant improvement in RFS only for tumors >6 cm. Secondly, the data as yet show no improvement in OS. Thirdly, the ACoSOG studies were not stratified by mitotic rate, now known to be a key prognostic factor. Finally, small, good prognosis GISTS may be cured with surgical resection alone-although at present there are no definite markers to identify these patients.
Safety and tolerability of imatinib in GIST patients
Common reactions reported with imatinib include fever, headaches, fluid retention (peripheral and periorbital edema), nausea and vomiting, dyspepsia, muscle cramps and pain, arthralgias, diarrhea, hemorrhage and anemia, neutropenia, upper respiratory infections, and elevated liver transaminases and bilirubin. Patients receiving imatinib should be monitored with liver function tests and consideration should be given for baseline troponins and electrocardiogram if they are being treated for hematologic disorders, and thyroid function tests if they have had a thyroidectomy.
Early results from the ACoSOG included a 2005 report by DeMatteo et al regarding the safety and tolerability of imatinib in patients with GIST; given orally 400 mg/daily for 1 year, the drug was well tolerated. No grade 4 or 5 toxicity was seen. Nineteen (17%) patients had grade 3 toxicity, consisting of neutropenia (2%), dermatitis (2%), and increased ALT (2%). The most frequent toxicities of any grade included edema (55%), fatigue (43%), nausea (42%), diarrhea (42%), and dermatitis (27%). Eighty-seven (82%) patients completed the 1 year of imatinib, and 72 (68%) tolerated full dose without a dose reduction.Citation21
Rare but serious reactions reported with imatinib include liver failure (ascites, anasarca, hepatotoxicity), left ventricular dysfunction (pulmonary edema, pleural effusions, congestive heart failure [CHF], pericardial effusions), thrombocytopenia and bleeding (GI hemorrhage, anemia), neutropenia, exfoliative dermatitis, hypokalemia, hypothyroidism, and – very rarely – Stevens-Johnson syndrome and erythema multiforme. As with any TKI, imatinib should be used with caution in patients with hypersensitivity to TKIs, cardiac risk factors, or impaired liver function, as the drug is extensively metabolized in the liver; only 12% is renally excreted.Citation20 Patients should avoid pregnancy and breast feeding.
Rarely, patients with advanced GIST on TKI therapy may develop complications such as intraluminal or intraperitoneal hemorrhage, rupture, abscess, fistula, or obstruction, necessitating emergency operation. All 3 operative deaths in one series occurred in patients undergoing emergency surgery.Citation24 Accordingly, pre-emptive operation should be considered in patients with evidence of fistulization, ongoing necrosis, or limited hemorrhage.Citation7
Advanced GIST: rationale for neoadjuvant and palliative imatinib
As mentioned above, imatinib was first tested in patients with advanced GIST. The rationale for this testing was clear: 95% of GIST express mutated c-KIT, and operative therapy alone for advanced GIST, as mentioned above, would be expected to fail in the majority of cases. Also as mentioned previously, subsequent trials, especially the Pivotal Trial, demonstrated that over 80% of patients with advanced GIST derive some clinical benefit.Citation17–Citation19
Given these findings, imatinib has been applied to patients with operable GIST in one neoadjuvant trial,Citation25 and to inoperable GIST in the hope of rendering the disease operable.Citation3,Citation7,Citation26 This combined medical-surgical approach in advanced GIST is based on the facts that there are few complete responses with imatinib alone in advanced disease, that responding lesions when biopsied/resected usually contain viable cells, data from other tumor types that cytoreduction may improve surgical outcomes, the general idea that recurrent GIST behaves like metastatic disease and therefore may best be treated with a multimodal approach, and that imatinib given preoperatively has the potential to increase resectability or reduce the extent of surgery.Citation7
