Abstract
Objective
To compare levels of persistency between cholinesterase inhibitors (ChEIs) among a Medicaid patient population of older adults.
Methods
Survival analysis was used to assess differences in discontinuation between ChEIs (donepezil versus rivastigmine and galantamine), and for difference in patient gender, age, race, and care setting.
Results
Rates of discontinuation increased from 42.7% (95% CI = 39.9–45.5) at 12 months to 84.8% (95% CI = 82.3–87.3) at 24 months. In multivariate models, no significant difference in discontinuation existed prior to 365 days. However, patients dispensed donepezil were less likely to discontinue as compared with users of the other two ChEIs after the first year (RR = 0.70; CI = 0.499–0.983; p < 0.04). Patients of white race were less likely to discontinue (RR = 0.549; 95% CI = 0.43–0.82; p = 0.0015), while gender, care setting, and age were not associated with discontinuation.
Conclusions
One-year persistence rates were similar between different ChEIs. Among patients persisting with ChEI medication for at least 12 months, users of donepezil were slightly more likely to continue to persist at 24 months. Nearly half of patients failed to persist with ChEI therapy for at least 12 months. Our findings underscore the limitations of the ChEI medications and the urgent need for effective and tolerable therapeutic options for patients having dementia.
Background
Alzheimer’s Disease (AD) is an irreversible progressive disorder characterized by neuronal deterioration that results in loss of cognitive functions, such as memory, communication skills, judgment and reasoning (CitationLanctot et al 2003). It is a common (CitationFratiglioni 1993; CitationZuard 2001) and chronic dementia disorder among elderly people (CitationFratiglioni 1993), responsible for nearly 70% of all dementias (CitationZuard 2001). Approximately 4.5 million Americans suffer from AD in the US population, and this number is expected to increase almost 3-fold, to 13.2 million by 2050 (CitationHebert et al 2003). The incidence and prevalence of AD increases exponentially between the ages of 65 and 85, approximately doubling every 5 years of age (CitationRocca et al 1991). The proportion of new onset cases who are 85 years of age or older is expected to increase from 42% in 1995 to 62% in 2050 (CitationHebert et al 2001). In 1995, 7.1% of all deaths in the US were attributable to AD, placing it on a par with cerebrovascular diseases as the third leading cause of death (CitationEwbank 1999). The 1991 estimate of total prevalent cost of the disease was $67.3 billion ($173,932 per case), with $20.6 billion in direct costs ($47,581 per case) (CitationErnst and Hay 1994). It is a disease with significant economic burden and a high societal impact, with the proportion of older adults increasing in the population (CitationFratiglioni 1993; CitationHebert et al 2003).
The FDA approved 4 cholinesterase inhibitor drug therapies for AD: tacrine, donepezil, galantamine, and rivastigmine, collectively known as acetyl-cholinesterase inhibitors (ChEIs). By inhibiting the breakdown of the enzyme acetylcholine-esterase, these agents are hypothesized to prolong the action of acetylcholine at the postsynaptic receptor by preventing its hydrolysis. Cholinesterase inhibitors are also prescribed for other conditions with cholinergic system dementia such as vascular dementia, Parkinson’s disease and multiple sclerosis dementia (CitationKloszewska 2002). Though not curative, these medications can slow the progression of AD rather than reverse its progressive decline and have been shown to have a modest beneficial impact on neuropsychiatric outcomes for AD patients (CitationTrinh et al 2003). The major therapeutic effect on ChEI is to maintain a cognitive function at a stable level during a 6- to 12-month period (CitationGiacobini 2000a, Citationb; CitationGiacobini 2001a, Citationb; CitationGiacobini 2002). Additional drug effects are to slow down cognitive deterioration, improve behavioral problems, and increase ability to perform daily living activities (CitationJann 1998; CitationGiacobini 2000a, Citationb; CitationGiacobini 2001a, Citationb; CitationGiacobini 2002) and improve the patient’s mood (CitationGrutzendler and Morris 2001). Recent studies show that the cognitive stabilization effect may be prolonged up to 24 (CitationGiacobini 2000a, Citationb; CitationGiacobini 2001a; CitationGiacobini 2002) to 36 months (CitationGiacobini 2001b). The four therapies for AD differ by selectivity and specificity for the brain tissue, as well as the ability to interact with other drugs, adverse events on the nervous system and gastrointestinal tract, and hepatotoxicity (CitationZuard 2001). Tacrine is no longer marketed in the US because of safety precautions (CitationClark and Karlawish 2003), and donepezil was the most frequently prescribed ChEI (CitationAuriacombe et al 2002; CitationBullock and Connolly 2002) at the time of the study.
