Abstract
The view that B lymphocytes are pathogenic in diverse pathological settings is supported by the efficacy of B-cell-ablative therapy in lymphoproliferative disorders, autoimmune diseases and graft rejection. Anti-B-cell antibodies (Abs) directed against CD20 have therefore been generated, and of these, rituximab was the first anti-CD20 monoclonal Ab (mAb) to be applied. Rituximab-mediated apoptosis, complement-dependent cytotoxicity and Ab-dependent cellular cytotoxicity differ from one disease to another, and, for the same disease, from one patient to another. This knowledge has prompted the development of new anti-CD20 mAbs in the hope of improving B-cell depletion. The inclusion of CD20/anti-CD20 complexes in large lipid rafts (LRs) enhances the results of some, but not all, anti-CD20 mAbs, and it may be possible to include smaller LRs. Lipid contents of membrane may be abnormal in malignant B-cells, and could explain resistance to treatment. The function of these mAbs and the importance of LRs warrant further investigation. A detailed understanding of them will increase results for B-cell depletion in lymphoproliferative diseases.
Introduction
B lymphocytes perform a variety of functions in the immune system. For example, they play a part in eradicating bacteria or viruses, and promote all antitumoral responses.Citation1 They may, however, run the risk of upregulating immune reactions. In autoimmunity, there is sufficient evidence to justify investigating B lymphocytes not only as antibody (Ab) producing cells at the end of the pathological sequence, but also in the early stages of pathophysiology.Citation2 These negative effects of B lymphocytes may be seen in graft rejection, which is associated with the production of Abs,Citation3 and in graft versus host (GVH) disease following allogeneic hematopoietic stem cell transplantation.Citation4 Moreover, there is anarchic proliferation of B lymphocytes in lymphoproliferative syndromes such as lymphomasCitation5 and chronic lymphocytic leukemia (CLL).Citation6 Not surprisingly, B-cell-ablative therapies have been proposed for B-cell non-Hodgkin’s lymphoma (NHL), representing a major advance in its treatment. One of these therapies involves anti-CD20 monoclonal Abs (mAbs), which are directed against the CD20-expressing members of the B-cell lineage, from late pre-B-cells through mature B-cells. Rituximab (RTX) was the first mouse–human chimeric mAb to be approved by the Food and Drug Administration (FDA) for the treatment of relapsed, or refractory, low-grade NHL.Citation7 RTX is now used in other malignant proliferations,Citation8 and is approved by the FDA for the treatment of autoimmune diseases (AIDS)Citation9 and the prevention of graft rejection.Citation10 To summarize the current situation, over 500,000 patients (including children) have been treated with RTX, and the improved patient-reported outcomes and cost-effectiveness have led to the production of other anti-B-cell agents. As a consequence, humanized anti-CD20 Abs are currently being developed.Citation11–Citation14
The effectiveness of anti-CD20 mAbs for a given disease is variable, as reported in patients with NHL or CLL.Citation15–Citation18 Furthermore, their self-efficacy differs from one B-cell disease to another. In fact, compared to observations with lymphoma, RTX has been shown to be weak when used as a single agent in CLL.Citation19,Citation20 These data therefore suggest that the results could be affected by factors in B lymphocytes themselves. The present review endeavors to chronicle therapeutic indications of anti-CD20 mAbs in these malignancies, to analyze the mechanisms of their action and to distinguish extrinsic or intrinsic factors, particularly lipid rafts (LRs) that could possibly be modulating their activities.
Diseases treated with rituximab
Malignant B-cell diseases
Non-Hodgkin B-cell lymphoma
Many clinical trials have been conducted as induction therapy for patients recently diagnosed with follicular lymphoma or who have suffered a relapse when RTX was used on its own, in combination with chemotherapy, or with other types of Abs. Generally speaking, these mAbs, or the related combination of drugs, prolong survival compared to chemotherapy alone.Citation21 Other trials have tested the efficacy of RTX in Waldenström’s macroglobulinemia (WM) and multiple myeloma.Citation22 In fact, Demopoulos et al have claimed that combining RTX with other drugs is the most commonly used primary treatment for WM.Citation23 Diffuse large B-cell lymphoma (DLBL), which accounts for approximately 40% of NHLs in adults, is a type of aggressive lymphoma with a diffuse growth pattern and a high-intermediate proliferation index. Combining RTX with chemotherapy represents a breakthrough in the treatment of malignancies, and could become the new standard therapy in elderly and young low-risk patients.Citation24 In addition, treatment of Burkitt’s lymphoma, marginal lymphoma and Hodgkin lymphoma with RTX is currently being investigated ().
Table 1 Diseases treated by rituximab
Its success notwithstanding, around 50% of NHL patients resisted treatment with RTX in that they did not respond to it, or they suffered an early relapse of the original disease.