Data from the Pivotal Trial demonstrated that the median time to overall response in patients with advanced GIST treated with imatinib was 13 weeks, and that 80% (of the 80% of patients who responded) did so within 6 months of therapy.Citation17 Conversely, it is rare to see incremental tumor shrinkage after 9 months, and the median time to progression on imatinib is approximately 2 years.Citation27 Accordingly, centers using imatinib for advanced GIST in the hope of rendering patients resectable generally follow patients (who are responding) out on at least 6 months of therapy before considering surgical exploration, but usually operate before 24 months.Citation7
Imatinib and surgery for advanced GIST: outcomes
The Radiation Therapy Oncology Group (RTOG) 0132 trial was a phase II study of neoadjuvant/adjuvant imatinib given for advanced primary and metastatic/recurrent operable GIST. Imatinib was given to 52 patients at 600 mg/day; 30 patients had primary GIST (group A) and 22 had recurrent/metastatic GIST (group B). All were felt to be operable; 7% of group A and 4.5% of group B had partial preoperative responses to imatinib, while 4.5% of group B progressed. The remainder had stable disease. Two-year PFS and OS were 83% and 93%, for Group A, and 77% and 91% for Group B, respectively. Complications of operation and toxicity of imatinib were judged to be minimal. Accordingly, the authors concluded that neoadjuvant imatinib is feasible, and not associated with notable postoperative complications.Citation25
One of the issues with this trial is that patients were evaluated for radiographic response to imatinib using the Response Evaluation in Solid Tumors (RECIST) criteria. These criteria, which are based on size changes on anatomic imaging, can under call response of GIST tumors to imatinib because 1) response, especially early on, can consist mostly of “dynamic” changes in the tumor (eg, decreased FDG-PET uptake and decreased tumor density on contrast-enhanced CT [CE-CT] scans), and 2) some responding GIST tumors may actually swell, a situation which may be labeled as progression by RECIST criteria if the increase in size of the sum of the greatest diameters of the tumors exceeds 20%. Accordingly, many oncologists favor the newer Choi Criteria for radiographic evaluation of GIST response to therapy, which include a dynamic measure of response (a 15% decrease in tumor density on CE-CT).Citation28
The data on combined medical-surgical therapy for advanced and inoperable (or “possibly operable”) GIST at present consists of small series; these studies report response rates a high as 76%, similar to the findings of the Pivotal Trial. Approximately 25% of unresectable primary and recurrent/metastatic lesions convert to resectable, and long term disease control has been documented.Citation7,Citation27,Citation30 The timing of resection can be tricky; surgeons generally want to wait until the patient has had a maximum response to TKI therapy but this cannot always be known in foresight-the minimum 6 months of TKI therapy is usually given as discussed in the previous section. After resection most surgeons resume TKI post op; in the French Sarcoma Group and RTOG S-0132 studies patients recurred quickly if the imatinib was stopped postoperatively.Citation7,Citation25,Citation31
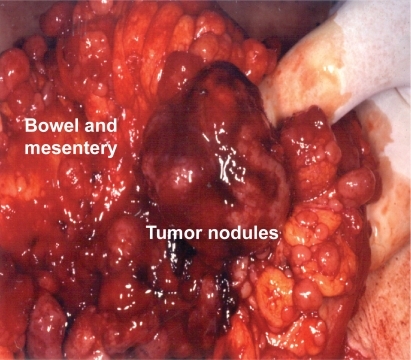
Six studies to date have provided surgical outcomes data for patients with advanced GIST going to operation after imatinib. Of patients who get resected, 48% to 91% have R0 or R1 resections, with documented improvement in survival. However, these resections have been done in specialty centers with highly selected patients using an aggressive approach (); 60% involved peritonectomy/omentectomy, 60% required multivisceral resections, and 40% involved resection of liver metastases.Citation24,Citation27,Citation29,Citation32–Citation34
Figure 2 Intraoperative photograph of advanced GIST after imatinib therapy showing multiple tumor nodules on the bowel and mesentery despite a good radiographic response. Resection of this disease requires an aggressive approach, which frequently includes peritonectomy/omentectomy, multivisceral resections, and resection of liver (courtesy Dr C Corless).