Persistence to these agents is expected to be suboptimal, as patients are often poorly adherent to chronic medications (CitationBarat et al 2001; CitationMcDonald et al 2002). In a number of chronic illnesses, noncompliance to medications has been shown to have a significant negative health impact (CitationLuscher et al 1985; CitationCol et al 1990; CitationPsaty et al 1990; CitationChin and Goldman 1997; CitationMcDermott et al 1997; CitationBergen et al 1998; CitationPaterson et al 2000; CitationTsuyuki and Bungard 2001), and is estimated to cost the US $25 billion annually when indirect costs are included (CitationSullivan and Hazlet 1990). Older adults are especially prone to be non-adherent (CitationGray et al 2001) because of susceptibility to adverse events (CitationMonane et al 1998; CitationGolden et al 1999), deficits in physical dexterity, cognitive skills and memory, and because of the large number of medications they are prescribed (CitationCramer 1998). Some researchers have found that older patients are generally more likely than younger patients to discontinue their medication (CitationApplegate 2002; CitationBenner et al 2002; CitationJackevicius et al 2002). Patients may discontinue ChEI drug therapy as a result of intolerable adverse events, rapid clinical deterioration, or failure to improve, stabilize, or reduce the rate of decline in AD (CitationFillit and Cummings 2000). While persistence with specific ChEI medications has not been adequately examined by researchers, switching between different ChEIs has been frequently reported (CitationAuriacombe et al 2002; CitationBullock and Connolly 2002; CitationEmre 2002). There is limited information outside the clinical trial setting (CitationMauskopf et al 2005) and results of previous studies examining persistence to ChEIs have been inconsistent (CitationSicras and Rejas-Gutierrez 2004; CitationMauskopf et al 2005; CitationSicras-Mainar et al 2006) or have included only one type of ChEI (CitationRoe et al 2002), thus preventing a comparison.
The objective of this research was to compare rates of persistence between donepezil and other types of ChEIs in usual care settings among a patient population of older adults enrolled in a state Medicaid program. Studies have reported donepezil to have better tolerability than the other ChEIs (CitationRogers et al 1998; CitationEmre 2002; CitationInglis 2002; CitationWilkinson et al 2002; CitationBirks 2006). Thus, we assessed persistence with each ChEI medications separately, and overall.
Methods
Study population and design
We conducted a retrospective cohort study among patients enrolled in the Rhode Island Medicaid program between January 1, 2001 and December 31, 2003, and who received at least one dispensing for a ChEI medication. New users of ChEIs were identified by selecting patients receiving an initial prescription for a ChEI medication between July 1, 2001 and December 31, 2003. Those included had no prior dispensing for a ChEI medication in the previous 6 months, and an initial prescription prior to June 30, 2003, such that all patients had at least 6-months of follow-up time. Cases were excluded if they were less than 50 years of age. Information describing demographic and other patient characteristics was made available. Members with greater than a 6-month period (180 days) between refills, or between the last refill and the end of the study period were considered to have discontinued the drug. Switching to other types of cholinesterase inhibitors was assessed separately among those that continued their medication at 6-months and at 1 year.
