Abstract
The worldwide introduction of the first, unique patch for hormonal contraception (ethinyl estradiol/norelgestromin, EE/NGMN patch) was widely recognized as a significant event in the development of drug delivery systems. This innovation offers a number of advantages over the oral route, and extensive clinical trials have proved its safety, efficacy, effectiveness, and tolerability. The weekly administration and ease of use/simplicity of the EE/NGMN patch contribute to its acceptability, and help to resolve the two main problems of non-adherence, namely early discontinuation and inconsistent use. The patch offers additional benefits to adolescents (improvement of dysmenorrhea and acne), adults (improvement in emotional and physical well-being, premenstrual syndrome, and menstrual irregularities), and perimenopausal women (correction of hormonal imbalance, modulation of premenopausal symptoms), thus providing high satisfaction rates (in nearly 90% of users). Since its introduction, the transdermal contraceptive patch has proved to be a useful choice for women who seek a convenient formulation which is easy to use, with additional, non-contraceptive tailored benefits for all the ages.
Introduction: transdermal drug delivery
Patient adherence is a key issue that must be addressed to ensure the efficacy of hormonal contraception. Combined hormonal contraceptives are effective forms of reversible contraception, whose benefits have been established by a wealth of studies (CitationD’Souza and Guillebaud 2002; CitationPetitti 2003). Provided that they are taken regularly and correctly on a once-daily regimen, combined hormonal contraceptives are more than 99% effective and almost 100% reliable, the first-year pregnancy rate being less than 0.5% among perfectly conscientious users (CitationPotter et al 1996). However, from 19% to 47% of oral contraceptive users miss one or more pills per cycle (CitationPierson et al 2003) and this gap translates into thousands of unintended pregnancies annually (CitationArcher et al 2002). A more convenient method of administration has thus been sought, to reduce the risk of “missing pills”.
Transdermal systems represent a milestone innovation in drug delivery, offering a number of advantages over the oral route (CitationPrausnitz et al 2004), They include multiday and more convenient dosing, especially for the sustained release of short half-life drugs (CitationBurkman 2007). The main drawback of this technology is that only a limited number of drugs can be delivered by passive diffusion from a patch, because low molecular weight, high lipophilic property, and a small required dose are essential for a molecule to permeate the skin (CitationBurkman 2007). Thus the introduction of the first, unique patch for transdermal hormonal contraception (ethinyl estradiol/norelgestromin, EE/NGMN patch) was widely acknowledged as a significant step in the development of transdermal drug delivery (CitationPrausnitz et al 2004).
The central aim of this article is to review the main evidence supporting the use of the transdermal contraceptive EE/NGMN patch, with particular reference to patient adherence and satisfaction.
Methods
A Medline search was made during April 2008, to identify all types of articles in English on the EE/NGMN patch, including prospective open label and controlled clinical studies. The keywords were: “transdermal”, “contraceptive”, “ethinyl estradiol”, “norelgestromin”, “compliance”, “adherence”, “persistence”, “acceptability”, “therapeutic alliance”, and “patient satisfaction”. Congress communications on the same topics were also searched, to include more recent clinical studies. When no refererences are cited, the reader can assume that the author’s clinical observations are presented.
Main characteristics and clinical development
The EE/NGMN patch is an innovative, three-layer hormonal contraceptive system that provides similar efficacy to oral contraceptives, with the substantial benefit of once-weekly administration. The patch is applied once weekly for 3 consecutive weeks, followed by a patch-free week. As a result, users need only actively comply with dosing once weekly on the same day (CitationPierson et al 2003).
The transdermal contraceptive EE/NGMN patch has the same mechanisms of action as combined oral contraceptives. After the patch is applied, hormones appear rapidly in the circulation, reaching a plateau after approximately 48 hours, which is maintained at this level during the 7-day wear period. On average, each EE/NGMN patch delivers 150 μg of norelgestromin (the primary active metabolite of norgestimate) and 20 μg of ethinyl estradiol daily to the systemic circulation (CitationO’Connel and Burkman 2007).
