Abstract
Background:
Mounting evidence indicates that herbal therapy is effective in alleviating anxiety, lessening cancer treatment-related side-effects, and facilitating rehabilitation. This is the first trial to examine the herbal therapy of combined yunzhi and danshen on quality of life among breast cancer patients.
Methods:
A multicenter, longitudinal, and self-control study was used. Eighty-two breast cancer patients were given combined yunzhi and danshen capsules for six months on a daily basis. Data collection including quality of life, vitality status and adverse effects were taken.
Results:
Results showed a significant improvement in physical function, role-physical, role-emotion and health transition (P < 0.05). Patients also reported less fatigue, better quality of sleep, better appetite, more regular bowel movements and more stable emotions (P < 0.05). As far as side-effects were concerned, only mild discomforts including sore throat (13.4%) and dry mouth (9.8%) were recorded.
Conclusion:
The findings add clinical evidence to support the beneficial effects of herbal therapy on quality of life and vitality status in breast cancer patients. Therefore, herbal therapy has a potentially important role to play in managing psychological distress in cancer patients. This study also suggests that herbal therapy is clinically acceptable and can be used safely with breast cancer patients.
Background
Breast cancer remains the most common form of cancer to affect women.Citation1 While cancer itself is a life-threatening disease, treatments for cancer can produce very unpleasant complications and side effects, including fatigue, anxiety, menopausal symptoms, nausea, lymphedema, and dermatitis. Such complications result in poor psychological adjustment, vitality status and quality of life.Citation2 Mounting evidence indicates that herbal therapy is effective in alleviating anxiety, lessening cancer treatment-related side-effects, and facilitating rehabilitation.Citation2–Citation4 The mushrooms yunzhi (Coriolus versicolor) and danshen (Salviae miltiorrhiza) are two commonly-used herbs for cancer prevention.Citation5 The results of clinical trials have further shown that the addition of yunzhi to radiotherapy and chemotherapy treatment for cancer patients could greatly improve their clinical symptoms and stabilize their immune function, thus greatly improving their quality of life.Citation5–Citation8 However, methodological flaws in these studies, including a small sample size, have limited the generalizability of their findings. Evidence for the effects of danshen (Salviae miltiorrhizae) on cancer patients is less substantial. In 1996, a preclinical study showed that danshen had an antioxidative effect which could promote the immune system indirectly so as to facilitate rehabilitation and improve the quality of life in patients with cancer.Citation9,Citation10 Using danshen as a supplement may further promote the immunomodulatory activities of yunzhi.
Our previous studies on the immunity of combined yunzhi and danshen (YZDS) in breast cancer patients have shown that a 6-month treatment with YZDS capsules could be beneficial for immunomodulatory functions by significantly enhancing cell-mediated immunity and humoral immunity in individuals whose immune systems might be suppressed by radiotherapy, chemotherapy, or estrogen replacement therapy.Citation11 The findings of this study were consistent with the conclusions of a review article that yunzhi and danshen had a beneficial effect on immunology.Citation5–Citation7,Citation9,Citation12 A more recent double-blind, randomized, placebo-control study found that combined YZDS significantly improved the physical well-being and immune status of both healthy participants and patients with nasopharyngeal carcinoma.Citation12,Citation13 The aim of this study is to further explore the effect of YZDS on the vitality status, quality of life, and safety profile of patients with breast cancer.