Independent variables included class of ChEI dispensed (donepezil versus rivastigmine and galantamine), gender, age (age 50–69 years; or age 70 years or greater), race (white versus nonwhite), and care setting (long-term care setting versus community-dwelling). Categorizing age at 70 years was determined after performing an assessment of the parametric form for the age variable.
Descriptive statistics were used to determine the frequencies of various patient characteristics. Survival analysis was used to assess differences in persistence among ChEIs product dispensed, and by the patient characteristics identified above. Kaplan-Meier (KM) curves were independently constructed for each of the predictor variables (class of ChEIs, gender, age, race, setting) and the log-rank statistic was used to evaluate group differences in persistence. Extended Cox proportional hazards models were used to estimate rate ratios (RR) and 95% confidence intervals (CI) for the association between patient characteristics and ChEIs discontinuation. Statistical analyses were carried out using SAS statistical package version 8.01.
Results
A total of 1564 patients met the study’s inclusion criteria. Baseline characteristics of the population are presented in . The mean age of these patients was 83 years and 76% were female. Most of the patient population were in long-term care (LTC) (86%) and 61% where of white race. Donepezil was the most widely dispensed ChEI accounting for 56.4% of the patients.
Table 1 Characteristics of new users of cholinesterase inhibitor (ChEI) medications (n = 1564)
presents the discontinuation rates at 12 months and 24 months, overall and according to patient characteristics. Overall discontinuation increased with time from 42.7% (95% CI = 39.9–45.5) at 12 months, to 84.8% (95% CI = 82.3–87.3) at 24 months. In univariate analyses, the initial type of dispensed ChEI medication was not associated with discontinuation (p = 0.22). During the first 12 months, males were more likely to discontinue than females (p = 0.04) and whites were less likely to discontinue (p < 0.0001) than non-white patients.
Table 2 Discontinuation rates of cholinesterase inhibitors medications at 12 and 24 months (n = 1564)
A small percentage of patients switched ChEI medication type during the study timeframe. Among patients who were started on donepezil and continued the medication for 6 and 12 months, 96% remained on donepezil at 6 months and at 12 months. At 6 months, 1% switched to rivastigmine and 3% switched to galantamine and at 12 months 2% were receiving rivastigmine and 2% were receiving galantamine. Among patients who were started on rivastigmine 92% were still on rivastigmine at 6 months while 4.4% were switched to donepezil and 3.5% to galantamine. At 12 months, 89% were still on rivastigmine, 7.8% were receiving galantamine and 3.2% were receiving donepezil. Finally, among patients who were started on galantamine, 98% remained on galantamine at 6 months, while 1.5% were switched to donepezil and 0.8 % to rivastigmine. At 12 months, 98.7% of these patients were receiving galantamine and 1.3% were receiving rivastigmine. The Medicaid plan had no influence on drug switches, as no restrictions on the use of any particular ChEI medication were in place during the study timeframe.
In multivariate models, persistence rates did not differ among ChEI medication use as assessed during the first 12 months of therapy (RR = 1.002; CI = 0.807–1.243; p = 0.9879). Among those persisting for at least 12 months, users of donepezil were less likely to discontinue during subsequent months as compared with users of the other two ChEIs (RR = 0.70; CI = 0.499–0.983; p < 0.0397). Overall, patients of white race showed better persistence than of non-white race (RR = 0.549; 95% CI = 0.43–0.82; p = 0.0015). This, however, was based on 66% of the population since 34% had missing values for race. Gender, care setting, and age were not associated with differences in discontinuation. These results are presented in . shows the survival curves for those dispensed donepezil vs other ChEI medications.
Figure 1 Survival curves of new users of donepezil versus other cholinesterase inhibitor medications (n = 1564).