The pharmacokinetic properties (CitationAbrams et al 2001; CitationBurkman 2007) of the EE/NGMN transdermal patch provide particular benefits over combined oral contraceptives ().
Table 1 Clinical advantages of a transdermal system for contraception
Efficacy
Three pivotal phase III clinical studies have consistently confirmed the contraceptive efficacy of the transdermal EE/NGMN patch, either in comparison with oral contraceptives (CitationHedon et al 2000; CitationAudet et al 2001) or according to an open, non-comparative trial design (CitationSmallwood et al 2001).
Pooled analyses of pivotal trials on more than 3,300 women and more than 22,000 treatment cycles showed favorable results: the EE/NGMN patch had an overall annual probability of pregnancy (method failure plus user failure) of 0.8% and a method failure probability of 0.6%. Efficacy and cycle control were similar to those of established oral contraceptives, and were comparable across age and racial groups (CitationZieman et al 2002). Follicular size and incidence of ovulation proved to be significantly reduced among patch users compared with those in women using oral contraceptives, both in normal cycles and after planned dosing errors (CitationPierson et al 2003).
Besides controlled studies, effectiveness was tested in real life conditions. Results of a large European, open-label study evaluating women’s experience with the transdermal contraceptive patch during routine use were recently disclosed at a congress (CitationJakimiuk et al 2006a; CitationJakimiuk et al 2006b). In the study, 573 healthy women were followed up for six, 4-week treatment cycles. The transdermal contraceptive patch was shown to be a reliable method of contraception, with a Pearl Index (the number of pregnancies per 100 women-years of use) of 0.43 (95% CI: −0.41 to 1.27) and a Pearl Index for patients who reported perfect compliance of 0.48 (95% CI: −0.47 to 1.43). Thus both indexes were comparable to those of other popular forms of hormonal contraception.
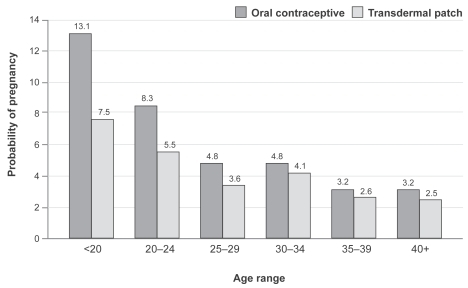
A further study (CitationSonnenberg et al 2005) estimated the contraceptive effectiveness of the patch versus oral contraceptives in real life conditions. The probability of pregnancy with the transdermal patch was shown to be lower than with oral contraceptives in all age groups, included younger women (). Therefore, the effectiveness of the patch is expected to exceed that of oral contraceptives, due to an increased rate of perfect use.
Figure 1 Age-specific pregnancy rates for oral contraceptives and patch calculated in real life conditions (From data of CitationSonnenberg et al 2005).
Adhesion could be a potential concern, because optimal drug delivery can be achieved only if the patch remains adhered to the user’s skin. In fact the adhesive reliability of the contraceptive patch has proved to be excellent and consistent: the two clinical trials showed that only 1.8% and 2.9% of patches required replacement because of complete or partial detachment, respectively. Furthermore, it was observed that patch adhesion tended to improve over treatment cycles, probably because participants learned the proper application technique with continued use. Finally, specific studies showed that the contraceptive patch detachment rate was unaffected by heat, humidity, and exercise (CitationZacur et al 2002).
In conclusion, the EE/NGMN patch provides, on a once-weekly schedule, at least the same efficacy as marketed oral contraceptives administered on a daily regimen, with the added benefits of sustained hormone concentrations and greater “forgiveness” of dosing errors. Even if a scheduled patch change is missed for 2 days during weeks 2 and 3 of a 4-week cycle, clinical efficacy is maintained, and backup contraception is not needed (CitationBurkman 2007). Adhesion properties are reassuring for women who enjoy participating in exercise, as they can maintain all their usual activities including bathing, swimming, jogging, and using a whirlpool or a sauna (CitationAbrams et al 2001; CitationZacur et al 2002).