Two-thirds of patients with metastatic GIST develop liver metastases at some time during their course, and the liver can often be the only site of metastatic disease. Such liver lesions are often multifocal and may require multiple or repeated medical and surgical interventions to render the liver without evidence of disease, including radiofrequency ablation and hepatic artery embolization.Citation35
One of the largest series of surgical outcomes using this approach was reported by Raut and colleagues at the Brigham and Women’s Hospital in Boston. These authors reported their surgical results in 69 consecutive patients in terms of the preoperative response (on imaging) to imatinib (), categorizing responses as “stable disease” (no progression of the index lesions by the time of operation), “limited progression” (progression of 1 target), or “generalized progression” (progression of more than 1 target lesion). R0 or R1 resections were accomplished in the majority of stable disease cases, whereas in almost half of generalized progression patients all gross disease could not resected. At 1 year the progression free and overall survival were high for stable disease patients, but all generalized disease had died of their tumors. The authors concluded that resection on imatinib is reasonable unless patients have generalized progression.Citation32
Table 1 Outcomes of resection in patients with advanced GIST on tyrosine kinase inhibitors (n = 69)
Imatinib-resistant GIST: options
For patients progressing on imatinib, options include the multitarget TKI sunitinib (Sutent®; Pfizer), usually given orally at 37.5 mg/day. This drug primarily targets kit exon 9 mutants, forms of GIST that often do not respond to 400 mg/day imatinib.Citation36 Studies of newer TKIs, such as dasatinib and nilotinib (which can act through signaling pathways other than c-KIT and PDGF-R),Citation37 are currently ongoing, as are studies with IPI-504, a heatshock protein 90 inhibitor. Work in GIST cell lines suggests that treatment with dasatinib or IPI-504 may provide a therapeutic alternative for GIST patients whose tumors carry the imatinib-resistant PDGR-F(D842V) mutant isoform.Citation38
RAD001, an mTOR inhibitor, is currently in trial given in combination with imatinib in imatinib-resistant GISTs. The use of imatinib combined with chemo agents can be considered, based on evidence from CML patients.Citation3,Citation39–Citation41
GIST genotyping: current recommendations
For all patients with GIST, especially those who develop advanced and/or imatinib resistant disease, the questions of whether and when to genotype the tumor (ie, perform mutational analysis) usually arise. The most recent (2007) NCCN guidelines recommend genotyping “ALL high risk and malignant (ie, advanced) GISTs”.Citation8
In fact, the vast majority of GIST tumors have a kit mutation, and most of these are at exon 11. Exon 11 mutations are usually associated with tumors that are located in the stomach, have a more favorable outcome, and initially respond to imatinib, although they may fail later. Fewer tumors have exon 9 mutations; these mutations are associated with nongastric sites, a less favorable prognosis, and respond less to imatinib at 400 mg/day – they may respond to 800 mg doses. Least favorable, and fortunately less common, are tumors with no kit mutation (“wild type”). These fail earlier and do not typically respond to TKIs. Pediatric GISTs are commonly wild type and this is reflected clinically by their aggressive morphology – these tumors are often multifocal, nodular, and node-positive. Specific gene mutations, such as codon 557–558 deletion and hTERT overexpression also mark high risk tumors.Citation16,Citation32
At our center, therefore, we do perform genotyping for specific indications where the information can be used clinically. We genotype all pediatric GISTs; while most are wild type and will do poorly on TKIs, a few have adult-type genotypes (exon 11 and 9 mutations) and TKIs may be indicated. The knowledge that a tumor expresses an exon 9 mutation marks cases where 800 mg/day of imatinib should be tried. For patients who are either being considered for or are already failing sunitinib, some mutations, such as the novel kit exon 16 mutation L783V, predict clinical sunitinib resistance and mark patients who may not respond to this drug. Secondary mutations of the kit activation loop confer cross-resistance to both imatinib and sunitinib, and mark patients for whom TKIs may not be effective. Some imatinib resistant tumors express mutated PDGF-R and may be targets for PDGF-R inhibitors.Citation4,Citation41
Patient-focused perspectives: quality of life, patient satisfaction, and support
The advent of targeted therapy has converted high risk and metastatic GIST from a deadly disease to one where the chief issues are disease control, timing of therapies, and quality of life (QOL). Accordingly, support groups for patients facing therapy for GIST have appeared and have a very real role in advocacy for affected patients and their caregivers. Two of the largest include GIST Support International (www.gistsupport.org), a world wide organization of patients and caregivers which provides information and one-time grants for patient treatment, and Life Raft Group (www.liferaftgroup.org), a 501c national group with local chapters, which provides information, support, and advocacy.