Methods
Study setting and samples
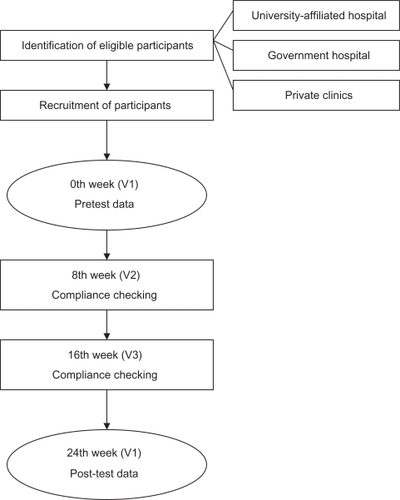
This was a multicenter, longitudinal, pre–posttreatment self-control study. Patients who had completed cancer treatments (surgery, radiotherapy, chemotherapy) within the past 3 years were recruited from breast cancer centers at a university-affiliated hospital, government hospitals and private clinics. To be included in the study, the patients had to be Chinese females aged ≥30, stage I–III breast cancer according to the TNM Classification of Malignant Tumors by the International Union Against Cancer and the American Joint Committee on Cancer (AJCC) Stage Grouping designated by the AJCC,Citation14 and who were not receiving any concurrent medical treatment. Patients were excluded if they had cancer in both breasts or cancer metastases were present, had abnormal liver and renal function, were either pregnant or breastfeeding, had a significant history of drug hypersensitivity, or were taking other alternative medicine or supplements. Based on previous studies, there is a 7%–10% difference in the quality of life between women with and without breast cancer.Citation15,Citation16 Taking a conservative approach, an 11% increase in the quality of life was used as the primary outcome. Eighty-two patients were required in order to detect group differences at a 5% two-sided significance level with a power of 90% and an attrition rate of 20%.
Procedure
Ethical approval for this study was obtained from the involved university and hospitals. Eligible patients who were referred by the collaborating hospitals and clinics were invited to participate in the study. After written consent was obtained, a baseline assessment (V1) of vitality status and quality of life was made. Patients then received 6-month YZDS treatment on a daily basis, taking 4 capsules 3 times a day. Checks were made at the 8th week (V2) and 16th week (V3), to ensure that the patients were on the right regime. At the 24th week (V4) patients attended a research clinic for a post-test assessment of vitality status, quality of life, and safety profile. Patients were asked to keep a diary recording their drug compliance and any side effects, and these diaries were collected at these three points. outlines the overall study protocol.
Auditing of adverse effects is less well-established in complementary and alternative medicine, including Chinese medicine, than in primary medicine. Very few studies have examined the rate of adverse effects, and the only documentation available consists of isolated case reports.Citation17 This being the case, we were particularly interested in including an assessment of adverse effects as one of the objectives of this study. All subjects were asked whether they had experienced any discomfort or adverse events, by means of an open-ended question at each study visit. Particular instances of discomfort or adverse events were then sorted into 3 categories: ‘Hot Constitution’ for complaints such as cold sore or sore throat; ‘Cold Constitution’ for dizziness or diarrhea; and ‘Others’ for vomiting or stomach discomfort. This classification was suggested by a Chinese medicine practitioner and based on established terminology used in Chinese medical literature.Citation18 Blood tests were carried out to assess renal and liver function and to ensure that the YZDS treatment was not endangering patients’ safety. Serious adverse events were recorded in the serious adverse events form and reported within 24 hours to the Ethics Committee.
Interviewing instrumentation
The Cantonese version of the SF-36 Health Survey questionnaire was used to evaluate the change in quality of life.Citation19,Citation20 Its 36 items are divided into 9 domains measuring (1) physical functioning with range 10–30, (2) role-physical with range 4–8, (3) bodily pain with range 2–12, (4) general health with range 5–25, (5) vitality with range 4–24, (6) social functioning with range 2–10, (7) role-emotional with range 3–6, (8) mental health with range 5–30, and (9) health transition with range 1–5. The raw scale scores in the first 8 domains were transferred into a 0%–100% scale, where higher percentage scores in each of the domains represented a better quality of life. The raw scale score in the health transition domain was directly presented with a lower scale score indicating a better subjective feeling of health status.
Five aspects of vitality status were used namely level of fatigue, appetite, bowel movement pattern, sleeping quality, and emotion status. These scores ranged from 1–7 for measures of the above 5 aspects, with higher scores representing a better vitality. The vitality status questionnaire was reviewed by an expert panel including 2 Chinese medicine practitioners, 2 physicians, 2 nurses, and 1 epidemiologist.