Tolerability and safety
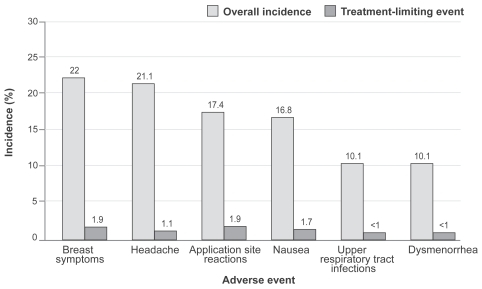
The pooled analysis of safety and tolerability data across three pivotal studies showed that the adverse effect profile of the EE/NGMN patch was fairly similar to that of oral contraceptives, the most frequent adverse events being headache and nausea (). A few notable differences were reported in patch users: transient, mild to moderate application site reactions, as expected, and breast discomfort symptoms, which generally resolved after 3 months of use. Local tolerability was shown to be good, with low potential for irritation and no potential for photo-toxicity (CitationSibai et al 2002). However, women with atopia and allergic skin diseases are more vulnerable to allergic reactions or local skin irritation.
Figure 2 Most common adverse events in the three pooled contraceptive studies with the patch (From data of CitationSibai et al 2002).
Further studies addressed some safety issues and showed that oral and transdermal contraception with similar hormones induced similar effects on vascular risk markers (CitationJohnson et al 2008; CitationKluft et al 2008). A review of post-marketing safety and surveillance data for progestin oral contraceptives containing norgestimate and ethinyl estradiol provided useful information about the relative rate of vascular events (CitationLippman and Shangold 1997). These data fully supported the favorable cardiovascular safety profile of norgestimate-containing oral contraceptives. A recently published, nested, case-control, epidemiologic study, which compared different routes of administration, and included a further 17 months of follow up, showed that the risk of non-fatal venous thromboembolism was similar in the contraceptive patch and norgestimate-containing oral contraceptives with 35 μg of ethinyl estradiol (CitationJick et al 2006; CitationJick et al 2007). Furthermore, the EE/NGMN patch showed no evidence of an increased risk of cerebral venous sinus thrombosis compared with levonorgestrel-containing, norgestimate-containing, and desogestrel-containing oral contraceptives (CitationJick and Jick 2006). Moreover, ischemic stroke and acute myocardial infarction were rarely reported among contraceptive patch users (CitationJick and Jick 2007). Only one epidemiologic study reported a more than two-fold increase in the risk of venous thromboembolism, but not arterial thromboembolic events, for the transdermal contraceptive system, compared with the same risk in users of norgestimate-containing oral contraceptives (CitationCole et al 2007). These data were not confirmed by other studies; moreover, the estimated incidence of venous thromboembolism per 100,000 women-years was 40.8 for contraceptive patch users, which is similar to that reported in studies of third generation progestins (CitationBurkman 2007).
Therefore the EE/NGMN contraceptive patch experience confirms previously reported findings on safety in hormonal oral contraceptives: provided that they are not prescribed to women at risk, and that they contain a low dose of ethinyl estradiol and suitable progestins (eg, norgestimate), their net health benefit is great, even when the health risks are taken into account (CitationPetitti 2003).
Perfect dosing
Patch users were better able to follow the dosing regimen than users of daily oral contraceptive (CitationCreasy et al 2001). Indeed transdermal contraception has been shown to improve the percentage of cycles with perfect dosing compared with oral contraceptives. Pooled data across the three pivotal studies showed that the percentage of cycles with perfect dosing was significantly higher with the patch than with oral contraceptives (CitationArcher et al 2004).