Because the health-related QOL and economic burden of GIST are timely from a payer, provider and patient perspective and may provide guidance for treatment decision making and reimbursement, it is not surprising that an extensive literature on these topics has appeared. Reddy and colleagues performed a systematic literature review of PubMed and 5 scientific meeting databases to identify and review 34 published studies and abstracts describing the epidemiologic, QOL and economic impact of GIST. Meta-analysis of these publications revealed that on the Functional Assessment of Chronic Therapy-fatigue (FACT-f) instrument, GIST patients scored 40.0 compared with 37.6 in anemic cancer patients (0 = worst; 52 = least fatigue). Reported total costs over 10 years for managing GIST patients with molecular targeted therapy averaged approximately £47,521 to £56,146 per patient compared with £4,047 to £4,230 per patient with best supportive care. They concluded from their review that the health-related QOL burden of GIST is similar to that with other cancers, and the value of new therapies in GIST need to consider not only cost but also anticipated benefits and the unmet medical need in this condition.Citation42
Summary: the place of surgery and targeted therapy in the treatment of GIST
GISTs, the most common sarcoma of the GI tract, have unique kinase mutations that serve as targets for medical therapy. Although an R0 surgical resection (microscopic negative specimen margins) remains the mainstay of treatment for these tumors, patients with resected GISTs have high relapse rates that can be reduced by 1 year of adjuvant imatinib. Data from studies supporting the use of imatinib in the adjuvant setting are still maturing, and the appropriate patient subsets and optimal duration of treatment are still unknown. For patients undergoing an R1 resection (tumor at 1 or more microscopic margins) options include either re-resection or imatinib, depending on the clinical setting.
Data clearly support the use of imatinib for patients with recurrent or unresectable GIST. In these patients the drug should be continued until progression, intolerance, or the patients are rendered resectable. Patients with advanced GIST who are successfully resected (R0 or R1) after imatinib treatment should be placed back on imatinib post-operatively. For patients who progress on 400 mg imatinib, the dose can be increased to 800 mg/day. Patients who develop generalized progression (progression at 2 or more sites) even on this higher dose should move to other treatments, such as newer TKIs or other targeted approaches currently under study. Genotyping of the tumor should be considered in all pediatric GISTs and high risk adult GISTs, especially if there is progression on imatinib. QOL and cost/benefit of new therapies are important issues for further study in patients with GIST.
Disclosures
The author discloses no conflicts of interest.
References
- CorlessCFletcherJAHeinrichMABiology of gastrointestinal stromal tumorsJ Clin Oncol20042238143824
- KirschRGaoZHRiddellRGastrointestinal stromal tumors: diagnostic challenges and practical approach to differential diagnosisAdv Anat Pathol20071426128517592256
- HuemanMTSchulickRDManagement of gastrointestinal stromal tumorsSurg Clin NA200888599614
- RubinBPHeinrichMCCorlessCLGastrointestinal stromal tumourLancet20073691731174117512858
- AgaimyAWunschPHHofstaedterFMinute gastric schlerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutationsAm J Surg Pathol20073111312017197927
- KawanowaKSakumaYSakuraiSHigh incidence of microscopic gastrointestinal stromal tumors in the stomachHuman Pathol2006371527153516996566
- RautCPAshleySWHow I do it: management of gastrointestinal stromal tumorsJ Gastrointest Surg2008121592159918317848
- NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma, V.2.2007Available at: http://www.globalgist.org/docs/NCCN_guidelines.pdf (accessed March 5th, 2009).
- BlumMGBilimoriaKYWayneJDSurgical considerations for the management and resection of esophageal gastrointestinal tumorsAnn Thor Surg20078417171723
- WayneJDBellRHJrLimited gastric resectionSurg Clin NA20058510091020
- NovitskyYMKetcherKWSingRFLong-term outcomes of laparoscopic resection of gastrointestinal stromal tumorsAnn Surg200624373874516772777
- ChoiSMKimMCJungGC GJLaparoscopic wedge resection for gastric GIST: long-term follow-up resultsEur J Surg Oncol20073344444717174060
- NishimuraJNakajimaKOmoriTSurgical strategy for gastrointestinal stromal tumors: laparoscopic vs. open resectionSurg Endosc 205;191109111116021371
- DeMatteoRPGoldJSSaranLTumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST)Cancer200811260818076015
- FletcherCDMBermanJJCorlessCDiagnosis of gastrointestinal stromal tumors: a consensus approachHuman Pathol20023345946512094370
- BlankeCDCorlessCLState-of-the art therapy for gastrointestinal stromal tumorsCancer Invest20052327428015945512
- DemetriGDvonMehrenMBlankeCDEfficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumorsN Engl J Med200234747248012181401
- BenjaminRSRankinCFletcherCPhase III dose-randomized study of imatinib mesylate (STI571) for GIST: Intergroup S0033 early resultsProceedings of the American Society for Clinical Oncology200322(abstract 3271).