Leukemias
These disorders are, in fact, bone marrow (BM) diseases. As in previous papers, we show the results of treating CLL and acute lymphocytic leukemia (ALL) with RTX. Many clinical trials have been conducted on CLL,Citation25 but the efficacy of RTX was weak compared to its effects with lymphoma. One explanation is that RTX reduces the membrane level of CD20 on CLL B-cells. This is reflected in the fact that fractionation of RTX into several low doses perpetuates the expression of CD20, and thereby prolongs the activity of the mAb.Citation26,Citation27 Finally, the results of combining RTX with chemotherapy in the treatment of ALL appear to be encouraging, with acceptable levels of toxicity ().Citation28
Autoimmune diseases
Autoimmune diseases where RTX is known to be efficient
A pilot study, RTX in AID, was set up in 2000 for the treatment of immune thrombocytopenic purpura. Objective clinical responses were observed in 30% of casesCitation29 and the beneficial effect of RTX was confirmed in others.Citation30,Citation31 Furthermore, the use of RTX with methotrexate has been approved by the FDA for the treatment of rheumatoid arthritis (RA), as it relieves symptoms in adult patients with moderate to severe active RA where treatment with one or more anti-TNF drugs has failed. RTX was also tested in Sjögren’s syndrome with interesting results,Citation32 and in systemic lupus erythematosus (SLE) where reduced activity of the disease and a clinical response of 86% were reported in one series of patients.Citation33,Citation34 However, certain SLE patients show only partial or brief B-cell depletion, while the clinical benefit, if any, is modest.Citation35 This failure suggests that SLE results from altered B-cell sensitivity, or possibly a complement (C) dysfunction.Citation36 Multiple sclerosis and antineutrophil cytoplasmic Abs-associated vasculitides have been studied for RTX efficiency, but controlled clinical trials have not yet been reported ().Citation37,Citation38
AID suspected of answering Abs
Finally, anecdotal cases of anti-CD20 B-cell depletion have been reported in autoimmune-hemolytic anemia, cryoglobulinemia, acquired factor VIII-inhibitor disease, glomerulonephritis, pemphigus vulgaris, systemic scleroderma, polymyositis and dermatomyositis.Citation39–Citation43 An ongoing phase-I trial is even treating inactive psoriasis with RTX. Intriguingly, however, a handful of case reports suggest that this B-cell ablative therapy may actually induce psoriasis.Citation44,Citation45
Graft rejection and graft versus host disease
The pathogenic role of B lymphocytes was highlighted in renal, cardiac, liver or pancreas transplantation, justifying B-cell depletion with RTX.Citation46–Citation48 Allogeneic hematopoietic stem cell transplantation may induce acute or chronic destruction of the host by the donor’s immune system. Whilst pathogenic mechanisms involved in these phenomena are not clearly understood, RTX therapy may be effective for some patients ().Citation49
Action mechanisms of rituximab
Complement-dependent cytotoxicity
C may be utilized by RTX, and C-dependent cytotoxicity (CDC) seems to be pivotal in the efficiency of RTX.Citation50 In fact, after RTX treatment, C deficiency or consumption is striking in patients with CLL,Citation51–Citation53 with the over expression of C inhibitors CD55 and CD59.Citation18,Citation22 Klepfish et al tried to enhance the activity of RTX in CLL by adding fresh frozen plasma in an attempt to correct the C imbalance.Citation52 Moreover, resistance to RTX therapy in NHL was ascribed to CDC inefficiency, as polymorphisms in the C1qA gene were affecting its clinical response and the duration of the response.Citation54,Citation55 Following treatment with RTX, the CD20 molecules are clustered in the LRs, concentrating IgG Fc regions locally and therefore inducing fixation of C1q. The C molecule, C3, has been shown to vary in affinity for LRs by supplementing cholesterol in cellular membrane and by virtue of the hypersensitivity of CD55 to phospholipases.Citation56,Citation57
Antibody-dependent cell-mediated cytotoxicity
ADCC occurs when B-cells are killed by monocytes, macrophages, natural killer (NK) cells and neutrophils following the binding of RTX to the Fc region. Among the Fc-gamma receptors (Fcγ), FcγRII was shown to inhibit ADCC, whereas FCγRIIIA behaved as an activator. However, a polymorphism of this latter receptor was also found to modulate ADCC. On the other hand, there is a dimorphism on residue 176 which can either be valine, with a high binding of IgG1, or phenylalanine with a low binding.Citation58 Cartron et al confirmed that a homozygous valine 158 polymorphism of FcγRIIIA leads to higher response rates and molecular complete remission rates in NHL patients after RTX treatment.Citation59 However, in CLL, ADCC was not influenced by the FcγR genotype expressed by autologous NK cells.Citation17
Apoptosis
RTX-induced apoptosis requires translocation of CD20 into the LRs.Citation60 This segregation increases cytosolic Ca2+ levels through association between CD20 and the B-cell receptor (BCR).Citation61 Interaction between CD20 and raft membrane protein permits activation of components such as the phosphotyrosine kinases Lyn, Fyn, and Lck, as well as p75/80 (Cbp/PAG). This appears to be necessary for RTX,Citation57 downstream signals mediated by the mitogen-activated protein kinase (MAPK) p38, cleavage of caspase-3, inhibition of Bcl-2, DNA fragmentation and, ultimately, apoptosis.Citation62 Growth inhibition of NHL cell lines by RTX may be caused by externalization, location in LRs with CD20 and acid sphingomyelinase production through ceramide synthesis, which also activates MAPK. However, Chan et al demonstrated that, in several cell lines at least, redistribution of CD20 was not crucial.Citation63
Why is the efficacy of RTX variable?
Is the quantity relevant?