Statistical analyses
The SPSS statistical package (version 13.0 for Windows; SPSS Inc., Chicago, IL) was used for data entry and analysis. Intention to treat using the mean within the group was applied to replace the missing data. Demographics data was presented by descriptive statistics. The outcome measures on quality of life and vitality status between pre- and post-treatment over 6 months were compared using paired t-test in parametric data and Wilcoxon signed-rank test in nonparametric data. Regression model was also used to explore the relationship between intervention effects and period since breast cancer diagnosis. Adverse event data was presented descriptively.
Results
Baseline characteristics of the participants
Between June and October 2002, 82 breast cancer patients were admitted to the study of whom 78 completed the 6-month study. Four patients withdrew from the study, two because they did not believe that YZDS would have any beneficial effect, one who was afraid of the blood test for the safety profile, and one who was afraid of contracting Severe Acute Respiratory Syndrome (SARS). Intention to treat using the fairest approach of mean replacement was applied and data of all 82 participants were included for data analysis. The mean age of the participants was 45.6 years (SD = 6.8). The mean period since cancer diagnosis and anti-cancer treatment completion was 56.0 weeks (SD = 28.3) and 31.7 weeks (SD = 27.7) respectively. Most of the patients had been diagnosed with stage II breast cancer (62.2%). As far as anticancer treatment was concerned, all patients had completed surgery (100%), 78.0% had had chemotherapy and completed, 74.4% had had radiotherapy and completed, and 70.7% were undergoing antiestrogen replacement (Tamoxifen) for prevention treatment during the study period. The demographics are shown in .
Table 1 Demographics of breast cancer subjects in YZDS clinical trial
Intervention compliance
The mean compliance rate over the 6-month treatment period was 97.0%. Patients were asked to report their compliance in the form of diary entries, and a check on the reliability of their reports was made by counting the number of capsules in the bottles returned in each study visit. The agreement between the diary reports and the capsule checks was 99.9%.
Intervention effects on quality of life and vitality status
summarizes the results of the study. As far as quality of life was concerned, the domains of physical function, role-physical, role-emotion and health transition were all significantly improved (P < 0.05) after 6-month YZDS treatment. No significant change was found in the domains of bodily pain, general health, vitality, social function, and mental health. Further analysis of the relationship between intervention effects and period since breast cancer diagnosis using regression found no significant difference.
Table 2 Effect of YZDS on breast cancer subjects in YZDS clinical trial
The vitality status findings were consistent with the quality of life using the SF-36 Health Survey questionnaire: participants experienced less fatigue (P < 0.001), better quality of sleep (P < 0.001), better appetite (P < 0.001), more regular bowel movements (P < 0.001) and more stable emotion (P < 0.001). Repeated analysis in respect of period since breast cancer diagnosis also found no significant difference in the change of vitality status.
Adverse events related to the intervention
None of the participants reported any serious adverse events during the 6-month study period. Only mild discomforts were recorded, and these are shown in . The most common discomforts were sore throat (13.4%) and dry mouth (9.8%) under the category of Hot Constitution.
Table 3 Mild adverse events of breast cancer subjects in YZDS clinical trial
There was no significant change in different parameters of the renal function test (sodium, potassium, urea creatinine) and liver function test (total protein, albumin, total bilirubin, alkaline phosphate, alanine transaminase) during the study period. All these parameters were generally within the normal range both before and after study.
Discussion
This study was the first trial to assess the effect of treatment with Chinese medicine on the quality of life of patients with breast cancer which strictly followed good clinical practice (GCP). The findings support the therapeutic effect of a combined treatment of YZDS on quality of life and vitality status. Rising stress levels make it very difficult for cancer patients to care for themselves, thereby causing many psychological and physical problems and placing a burden on health care costs. Yet the evidence suggests that a significant reduction in and management of disease- and treatment-related symptoms could improve the quality of life among women with breast cancer.Citation16 Therefore, herbal therapy has a potentially-important role to play in managing psychological distress in cancer patients. This study also suggests that this herbal therapy is clinically acceptable and can be used safely with breast cancer patients.