- VerweijJCasaliPGZalcbergJProgression-free survival in gastrointestinal stromal tumors with high-dose imatinib; randomised trialLancet20043641127113415451219
- DrukerBJLydonNBLessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemiaJ Clin Invest20001053710619854
- DeMatteoRPAntonescuCRChadaramVAdjuvant imatinib mesylate in patients with primary high risk gastrointestinal stromal tumor (GIST) following complete resection: safety results from the US Intergroup Phase II trial ACOSOG Z9000Proceedings of the American Society for Clinical Oncology20052316S9009
- DeMatteoRPOwzarKAntonescuCREfficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST)at high risk of recurrence: the US Intergroup phase II trial ACOSOG Z9000. [Abstract]American Society of Clinical Oncology 2008 Gastrointestinal Cancers SymposiumJanuary 25–27, 2008Orlando, FLA-82008
- DeMatteoRPOwzarKMakiRGAdjuvant imatinib mesylate increases recurrence-free survival (RFS) in patients with completely resected localized primary gastrointestinal stromal tumor (GIST):North American Intergroup Phase III trial ACOSOG Z9001Proceedings of the American Society for Clinical Oncology 2007Abstract 10079.
- BonvalotSEldwenyHPechouxCLImpact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib mesylate eraAnn Surg Oncol2006131596160316957966
- EisenbergBLHarrisJBlankeCDPhase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665J Surg Oncol20081021(Epub ahead of print)
- DemetriGDBenjaminRSBlankeCDNCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)-update of the NCCN clinical practice guidelinesJ Natl Compr Canc Netw20075SupplS1S2917624289
- DeMatteoRPMakiRGSingerSResults of tyrosine kinase inhibitor therapy followed by surgical resection in metastatic gastrointestinal stromal tumorAnn Surg200724534735217435539
- BenjaminRSChoiHMacapinlacHAWe should desist in using RECIST, at least in GISTJ Clin Oncol2009251760176417470866
- AndtbackaRHNgCHScaifeCLSurgical resection of gastrointestinal tumors after treatment with imatinibAnn Surg Oncol200714142417072676
- GutierrezJCPerezEAMoffatFLShould soft tissue sarcomas be treated at high volume centers? An analysis of 4205 patientsAnn Surg200724595295817522521
- BlayJYLeCesneARay-CloquardIProspective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma groupJ Clin Oncol2007251107111317369574
- RautCPPosnerMDesaiJSurgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitorsJ Clin Oncol2006242325233116710031
- GronchiAFioreMMiselliFSurgery of residual disease using molecular-targeted therapy with imatinib mesylate in advanced/metastatic GISTAnn Surg2007245245346
- RutkowskiPNoweckiZNyckowskiPSurgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylateJ Surg Oncol20069330431116496358
- MaeharaNChijiiwaKEtoTSurgical treatment for gastric GIST with special reference to liver metastasesHepatogastroenterology20085551251618613398
- JudsonIRPrognosis, imatinib dose, and benefit of sunitinib in GIST: knowing the genotypeJ Clin Oncol2008265322532518955449
- BraconiCBracciRCellerinoRMolecular targets in Gastrointestinal Stromal Tumors (GIST) therapyCurr Cancer Drug Targets2008835936618690842
- DewaeleBWasagBCoolsJActivity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842V mutationClin Cancer Res200815;145749575818794084
- KitamuraYGastrointestinal stromal tumors: past, present, and futureJ Gastroenterol200843488508
- ReichertPNovel approaches to imatinib-resistant GISTCurr Oncol Rep20081034434918778561
- HeinrichMCCorlessCLLieglBMechanisms of sunitinib malate resistance in gastrointestinal stromal tumorsProceedings of the American Society for Clinical Oncology20072518S10006(Abstract), 656(S).
- ReddyPBociKCharbonneauCThe epidemiologic, health-related quality of life, and economic burden of gastrointestinal stromal tumoursJ Clin Pharm Ther20073255756518021332