Number of lymphocytes
The efficacy of anti-CD20 mAb depends of the number of tumor cells. In fact, when the tumor burden is high, the various effector mechanisms engaged by RTX are saturated.Citation19 Kennedy et al confirmed this observation through an analysis of elevated B CLL cell concentration binding by RTX, which led to complete consumption of C lytic activity and depletion of several C components.Citation53 Their replacement with purified C proteins is sufficient to restore the cytotoxic activity of RTX. In aggressive DLBL, association between RTX and chemotherapy improves the rate and increases the duration of the response.Citation64
Other variables for a fixed number of B-cells
Number of CD20 molecules
CD20, a nonglycosylated phosphoprotein, is expressed in large quantities on the surface of almost all normal and malignant B-cells, up to 250,000 molecules per cell. However, resistance to RTX was identified in certain diseases, and especially in CLL. Mankai et al and others have established that this is related to a lower density of CD20 on B-cells in CLL compared to B-cells from other lymphomas,Citation65 associated with a down-regulation of the purine-rich box-1 (PU.1) transcription factor, so that transfection of B-cells with its cDNA restores RTX-induced lysis. Similarly, stimulation of B-cells with CpG increases the expression of CD20 and ameliorates the CDC by RTX.Citation66 The same results have been observed in lymphoma,Citation20 but Weitzman et al claim that there was no correlation between ADCC and CD20 density.Citation17
Location of the B-cells
After an infusion of RTX, circulating B-cells are rapidly removed. In contrast, germinal-center and marginal-zone B-cells resist this destruction.Citation67 This phenomenon seems to be linked to factors such as BAFF (B-cell-activating factor) from the microenvironment, integrins and the lack of vascularization, rather than to poor tissue penetration by RTX. This factor is more decisive for RTX susceptibility than for the density of Fcγ receptors on the membrane of all effectors.Citation68 We can note that the level of BAFF in sera from patients with lymphoma may be a useful indicator in predicting the efficacy of treatment with RTX and chemotherapy.Citation69 Furthermore, baseline serum levels of BAFF are inversely correlated with the induration of B-cell depletion after RTX treatment in primary Sjögren’s syndrome.Citation70 These findings provide new options for treating AID and hematological malignancies.
Quality implication
Lipid rafts
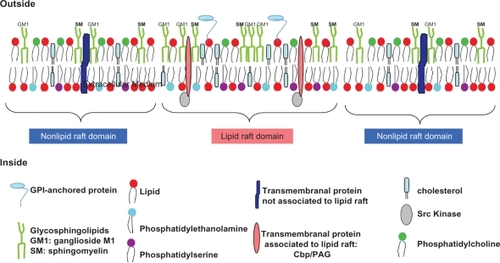
LRs have been described as stable structures, 100–500 nm in diameter, which resist extraction by nonionic detergents at low temperature due to their high cholesterol and glycosphingolipid content.Citation71 This definition is misleading and may not apply to the composition of LRs in vivo. In fact, this structure is no longer accepted, and LRs are considered to be very heterogeneous, differing in the temporal stability of the composition of their lipids and proteins such as Src kinases. The generally accepted definition of LRs was agreed at the 2006 Keystone symposium on LRs and cell function: “Lipid rafts are small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes”. The largest components of LRs are glycosphingolipids such as ganglioside M1 (GM1), sphingomyelin (SM), cholesterol and phosphatidyl serine, the last three in approximately 50% greater quantities than in plasma membranes (). “Small rafts can sometimes be stabilized to form larger platforms through protein–protein and protein–lipid interactions”.Citation72–Citation74 When plasma membrane lipids and protein are clustered in the LRs, they can mediate transduction pathways which favor cellular adhesion, trans-membrane signaling, virus budding, control of ionic pumps and channels, and mediation of vesicle fusion.Citation75,Citation65
Proteins connected to LRs have been described as insoluble in the nonionic detergent Triton X-100 at low temperature, in contrast to the other membrane lipids.Citation70 This means we should be careful when investigating proteins linked to rafts, as analyses of fluorescence microscopy or fluorescence resonance energy transfer confirm these data.Citation77 Many of these signaling proteins either reside in, or are transferred into, the LRs during signal transmission. Their fine-tuned regulation is probably influenced by nanoscale membrane microdomains, which are defined as dynamic and which apportion them to the membrane.Citation78 These include CD20,Citation79 BCR,Citation77 Fas, HLA II,Citation80 TNF receptor, CD40,Citation81 phosphatidyl inositol 3-kinase and several protein kinase C isoforms. Cbp/PAG, a ubiquitous, highly tyrosine-phosphorylated adaptor protein, only occurs in LRs, and plays an important part in the recruitment of Lyn, Fyn and Lck kinases into these LRs ().Citation82
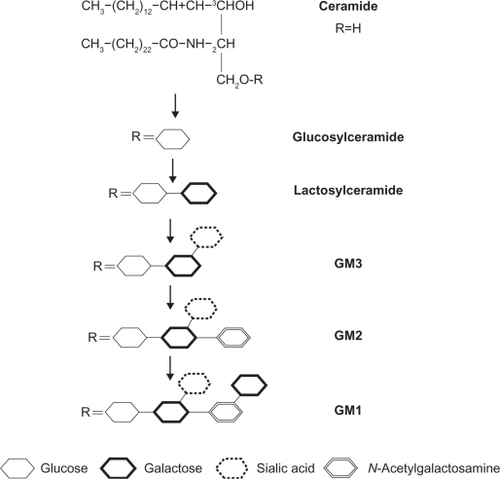
Glycosphingolipids are involved in cell proliferation and differentiation. Glycosyl ceramides are the most widely distributed glycosphingolipids in cells, and act as precursors for the biosynthesis of many glycosphingolipids. GM1, widely used as a marker for LRs, is a sialoglycosphingolipid composed of a ceramide hydrophobic portion and a oligosaccharide sequence (). It is integrated into the external leaf of the plasma membrane, and is at the forefront of the interaction of cells with their environment. For example, GM1 is known as a receptor for toxins and viral particles.Citation83,Citation84 Furthermore, close interaction between SM and cholesterol is necessary for LR formation, and sphingomyelinase can transform small LRs into large, ceramide-enriched ones. In addition, glycosphingolipids can be metabolized into lysosphingolipids and sphingoid bases. This function as a metabolite acts as second messenger in signal transduction pathways of growth and apoptosis.Citation85,Citation86