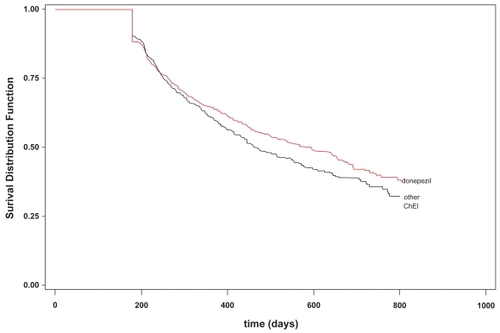
Table 3 Adjusted associations between patient characteristics and discontinuation of cholinesterase inhibitors (ChEI) medications (n = 1564)
Discussion
Our findings suggest that discontinuation rates for ChEIs are high, as previously reported (CitationRoe et al 2002; CitationSicras and Rejas-Gutierrez 2004; CitationMauskopf et al 2005; CitationSicras-Mainar et al 2006), and indicate slightly better persistence with donepezil than other ChEIs beyond 1 year of therapy, adjusting for gender, age, race, and living arrangements (adjusted RR = 0.70; CI = 0.499–0.983; p < 0.0397). Similar rates of persistence for all ChEI medications for the first 12 months of use were observed (adjusted RR = 1.002; CI = 0.807–1.243; p = 0.9879).
Patients of white race were shown to have better persistence than nonwhites (41% vs 69%), although this finding was based on an analysis of only 66% of the study population, as 34% of individuals had no information describing race in the available data sources. Despite the high rate of missing values for this variable, we believed that it was important to include this covariate in our analysis given the magnitude of difference in the percentages persisting, and because race has also been reported to be among factors associated with noncompliance (CitationBalkrishnan 1998). The smaller percentage of nonwhite cases overall merits further analysis, as it is possible that nonwhite patients were less frequently prescribed ChEI medications. In the multivariate analysis, we did not find persistence rates to differ significantly when assessing by patient age, gender, or by living arrangement. Other researchers evaluating these factors have described inconsistent findings (CitationCoons et al 1994; CitationBalkrishnan 1998).
Two studies carried out in primary care health centers in Spain (CitationSicras and Rejas-Gutierrez 2004; CitationSicras-Mainar et al 2006) also demonstrated better persistence of donepezil than rivastigmine and galantamine. CitationMauskopf et al (2005) found similar rates of persistence between rivastgmine and donepezil in a retrospective community-based study. This research, however, looked at 6 months of medication persistence and did not evaluate persistence rates after 1 year. Thus, our results are consistent since we found no significant difference in persistence prior to 1 year of therapy. Furthermore, CitationMauskopf et al (2005) recognized that the limited sample size of rivastgmine patients might have limited the authors’ ability to detect differences in persistence.
Leading reasons for discontinuation of ChEI therapy include patient or physician-perceived ineffectiveness, intolerance of side effects, or an inconvenient dosing schedule (CitationMauskopf et al 2005). Reported side effects include nausea, vomiting, and diarrhea (CitationRogers and Friedhoff 1996; CitationRogers et al 1998; CitationEmre 2002; CitationMauskopf et al 2005). Donepezil has been reported to have better tolerability and a milder side effect profile than other ChEIs (CitationRogers et al 1998; CitationEmre 2002; CitationInglis 2002; CitationWilkinson et al 2002) which might explain the observed difference in persistency rates beyond 1 year. This, nonetheless, does not explain why similar persistency rates were observed prior to 1 year, since side effects are expected to start before 1 year. Additionally, based on comparison of persistence rates, our results do not support reports that rivastigmine provides a higher magnitude of benefits than donepezil (CitationRogers et al 1998; CitationEmre 2002; CitationInglis 2002; CitationWilkinson et al 2002).
The cost-effectiveness of ChEI therapy has been questioned, particularly given the high direct cost for these medications (CitationClegg et al 2001, Citation2002; CitationFillit and Hill 2004; CitationCurtiss 2005; CitationLoveman et al 2006). Outcome evaluations should also consider the quality of life of the patient and of care givers, and the importance of developing a quality of life instrument for both (CitationLoveman et al 2006). It is difficult to quantify benefits as reported in the literature since improvements in tests such as ADAS-cog (Alzheimer’s Disease Assessment Scale cognitive subscale) may not be reflected in changes of daily life (CitationClegg et al 2001).