The conspicuous success of herbal therapy in improving the quality of life of breast cancer patients can be explained by its favorable effects on the immune functions.Citation11,Citation12,Citation21 These findings conform to those of polysaccharide peptide (PSP) studies on the quality of life in patients with gynecological malignancies.Citation22 The significant improvement in vitality status, including fatigue level, quality of sleep, appetite, bowel movement, and emotion status, is also in line with the results of a PSP study by Chai and Shen.Citation23
Although YZDS treatment might have been expected to enhance the domains of general health, vitality, and mental health in the SF-36 survey, the current study indicates the absence of such improvement in breast cancer patients. This observation may be explained by the sensitivity of the SF-36 questionnaire, which is designed to evaluate the efficacy of conventional treatment and may be unable to assess the positive psychological effects of herbal therapy in explicit terms. Nevertheless, as a significant trend of improvement in vitality status was found when the vitality questionnaire derived by an expert panel of Chinese medicine practitioners was used, the beneficial effect of YZDS should not be undermined.
Although the current study adopted a specially-designed vitality questionnaire to evaluate the effect of herbal therapy, the absence of a control group leaves open the possibility that its positive findings are attributable to the placebo effect of herbal therapy. Also, the vitality questionnaire needs to be further studied for its reliability. In this respect, the low power of the current study in detecting the intervention effect should be taken into account. Herbal therapy is becoming an increasingly-popular treatment method, and it is possible that its effect on the quality of life of some patients was exaggerated because they placed high confidence in its effectiveness. Although the possibility of a placebo effect cannot be entirely ruled out, the immunological results of the current study,Citation11 its broad sample source, its low dropout rate, and its good compliance rate may not limit the generalizability of the findings.
The study design had two limitations. It was originally designed as a double-blind placebo-control cross-over study, but it proved very difficult to persuade participants at risk from a life-threatening disease to observe the conditions of the study. The use of a placebo was a particular stumbling block. As nearly all the participants were afraid of a recurrence of cancer, 97% of them breached the terms of the study by taking their own herbal supplements, and over half (60%) were found to be taking 2 or more types of health supplement at the same time. The scale of this subversive behavior became apparent 4 months after the commencement of the study. We decided that the best and most practical course of action was to convert the study into a self-control comparative study without placebo, to ensure that the participants would comply with the study protocol. After we modified the study design, 97% of the participants promised to stop taking their own herbal supplements during a 1-month washout period and restart the 6-month clinical trial. Although such an open-label outcome study is obviously of lower research value by modern medicine standards and is not a substitute for the double-blind placebo methodology, it fulfilled an important role in defining the variables in the clinical use of Chinese medicine. Another limitation is the lack of information on the menstruation status of participants which might be important adjusted factors in exploring the relationship between the herbal effect and the quality of life among breast cancer patients receiving Tamoxifen. It should be taken into consideration in any future studies.
Conclusion
Psychological distress and poor quality of life is a serious problem that significantly affects the morbidity of breast cancer patients. Herbal therapy seems to offer a useful complementary treatment that can enhance the health outcome of this group of patients. This study, using self-control design, is the first step to explore the efficacy of herbal therapy on quality of life in breast cancer patients. Results from the outcome studies could provide evidence for efficacy and might be used to design more future comprehensive studies.
Authors’ contribution
All investigators contributed to the study design. CK Wong and WKC Lam provided the laboratory support and analysis. LYE Wong and PC Leung provided the results analysis. The manuscript was prepared by LYE Wong and vetted by other investigators.
Acknowledgements
We thank Dr MCM Chan, Department of Surgery, Kwong Wah Hospital; Dr BKB Law, Department of Surgery, Prince of Wales Hospital; Dr WMM Yeo, Department of Clinical Oncology, the Chinese University of Hong Kong; and Dr PSY Cheung, Breast Surgery Associates, Hong Kong; for the recruitment of cancer patients for this study. This study was supported by the Innovation and Technology Fund, Hong Kong.
Disclosure
All the investigators have no conflict interest.