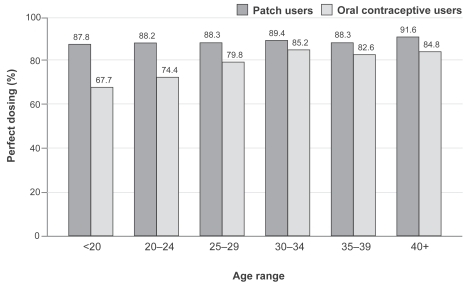
Age has often been reported as a factor affecting correct and consistent oral contraceptive use. Significantly, in the main comparative clinical trial conducted in North America, perfect use was consistent across age groups for the patch, while, as expected, rates of perfect use for oral contraceptives differed significantly by age (CitationArcher et al 2002). Adolescents (the age group most vulnerable to forgetting to take oral contraceptives and/or unintentional mistakes and thus inadequate adherence) had the greatest increase in compliance with transdermal contraception over oral contraceptives (87.7% vs 67.7%) (CitationAudet et al 2001). This significant advantage should be considered especially when counseling adolescents on contraceptive choices.
As a consequence of improved compliance, a base-case analysis showed that increased perfect use of the patch would result in a saving of US$249 per woman over 2 years compared with oral contraceptives (CitationSonnenberg et al 2005).
Transdermal contraception has further benefits, encouraging perfect use, in addition to its effectiveness in preventing unwanted pregnancies and its favorable safety profile. Most women consider the patch a convenient and simple method: in a recent study, more than 70% preferred or strongly preferred this contraceptive method compared with previous forms of contraception (CitationJakimiuk et al 2006b, ). Convenience and simplicity of use were predominant factors in this high level of preference and contributed to the good acceptability of the patch.
Table 2 Satisfaction, compliance and preference for the patch
Patient adherence and acceptability
When considering patient choices among contraceptive methods, the reasons for treatment compliance and adherence need to be carefully considered. As originally defined by Sackett in his 1976 landmark publication (CitationSackett et al 1976), the term compliance refers to the extent to which the behavior of the patient coincides with the doctor’s order. The term compliance has long been criticized as denoting obedience by passive patients to doctors as authoritity figures, in the traditional hierarchical doctor/patient relationship. Therefore, a more consistent definition encompassing consensus was sought.
Adherence can also refer to a therapeutic plan, mutually agreed between patient and doctor, in which the patient expects to understand the information provided, and to cooperate by adding their personal perception and experience (CitationProbstfield 1991). Because they actively “adhere” to therapeutic decisions, patients are involved in mutual decision making with their doctor, reaching a “therapeutic alliance”, a valuable predictor of favorable outcome.
However, adherence needs to be combined with persistence for the duration of treatment, to optimize efficacy and satisfaction with use. In this way, personal reward, can encourage repetition of the same behavior.
The major difference detected in the oral contraceptive failure rate with perfect use (0.1%), and the failure rate in real life (6.9% after 12 months) probably reflects at least some degree of dissatisfaction with available methods (CitationTrussel and Vaughan 1999). Every physician prescribing a contraceptive should carefully evaluate patient satisfaction, and factors that could potentially enhance it. This satisfaction is closely related to the success of treatment, just as a favorable patient/physician relationship influences treatment adherence. Oral contraceptive non-adherence can be explained in several ways, that is, early discontinuation (drop-outs), sporadic non-adherence due to forgetfulness and/or unintentional mistakes (inconsistent use), and systematic non-adherence over time ().
Table 3 Main determinants of compliance, adherence, and persistence
Early discontinuation is the most critical issue, as women who discontinue oral contraceptives often choose a less reliable contraceptive or no method at all, thus experiencing more unintended pregnancies. A significant predictor of early discontinuation is the occurrence of adverse events, with an increased risk of discontinuation as the number of adverse effects increases (CitationRosenberg et al 1995). Weight gain is the most commonly reported adverse event leading to drop out. It seems to be a subjective complaint, however, as no evidence of a causal association between hormonal contraception and weight gain was found by the Cochrane group in their recent systematic review (CitationGallo et al 2006).
Key predictors for inconsistent use are lack of an established routine for pill-taking and failure to understand instructions, thus emphasizing that quality of information provided by the physician and mutual decision making are influential in achieving long-term adherence.