Because there is currently no cure for AD, one cannot expect the initial cognitive improvement observed in the first few months of therapy to be sustained indefinitely. However, one should expect that some patients who are treated early and persistently with AD medications will show less evidence of behavioral and cognitive deterioration over a period of time than one would expect in the absence of pharmacotherapy, and less decline over the long term (CitationGeldmacher et al 2006). By reducing cognitive and functional declines over time, long-term therapy may enable patients to stay at home longer and decrease the burden faced by patients, caregivers, and society (CitationGeldmacher 2003). CitationGeldmacher et al (2003) reported that taking donepezil for 9–12 months delays nursing home placement. CitationHill et al (2002) demonstrated lower costs for 204 AD patients in a large Medicare managed care plan on donepezil compared with 204 patients not receiving therapy with matched characteristics, where annual costs of prescriptions and medical services were $3,891 lower for the study group. Longer term therapy (≥270 days) also achieved lower costs compared to shorter term therapy. In our study population, 686 (57.3%) patients remained on therapy for 12 months, a figure that may represent a cost-savings considering the potential outcomes described above.
Several limitations to this study can be described. Clinical data regarding diagnosis, doses used, and exact reason for discontinuation were lacking. Prescription dispensing data were used to evaluate persistence, and filling the prescription does not ensure that the drug was actually consumed. Nonetheless, refill data are considered more objective than self-report which can overestimate compliance (CitationChoo et al 1999), and is a useful tool to assess the drug use in population-based studies (CitationSteiner and Prochazka 1997). We could not account for use of samples or hospitalizations during a follow-up period. However, we believe that our criterion of 6 months without a prescription being dispensed to be classified as nonpersistent mitigates the potential influence of these factors because it would be difficult to obtain drug samples that cover such a long period of time and it is a long period for a continuous hospitalization. Severity of AD was not assessed, but including newly treated patients in the study addresses this concern to some degree, and these medications were approved for only use in mild to moderate disease as of the time of the study. We were unable to control for potential confounders pertaining to patient co-morbidities, and 34% of the population had missing values for race. Additionally, as all the patients were enrolled in a Medicaid program, they are expected to be of lower incomes and to have no co-payments for medications. This, however, might limit generalizability of the results to other populations.
While the improvements gained with the use of ChEI medications may be small or modest, sustained benefits of therapy can be realized only by patients who persist with therapy. Yet our results indicate that persistence rates with these medications are quite low. Persistence rates were higher for donepezil users compared with those who received other ChEI medication beyond 12 months, yet given the marginal difference and the limitations of our data source, we are unable to conclude that this difference suggests superiority of donepezil.
Conclusions
We found similar persistence rates for each of the three available ChEI medications, with a 42% rate of persistence at 12 months, and those dispensed donepezil were slightly more likely to persist at 24 months. While we were not able to ascertain if discontinuation was due to lack of efficacy or lack of tolerability, our analyses revealed that nearly half of patients failed to persist with ChEI therapy for at least 12 months. Our findings underscore the limitations of the ChEI medications and the urgent need for effective and tolerable therapeutic options for patients having dementia. From a drug policy perceptive, the consideration of the utility of ChEI medications should include both results from clinical trials and insights from observational studies such as ours, which reveal that for many patients ChEI medications cannot be relied upon to provide longer-term benefit in managing dementia. Reports describing measures of therapy persistence can be important to caregivers and patients in forming expectations for pharmacotherapy.