Variation in their composition
A recent study analyzing LR constitution in mantle cell lymphoma has revealed a downregulation of key LR proteins such as raftlin and Cbp/PAG.Citation87 These are involved in B-cell transduction and in the pathology of lymphoma. Moreover, the presence of Cbp/PAG in the LRs is necessary for RTX to effect transmembrane signaling.Citation57 The resistance of tumor B-cells to RTX may be explained by a deficiency in Cbp/PAG. If we take this a step further, changes in the expression of a number of glycosphingolipids on the cell surface have been correlated with typical cancer phenotype and tumor progression. In this respect, GM1 expression differs between lymphoma subtypes, even within one lymphoma subgroup. A recent study has demonstrated a correlation between the expression of GM1 and response to RTX in NHL and CLL.Citation88 In this latter leukemia, B lymphocyte membrane appears with increased fluidity, and produces lipid dynamics and lipid-protein interactions.Citation89,Citation90 Furthermore, glucosylceramide, lactosylceramide and SM improve activity of P-glycoprotein, a membrane efflux transporter, which frequently underlies cancer cell and bacterial resistance.Citation91 There are excessive quantities of B lymphocytes in CLL patients.Citation92,Citation93 In addition, glucosylceramide synthase inhibitors stimulate CLL cells to react to conventional cytotoxic and cytostatic drugs used in treatment.Citation94
Finally, the disialoganglioside (GD3), a ganglioside weakly expressed in most normal tissues, is over-expressed during development and in pathological conditions such as cancer. It is responsible for a variety of events such as proliferation, differentiation and apoptosis.Citation95 However, the relationship between ganglioside GD3 and CLL is not fully understood. It should be noted that alterations in the LR localization of certain signaling molecules contribute to the acuteness of B-cell-dependent autoimmune disease in some cases. On the other hand, the majority of SLE patients exhibited a low level of Lyn expression as well as a diminished association with LRs, due to an increase in ubiquitination through translocation of c-Cbl into LRs.Citation96,Citation97 This is associated with the abnormal expression of CD45, a membrane protein, tyrosine phosphatase, which controls Lyn expression by modulating its phosphorylation.Citation98 Lyn serves as a negative regulator for B-cell signaling, and reduced negative signaling in LRs hyperactivates B-cells, leading to uncontrolled production of IL-10 and auto-Abs. The development of SLE induced by abnormalities in the sub-cellular localization of the BCR signaling pathway was confirmed by the discovery of a single polymorphism in the FcγRIIB gene.Citation99 This mutation excludes FcγRIIB from the LRs and impedes its inhibitory effects on BCR signals.Citation100
Efficacy of other anti-CD20 mAbs
There is a clear need to develop new agents in order to improve the efficacy of B-cell depletion. In fact, there are many forms of resistance to RTX in autoimmune conditions and B-cell malignancies. On the other hand, other anti-CD20 mAbs have been, or are currently being developed ().
Table 2 Major characteristics of anti-CD20 antibodies compared to activity of rituximab
CDC-improving anti-CD20 mAbs
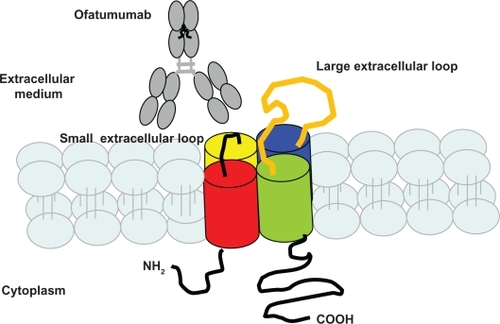
Ofatumumab (2F2, HuMax-CD20, Genmab/GSK) is a fully-humanized, anti-CD20 mAb which binds to a portion closer to the B-cell membrane, and which is composed of the 74–80 amino acids in its small extracellular loop (). Preclinical studies have thus far established that Ofatumumab has a slower rate of dissociation from CD20 than RTX and greater CDC power. Lysis of refractory RTX-resistant B-cells is also effected through this mAb,Citation50 with sustained success.Citation14 Moreover, infusion reactions which appear after use of Ofatumumab are similar to those which appear with RTX, and it is more effective in CLL patients.Citation101
Veltuzumab (IMMU-106, hA20, Nycomed/Immunomedics) is a humanized mAb with complementarity-determining regions (CDRs) identical to RTX, except at position 101 in the CDR3 of the variable heavy chain (VH) in aspartic acid substitutes for asparagines, and in framework regions of Epratuzumab, another humanized anti-CD22 Ab. This difference induces ADCC identical to that of RTX in CLL.Citation17 There is a reduced off-rateCitation102 and greater efficacy in lysing tumor cells in vitro by CDC, and also in vivo with a phase I/II for recurrent/refractory NHL.Citation13
ADCC-increasing anti CD20 mAbs
Ocrelizumab (2H7) is a 90%–95% humanized mAb (Genentech/Roche/Biogen-Idec), and binds to an epitope which is different to that of RTX but which overlaps with it, and which is in the extracellular domain of CD20.Citation103 Because C activation leads to side effects associated with RTX, a modification of its Fc portion is necessary. Its gives mAb a reduced CDCCitation12 and increases its tolerability in autoimmune diseases. On the other hand, ADCC is higher than with RTX, due to amplified binding affinity for the low-affinity variants of FcγRIIIa.