References
- Fact sheet N297 on cancer [http://www.who.int/mediacentre/factsheets/fs297/en/print.html]
- EustachiAComplementary Therapies in Breast Cancer PatientsBreast Care200724209216
- KiddPMThe use of mushroom glucans and proteoglycans in cancer treatmentAltern Med Rev20005142710696116
- TsangKWLamCLYanCCoriolus versicolor polysaccharide peptide slows progression of advanced non-small cell lung cancerRespir Med200397661862412814145
- WongLYTangJLLeungPCPrevalence and attitude of complementary and alternative medicine by healthy people in Hong KongThe 4th TWGHs-CUHK Eddie Wang Symposium on Complementary Chinese and Western Medicine-Integrated Approach 2004Hong Kong: TWGHs 2004115
- ShiuWCTLeungTWTTaoMA clinical study of PSP on peripheral blood counts during chemotherapyPhytother Res199264217218
- SunZYLiuJXThe clinical efficacy of PSP in 485 cancer patients: A double blind studyChinese Medicine and Public Health, No 23–24Hong KongUniversity of Hong Kong1996
- WongCKLeungKNFungKPChoyYMImmunomodulatory and anti-tumor polysaccharides from medicinal plantsJ Int Med Res1994222993127895893
- CaoEHLiuXQWangJJXuNFEffect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cellsFree Radic Biol Med19962068018068728027
- de la FuenteMFerrandezMDBurgosMSSolerAPrietoAMiquelJImmune function in aged women is improved by ingestion of vitamins C and ECan J Physiol Pharmacol19987643733809795745
- WongCKBaoYXWongELYLeungPCFungKPLamCWKImmunomodulatory activities of yunzhi and danshen in post-treatment breast cancer patientsAm J Chin Med200533338139516047556
- WongCKTsePSWongELYLeungPCFungKPLamCWKImmunomodulatory effects of Yun Zhi and danshen capsules in healthy subjects – a randomized, double-blind, placebo-controlled, crossover studyInt J Immunopharmacol200442201211
- BaoYXWongCKLeungSFClinical studies of immunomodulatory activities of yunzhi-danshen in patients with nasopharyngeal carcinomaJ Altern Complement Med200612877177617034283
- WoodwardWAStromEATuckerSLChanges in the 2003 American Joint Committee on Cancer Staging for Breast Cancer Dramatically Affect Stage-Specific SurvivalJ Clin Oncol200321173244324812947058
- HelgessonOLissnerLManssonJBengtssonCQuality of life in cancer survivors as observed in a population study of Swedish womenScand J Prim Health Care200725422022517924285
- JanzNKMujahidMChungLKSymptom experience and quality of life of women following breast cancer treatmentJ Womens Health (Larchmt)20071691348136118001192
- RampesHJamesRComplications of acupunctureAcupunct Med19951312633
- TamKTheory of Chinese MedicineTaiwanCheng Chung Book Co. Ltd.1998
- LamCLKGandekBRenXSChanMSTests of scaling assumptions and construct validity of the Chinese (Hong Kong) version of the SF-36 health surveyJ Clin Epidemiol199851113911479817131
- LamCLKLauderIJLamTPGandekBPopulation based norming of the Chinese (Hong Kong) version of the SF-36 health surveyHK Pract199921460470
- MorimotoTOgawaMOritaKPostoperative adjuvant randomised trial comparing chemoendocrine therapy, chemotherapy and immunotherapy for patients with stage II breast cancer: 5-year results from the Nishinihon Cooperative Study Group of Adjuvant Chemoendocrine Therapy for Breast Cancer (ACETBC) of JapanEur J Cancer199632A2352428664034
- SunTWZhuYPThe effect of PSP on immune function and living quality in patients receiving chemotherapy for gynecological malignanciesAdvanced Research in PSPYangQYHong KongThe Hong Kong Association for Health Care1999
- ChaiZKShenHMYunzhi polysaccharopeptide capsule III period clinical researchAdvanced Research in PSPYangQYHong KongThe Hong Kong Association for Health Care19992550