References
- ApplegateWB2002Elderly patients’ adherence to statin therapyJAMA288495712132982
- AuriacombeSPereJJ2002Efficacy and safety of rivastigmine in patients with Alzheimer’s disease who failed to benefit from treatment with donepezilCurr Med Res Opin181293812094822
- BalkrishnanR1998Predictors of medication adherence in the elderlyClin Ther20764719737835
- BaratIAndreasenF2001Drug therapy in the elderly: what doctors believe and patients actually doBr J Clin Pharmacol516152211422022
- BennerJSGlynnRJ2002Long-term persistence in use of statin therapy in elderly patientsJAMA2884556112132975
- BergenJHuntG1998Six-month outcome following a relapse of schizophreniaAust N Z J Psychiatry328152210084346
- BirksJ2006Cholinesterase inhibitors for Alzheimer’s diseaseCochrane Database Syst Rev1CD00559316437532
- BullockRConnollyC2002Switching cholinesterase inhibitor therapy in Alzheimer’s disease--donepezil to rivastigmine, is it worth it?Int J Geriatr Psychiatry173288911921158
- ChinMHGoldmanL1997Factors contributing to the hospitalization of patients with congestive heart failureAm J Public Health8764389146445
- ChooPWRandCS1999Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapyMed Care378465710493464
- ClarkCMKarlawishJH2003Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategiesAnn Intern Med1384001012614093
- CleggABryantJ2001Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic reviewHealth Technol Assess51137
- CleggABryantJ2002Clinical and cost-effectiveness of donepezil, rivastigmine, and galantamine for Alzheimer’s disease. A systematic reviewInt J Technol Assess Health Care1849750712391943
- ColNFanaleJE1990The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderlyArch Intern Med15084152327844
- CoonsSJSheahanSL1994Predictors of medication noncompliance in a sample of older adultsClin Ther1611078205597
- CramerJA1998Enhancing patient compliance in the elderly. Role of packaging aids and monitoringDrugs Aging127159467683
- CurtissFR2005Does persistence with drugs for Alzheimer’s disease matter?J Manag Care Pharm11260215804211
- EmreM2002Switching cholinesterase inhibitors in patients with Alzheimer’s diseaseInt J Clin Pract Suppl127647212139369
- ErnstRLHayJW1994The US economic and social costs of Alzheimer’s disease revisitedAm J Public Health84126148059882
- EwbankDC1999Deaths attributable to Alzheimer’s disease in the United StatesAm J Public Health899029987474
- FillitHCummingsJ2000Practice guidelines for the diagnosis and treatment of Alzheimer’s disease in a managed care setting: Part II--Pharmacologic therapy. Alzheimer’s Disease (AD) Managed Care Advisory CouncilManag Care Interface1351610747691
- FillitHHillJ2004The economic benefits of acetylcholinesterase inhibitors for patients with Alzheimer disease and associated dementiasAlzheimer Dis Assoc Disord18Suppl 1S24915249845
- FratiglioniL1993Epidemiology of Alzheimer’s disease. Issues of etiology and validityActa Neurol Scand Suppl1451708333250
- GeldmacherDS2003Long-term cholinesterase inhibitor therapy for Alzheimer’s disease: practical considerations for the primary care physicianPrim Care Companion J Clin Psychiatry5251915213795
- GeldmacherDSFrolichL2006Realistic expectations for treatment success in Alzheimer’s diseaseJ Nutr Health Aging104172917066215
- GeldmacherDSProvenzanoG2003Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s diseaseJ Am Geriatr Soc519374412834513
- GiacobiniE2000aCholinesterase inhibitor therapy stabilizes symptoms of Alzheimer diseaseAlzheimer Dis Assoc Disord14S31010850724
- GiacobiniE2000bCholinesterase inhibitors stabilize Alzheimer diseaseNeurochem Res2511859011059792
- GiacobiniE2001aDo cholinesterase inhibitors have disease-modifying effects in Alzheimer’s disease?CNS Drugs15859111460892
- GiacobiniE2001bIs anti-cholinesterase therapy of Alzheimer’s disease delaying progressionAging132475411442306
- GiacobiniE2002Long-term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer’s diseaseJ Neural Transm Suppl62181712456062
- GoldenAGPrestonRA1999Inappropriate medication prescribing in homebound older adultsJ Am Geriatr Soc479485310443855