Modifications to anti-CD20 Ab glycosylation, aimed at increasing ADCC, have generated three anti-CD20 mAbs. GA-101 is a humanized third-generation and glyco-engineered version of anti-CD20 mAb (Glycart Biotechnology AG, Genentech Inc, F Hoffmann-LaRoche Ltd, Biogen Idec Inc and Chugai Pharmaceutical Co Ltd) which produces greater ADCC, superior direct cell death and greater efficacy in depleting B-cells than RTX in the potential treatment of NHL or CLL.Citation104 EMAB-6, with a low fucose content, triggers similar apoptosis and CDC to that observed for RTX, but promotes ADCC in CLL. The different levels of efficacy are more pronounced with low doses and when target cells express fewer CD20 molecules.Citation105 BLX-301 (Biolex/Aragen) is a humanized anti-CD20 mAb with an optimized glycosylation structure, enhanced ADCC, potent B-cell depletion, and potentially low side effects. BLX-301 is being developed for treatment of NHL.
Finally, the objective of protein engineering is to manufacture an anti-CD20 Ab with greater potency and efficacy in all patients. Thus, vaccines are being developed with CD20 mimotope peptidesCitation106 and AME-133v (Lilly), which improve the binding of anti-CD20 mAb to the Fc receptor on their immune effectorcells.
Anti-CD20 mAbs which increase programmed cell death
Zevalin, an anti-CD20 mAb linked to Yttrium-90 (IDECY2B8, Ibritumomab Bayer Biogen Idec) and Bexxar (Tositumomab, B1 coupled to iodine I131-GlaxoSmithKline) were approved by the FDA in 2002 and 2003 respectively for treatment of NHL and for trials in other malignant diseases such as CLL.Citation107 It is worthy of note that unradiolabeled tositumomab was described as inducing stronger apoptosis and ADCC than RTX.Citation108
The Amgen/AstraZeneca mAb 1.5.3 better enhances proapoptotic activity in vitro than RTX, and mediates both CDC and ADCC, with superior ADCC when NK donor cells present an FcγIIIa F/F allotype. In a primate pharmacodynamic model, this promotes higher B-cell depletion in lymph node organs and BM.Citation109
The genetically-engineered tetravalent Ab (TetraMcAb), derived from the anti-CD20 mAb RTX and ofatumumab, exhibits more potent antiproliferative and apoptosis activities than ofatumumab or RTX.Citation110 Hex-hA20, which comprises six Fabs with one Fc, translocates CD20 in LRs, affects ADCC but not CDC, and inhibits proliferation of NHL cell lines in vitro at low level concentration without requiring antibody cross-linking.Citation111
Conclusion
The efficacy of anti-CD20 mAbs seems to depend, at least in part, on their ability to translocate CD20 molecules into LRs. Apoptosis is higher in those which cannot do so, where mAbs which can do so are characterized by CDC. ADCC is the same in both types. New anti-CD20 mAbs have been developed based on this classification. Mechanisms differ according to the type of B-cell-disease and the type of patient. These mAbs will be able to be used to best effect when their precise roles are known, and when B-cell-disorder physiopathology is better understood. For the time being, performance could be improved by increasing doses of mAbs, changing the type of mAb for each disease, associating different drugs simultaneously or successively, and modifying the LRs.
Acknowledgements
Work on this review was funded by grants from the “Conseil Régional de Bretagne”, the “Ligue contre le Cancer” and the “Fédération Leucémie Espoir”.
Disclosure
The authors report no conflicts of interest in this work.
References
- VollmersHPBrändleinSNatural antibodies and cancerN Biotechnol200925529429819442595
- ChanOTMadaioMPShlomchikMJB cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunityJ Immunol199916373592359610490951
- XuHHeXSunJShiDZhuYZhangXThe expression of B-cell activating factor belonging to tumor necrosis factor super family (BAFF) significantly correlated with C4D in kidney allograft rejectionTransplant Proc200941111211619249491
- CutlerCMiklosDKimHTRituximab for steroid-refractory chronic graft-versus-host diseaseBlood2006108275676216551963
- MartinBCraigPGrattanCLow-grade B-cell proliferation progressing to high-grade B-cell lymphomaAm J Dermatopathol200931657858119590416
- DefoicheJDebacqCAsquithBReduction of B cell turnover in chronic lymphocytic leukemiaBr J Haematol2008143224024718710389
- MaloneyDGGrillo-LópezAJWhiteCAIDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphomaBlood1997906218821959310469
- WinklerUJensenMManzkeOSchulzHDiehlVEngertACytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8)Blood19999472217222410498591
- PersJODevauchelleVDaridonCBAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximabtreated patients with Sjögren’s syndromeArthritis Rheum20075651464147717469105
- GarrettHEJrGroshartKDuvall-SeamanDCombsDSuggsRTreatment of humoral rejection with rituximabAnn Thorac Surg20027441240124212400781
- ChesonBBexxar (Corixa/GlaxoSmithKline)Curr Opin Investig Drugs200231165170
- GenoveseMCKaineJLLowensteinMBOcrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I/II randomized, blinded, placebo-controlled, dose-ranging studyArthritis Rheum20085892652266118759293
- MorschhauserFLeonardJPFayadLHumanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II resultsJ Clin Oncol200927203346335319451441
- HagenbeekAGadebergOJohnsonPFirst clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trialBlood2008111125486549518390837
- StolzCSchulerMMolecular mechanisms of resistance to Rituximab and pharmacologic strategies for its circumventionLeuk Lymphoma200950687388519373595
- PawluczkowyczAWBeurskensFJBinding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTXJ Immunol2009183174975819535640
- WeitzmanJBetancurMBoisselLRabinowitzAPKleinAKlingemannHVariable Contribution of Monoclonal Antibodies to ADCC in patients with chronic lymphocytic leukemiaLeuk Lymphoma20095081361136819562616
- GolayJZaffaroniLVaccariTBiologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysisBlood200095123900390810845926
- GlennieMJFrenchRRCraggMSTaylorRPMechanisms of killing by anti-CD20 monoclonal antibodiesMol Immunol200744163823383717768100
- CvetkovićRSPerryCMSpotlight on rituximab in non-Hodgkin lymphoma and chronic lymphocytic leukemiaBioDrugs200620425325716831024
- BolandABagustAHockenhullJDavisHChuPDicksonRRituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphomaHealth Technol Assess200913Suppl 24148
- TreonSPMitsiadesCMitsiadesNTumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignanciesJ Immunother2001243263271
- DimopoulosMAKastritisERoussouMRituximab-based treatments in Waldenström‘s macroglobulinemiaClin Lymphoma Myeloma200991596119362975
- SonetABoslyARituximab and chemotherapy in diffuse large B-cell lymphomaExpert Rev Anticancer Ther20099671972619496708
- ChristianBALinTSAntibody therapy for chronic lymphocytic leukemiaSemin Hematol20084529510318381104
- AueGLindorferMABeumPVFractionated subcutaneous Rituximab is well tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemiaHaematologica201095932933219679883
- WilliamsMEDensmoreJJPawluczkowyczAWThrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemiaJ Immunol2006177107435744317082663
- GriffinTCWeitzmanSWeinsteinHChildren‘s Oncology Group. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology GroupPediatr Blood Cancer200952217718118816698
- SalehMNGutheilJMooreMA pilot study of the anti-CD20 monoclonal antibody rituximab in patients with refractory immune thrombocytopeniaSemin Oncol2000276 Suppl 129910311226008
- AggarwalACatlettJPRituximab: an anti-CD20 antibody for the treatment of chronic refractory immune thrombocytopenic purpuraSouth Med J200295101209121212425512
- DierickxDVerhoefGVan HoofARituximab in auto-immune haemolytic anaemia and immune thrombocytopenic purpura: a Belgian retrospective multicentric studyJ Intern Med2009266548449119549092
- Devauchelle-PensecVPennecYMorvanJImprovement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20)Arthritis Rheum200757231031717330280
- DingCFooteSJonesGB-cell-targeted therapy for systemic lupus erythematosus: an updateBioDrugs200822423924918611066
- WeideRHeymannsJPandorfAKöpplerHSuccessful long-term treatment of systemic lupus erythematosus with rituximab maintenance therapyLupus2003121077978214596428
- LeandroMJCambridgeGEdwardsJCEhrensteinMRIsenbergDAB-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patientsRheumatology (Oxford)200544121542154516188950
- MandersonAPBottoMWalportMJThe role of complement in the development of systemic lupus erythematosusAnnu Rev Immunol20042243145615032584
- RobakTOfatumumab, a human monoclonal antibody for lymphoid malignancies and autoimmune disordersCurr Opin Mol Ther200810329430918535937
- RoccatelloDBaldovinoSAlpaMEffects of anti-CD20 monoclonal antibody as a rescue treatment for ANCA-associated idiopathic systemic vasculitis with or without overt renal involvementClin Exp Rheumatol2008263 Suppl 49S67S7118799057