- GraySLMahoneyJE2001Medication adherence in elderly patients receiving home health services following hospital dischargeAnn Pharmacother355394511346058
- GrutzendlerJMorrisJC2001Cholinesterase inhibitors for Alzheimer’s diseaseDrugs61415211217870
- HebertLEBeckettLA2001Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050Alzheimer Dis Assoc Disord151697311723367
- HebertLEScherrPA2003Alzheimer disease in the US population: prevalence estimates using the 2000 censusArch Neurol6011192212925369
- HillJWFuttermanR2002The effect of donepezil therapy on health costs in a Medicare managed care planManag Care Interface15637011925682
- InglisF2002The tolerability and safety of cholinesterase inhibitors in the treatment of dementiaInt J Clin Pract Suppl127456312139367
- JackeviciusCAMamdaniM2002Adherence with statin therapy in elderly patients with and without acute coronary syndromesJAMA288462712132976
- JannMW1998Pharmacology and clinical efficacy of cholinesterase inhibitorsAm J Health Syst Pharm155S2259809108
- KloszewskaI2002Acetylcholinesterase inhibitors—beyond Alzheimer’s diseasePsychiatr Pol366 Suppl1334112647432
- LanctotKLHerrmannN2003Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysisCMAJ1695576412975222
- LovemanEGreenC2006The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s diseaseHealth Technol Assess10iii-ivix-xi1160
- LuscherTFVetterH1985Compliance in hypertension: facts and conceptsJ Hypertens, Suppl31S393916440
- MauskopfJAParamoreC2005Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settingsJ Manag Care Pharm112315115804207
- McDermottMMSchmittB1997Impact of medication nonadherence on coronary heart disease outcomes. A critical reviewArch Intern Med157192199308504
- McDonaldHPGargAX2002Interventions to enhance patient adherence to medication prescriptions: scientific reviewJAMA28828687912472329
- MonaneMMatthiasDM1998Improving prescribing patterns for the elderly through an online drug utilization review intervention: a system linking the physician, pharmacist, and computerJAMA2801249529786375
- PatersonDLSwindellsS2000Adherence to protease inhibitor therapy and outcomes in patients with HIV infectionAnn Intern Med133213010877736
- PsatyBMKoepsellTD1990The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockersJAMA263165371968518
- RoccaWAHofmanA1991Frequency and distribution of Alzheimer’s disease in Europe: a collaborative study of 1980–1990 prevalence findings. The EURODEM-Prevalence Research GroupAnn Neurol30381901952826
- RoeCMAndersonMJ2002How many patients complete an adequate trial of donepezil?Alzheimer Dis Assoc Disord16495111882749
- RogersSLFarlowMR1998A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study GroupNeurology50136459443470
- RogersSLFriedhoffLT1996The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. The Donepezil Study GroupDementia72933038915035
- Sicras-MainarAVergaraJ2006Retrospective comparative analysis of antidementia medication persistence patterns in Spanish Alzheimer’s disease patients treated with donepezil, rivastigmine, galantamine and memantineRev Neurol434495317033976
- SicrasARejas-GutierrezJ2004Drug-cholinesterase-inhibitors persistence patterns in treated patients with dementia of Alzheimer type: retrospective comparative analysis of donepezil, rivastigmine and galantamine]Rev Neurol39312615340887
- SteinerJFProchazkaAV1997The assessment of refill compliance using pharmacy records: methods, validity, and applicationsJ Clin Epidemiol50105169048695
- SullivanSDHazletTK1990Noncompliance with medication regimens and subsequent hospitalizations: A literature analysis and cost of hospitalization estimateJ Res Pharmac Econom21933
- TrinhNHHoblynJ2003Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysisJAMA289210612517232
- TsuyukiRTBungardTJ2001Poor adherence with hypolipidemic drugs: a lost opportunityPharmacotherapy215768211349746
- WilkinsonDGPassmoreAP2002A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s diseaseInt J Clin Pract56441612166542
- ZuardEG2001New treatments for Alzheimer’s Disease: A reviewDrug benefit trends132740