- BerentsenSTjønnfjordGEBrudevoldRFavorable response to therapy with the anti-CD20 monoclonal antibody rituximab in primary chronic cold agglutinin diseaseBr J Haematol20011151798311722415
- FieldJJFenskeTSBlinderMARituximab for the treatment of patients with very high-titre acquired factor VIII inhibitors refractory to conventional chemotherapyHaemophilia2007131465017212724
- CollinsMNavaneethanSDChungMRituximab treatment of fibrillary glomerulonephritisAm J Kidney Dis20085261158116218823685
- ZambrunoGBorradoriLRituximab immunotherapy in pemphigus: therapeutic effects beyond B-cell depletionJ Invest Dermatol2008128122745274718997839
- CooperMAWillinghamDLBrownDEFrenchARShihFFWhiteAJRituximab for the treatment of juvenile dermatomyositis: a report of four pediatric patientsArthritis Rheum20075693107311117763414
- DassSVitalEMEmeryPDevelopment of psoriasis after B cell depletion with rituximabArthritis Rheum20075682715271817665440
- MielkeFSchneider-ObermeyerJDörnerTOnset of psoriasis with psoriatic arthropathy during rituximab treatment of non-Hodgkin lymphomaAnn Rheum Dis20086771056105718556453
- VenetzJPPascualMNew treatments for acute humoral rejection of kidney allograftsExpert Opin Investig Drugs2007165625633
- BillingHRiegerSOvensJSuccessful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipientsTransplantation20088691214122119005402
- KesslerLParissiadisABayleFOn behalf of the GRAGIL Study Group. Evidence for Humoral Rejection of a Pancreatic Islet Graft and Rescue with Rituximab and IV Immunoglobulin TherapyAm J Transplant2009981961196619522877
- TeshimaTNagafujiKHenzanHRituximab for the treatment of corticosteroid-refractory chronic graft-versus-host diseaseInt J Hematol200990225326019543951
- BeumPVLindorferMABeurskensFComplement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysisJ Immunol2008181182283218566448
- van der KolkLEGrillo-LópezAJBaarsJWHackCEvan OersMHComplement activation plays a key role in the side-effects of rituximab treatmentBr J Haematol2001115480781111843813
- KlepfishAGillesLIoannisKEliezerRAmiSEnhancing the action of rituximab in chronic lymphocytic leukemia by adding fresh frozen plasma: complement/rituximab interactions and clinical results in refractory CLLAnn N Y Acad Sci2009117386587319758239
- KennedyADBeumPVSolgaMDRituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemiaJ Immunol200417253280328814978136
- ManchesOLuiGChaperotLIn vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomasBlood2003101394995412393572
- RacilaELinkBKWengWKA polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphomaClin Cancer Res200814206697670318927313
- CohenAMShinitzkyMModulation of complement lysis of human erythrocytes by the membrane lipid viscosityVox Sang198243123277113115
- SemacIPalombaCKulangaraKAnti-CD20 therapeutic antibody rituximab modifies the functional organization of rafts/microdomains of B lymphoma cellsCancer Res200363253454012543813
- WuJEdbergJCRedechaPBA novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune diseaseJ Clin Invest19971005105910709276722
- CartronGDacheuxLSallesGTherapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa geneBlood200299375475811806974
- JanasEPriestRWildeJIWhiteJHMalhotraRRituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosisClin Exp Immunol2005139343944615730389
- PetrieRJDeansJPColocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid raftsJ Immunol200216922886289112218101
- PedersenIMBuhlAMKlausenPGeislerCHJurlanderJThe chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanismBlood20029941314131911830481
- ChanHTHughesDFrenchRRCD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane raftsCancer Res200363175480548914500384
- FeugierPVan HoofASebbanCLong-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’AdulteJ Clin Oncol200523184117412615867204
- MankaïABordronARenaudineauYPurine-rich box-1-mediated reduced expression of CD20 alters rituximab-induced lysis of chronic lymphocytic leukemia B cellsCancer Res200868187512751918794139
- MankaïABuhéVYouinouPGhediraIBerthouCBordronAImprovement of rituximab efficiency in chronic lymphocytic leukemia by CpG-mediated up-regulation of CD20 expression independently of PU.1Ann N Y Acad Sci2009117372172819758221
- GongQOuQYeSImportance of cellular microenvironment and circulatory dynamics in B cell immunotherapyJ Immunol2005174281782615634903
- AhujaAShupeJDunnRKashgarianMKehryMRShlomchikMJDepletion of B cells in murine lupus: efficacy and resistanceJ Immunol200717953351336117709552
- KimSJLeeSJChoiIYSerum BAFF predicts prognosis better than APRIL in diffuse large B-cell lymphoma patients treated with rituximab plus CHOP chemotherapyEur J Haematol200881317718418510703
- PersJODevauchelleVDaridonCBAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren’s syndromeArthritis Rheum20075651464147717469105
- BrownDARoseJKSorting of GPI-anchored proteins to glycolipid enriched membrane subdomains during transport to the apical cell surfaceCell19926835335441531449
- PikeLJHanXChungKNGrossRWLipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysisBiochemistry20024162075208811827555
- FridrikssonEKShipkovaPASheetsEDHolowkaDBairdBMcLaffertyFWQuantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometryBiochemistry199938258056806310387050
- PikeLJRafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell FunctionJ Lipid Res20064771597159816645198
- RajendranLSimonsKLipid rafts and membrane dynamicsJ Cell Sci2005118Pt61099110215764592
- Hanzal-BayerMFHancockJFLipid rafts and membrane trafficFEBS Lett200758111209104
- SohnHWTolarPPierceSKMembrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapseJ Cell Biol2008182236737918644892
- LingwoodDSimonsKLipid rafts as a membrane-organizing principleScience20103275961465020044567
- LiHAyerLMPolyakMJThe CD20 calcium channel is localized to microvilli and constitutively associated with membrane rafts: antibody binding increases the affinity of the association through an epitope-dependent cross-linking-independent mechanismJ Biol Chem200427919198931990114976189
- SetterbladNBécartSCharronDMooneyNB cell lipid rafts regulate both peptide-dependent and peptide-independent APC-T cell interactionJ Immunol200417331876188615265920
- XiaMWangQZhuHLipid rafts regulate cellular CD40 receptor localization in vascular endothelial cellsBiochem Biophys Res Commun2007361376877417678876
- OneyamaCHikitaTEnyaKThe lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-SrcMol Cell200830442643618498747
- SpiegelSInsertion of ganglioside GM1 into rat glioma C6 cells renders them susceptible to growth inhibition by the B subunit of cholera toxinBiochim Biophys Acta198896932492562835987
- FantiniJMarescaMHammacheDYahiNDelézayOGlycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: activation of signal transduction pathways and perturbations of intestinal absorption and secretionGlycoconj J2000173–417317911201788
- HannunYABellRMFunctions of sphingolipids and sphingolipid breakdown products in cellular regulationScience198924348905005072643164
- OliveraABuckleyNESpiegelSSphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblastsJ Biol Chem19922673626121261271464623
- BoydRSJukes-JonesRWalewskaRBrownDDyerMJCainKProtein profiling of plasma membranes defines aberrant signaling pathways in mantle cell lymphomaMol Cell Proteomics2009871501151519346216
- Meyer zum BüschenfeldeCFeuerstackeYGötzeKSScholzeKPeschelCGM1 expression of non-Hodgkin’s lymphoma determines susceptibility to rituximab treatmentCancer Res200868135414542218593944
- DaeflerSKruegerGRLack of dynamic lipid changes after binding of interleukin 2 in chronic lymphatic leukemia lymphocytes indicates defective transmembrane signalingAnticancer Res1989937437482788388
- DaeflerSKruegerGRExpression of proliferation and differentiation antigens in response to modulation of membrane fluidity in chronic lymphocytic leukemia lymphocytesAnticancer Res1989925015062751273
- Gouaze-AnderssonVCabotMCGlycosphingolipids and drug resistanceBiochim Biophys Acta20061758122096210317010304
- ConsoliUSantonocitoAStagnoFMultidrug resistance mechanisms in chronic lymphocytic leukaemiaBr J Haematol2002116477478011886380
- JamroziakKSmolewskiPCebulaBSzmigielska-KaplonADarzynkiewiczZRobakTRelation of P-glycoprotein expression with spontaneous in vitro apoptosis in B-cell chronic lymphocytic leukemiaNeoplasma200451318118715254670
- GerrardGButtersTDGaneshaguruKMehtaABGlucosylceramide synthase inhibitors sensitize CLL cells to cytotoxic agents without reversing P-gp functional activityEur J Pharmacol20096091–3343919285492
- MalisanFTestiRGD3 ganglioside and apoptosisBiochim Biophys Acta200215852–317918712531552
- Flores-BorjaFKabouridisPSJuryECIsenbergDAMageedRAAltered lipid raft-associated proximal signaling and translocation of CD45 tyrosine phosphatase in B lymphocytes from patients with systemic lupus erythematosusArthritis Rheum200756129130217195233
- Flores-BorjaFKabouridisPSJuryECIsenbergDAMageedRADecreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosusArthritis Rheum200552123955396516320343
- YanagiSSugawaraHKurosakiMSabeHYamamuraHKurosakiTCD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cellsJ Biol Chem19962714830487304928940015
- ClatworthyMRWillcocksLUrbanBSystemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malariaProc Natl Acad Sci U S A2007104177169717417435165
- KonoHKyogokuCSuzukiTFcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signalingHum Mol Genet200514192881289216115811
- CoiffierBLepretreSPedersenLMSafety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 studyBlood20081111094110018003886
- GoldenbergDMRossiEASteinRProperties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibodyBlood200911351062107018941114
- DuJWangHZhongCCrystal structure of chimeric antibody C2H7 Fab in complex with a CD20 peptideMol Immunol2008452861286818346788
- RobakTGA-101, a third-generation, humanized and glyco-engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignanciesCurr Opin Investig Drugs2009106588596
- De RomeufCDutertreCALe Garff-TavernierMChronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16Br J Haematol2008140663564318302712
- LiMYanZHanWZhangYMimotope vaccination for epitopespecific induction of anti-CD20 antibodiesCell Immunol2006239213614316814270
- JainNWierdaWFerrajoliAA phase 2 study of yttrium-90 ibritumomab tiuxetan (Zevalin) in patients with chronic lymphocytic leukemiaCancer2009115194533453919637351
- CraggMSWalsheCAIvanovAOGlennieMJThe biology of CD20 and its potential as a target for mAb therapyCurr Dir Autoimmun2005814017415564720
- BornsteinGGQuévaCTabriziMDevelopment of a new fully human anti-CD20 monoclonal antibody for the treatment of B-cell malignanciesInvest New Drugs2009
- LiBShiSQianWDevelopment of novel tetravalent anti-CD20 antibodies with potent antitumor activityCancer Res2008682400240818381448
- RossiEAGoldenbergDMCardilloTMSteinRWangYChangCHNovel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeuticsCancer Res2008688384839218922911