As shown by measuring compliance using electronic devices, women tend to become less careful about their pill-taking behavior over time (CitationPotter et al 1996). This change may reflect personalized schedules of use, learning to make up for occasional missed pills, or a kind of fatigue during continuous use. In addition, the same study showed that women tend to under-report their missed pills: the proportion of women missing at least three pills in a cycle according to the electronic data was triple that derived from the women’s diaries (30%–51% vs 10%–14%). Missing pills were more likely to be clustered at the weekends and on consecutive days (CitationPotter et al 1996), with a consequent increased risk of irregular bleeding and unintended pregnancies.
An analysis of women’s self described reasons for missing pills showed that the three most reported reasons were being away from home, simply forgetting to take a pill, and not having the pill pack with them/being unable to obtain a new pack of pills in time for the beginning of the new cycle (CitationSmith and Oakley 2005). Further predictors for inconsistent use are reported in .
Table 4 Key predictors for inconsistent contraceptive use
To date, systematic “reasoned” non-adherence, whereby patients decide to change the dosage and/or dosing interval without informing the doctor, does not seem to have been adequately investigated. This kind of non-adherence can be avoided to some extent, by jointly establishing the ultimate objective, which differs according to the age of the woman. Evaluating a woman’s particular needs or specific fears (including fear of forgetting to taking the pill, or fear of a negative effect on the body) and other such emotions including general anxiety which could foster inconsistent usage (CitationWalsemann and Perez 2006) can help in doctor/patient decision making. In a recent cross-sectional multicenter study, designed to assess the reasons for selecting the contraceptive pill, the skin patch, or the vaginal ring in 9700 women, the main reasons for choosing one type of hormonal treatment over another, consistent with the findings of previous studies, were convenience and frequency of use associated with lower probability of inadvertent omission (CitationLete et al 2007). In addition, including the husband in family planning programs has been shown to increase the use of modern contraception (CitationTerefe and Larson 1993).
Adherence to treatment has been shown to be statistically superior for the transdermal patch compared with that observed with oral contraceptives (), in all age groups (CitationArcher et al 2004) and in all treatment cycles (CitationDittrich et al 2002). The same is true for user satisfaction: in a study presented at a recent conference (CitationJakimiuk et al 2006b), the mean satisfaction score was shown to increase when women switched from previous contraceptive methods to the transdermal patch ().
Figure 3 Percentage of cycles with perfect adherence, by age group. Comparison between patch and oral contraceptive users (From data of CitationArcher et al 2004).
Furthermore, in a recently published Cochrane review comparing contraceptive effectiveness and compliance in non-oral methods versus oral contraceptives, the authors concluded that although effectiveness was similar, the contraceptive patch group reported better compliance than the oral contraceptive group (odds ratio = 2.05 and 2.76 in two trials). In one crossover ring trial greater non-compliance was reported by ring users (CitationLopez et al 2008).
Benefits of hormonal contraception: tailoring treatment
Today women are still largely unaware of the non-contraceptive health benefits associated with hormonal contraceptives (). Choosing a contraceptive method is a mutual decision that should take into account, besides the risks, the expected non-contraceptive benefits. A doctor should evaluate these with the woman in order to agree on a tailored program to achieve her adherence and persistence of use.
Table 5 Well-established non-contraceptive benefits of hormonal methods
Adolescents
The most popular form of birth control among adolescents is oral contraceptives (CitationRubinstein et al 2004). However, adolescents tend to miss oral contraceptive pills, with a rate of failure close to 5% to 18%, that is, approximately 55% higher than that found in adult users, leading to a greater risk of unintended pregnancies (CitationRubinstein et al 2004; CitationHarel et al 2005). In addition, less than 60% of adolescents report perfect compliance with oral contraceptives, the rate of discontinuation within 1 year being approximately 64% (CitationRubinstein et al 2004).
A number of specific studies have examined adolescent use of the transdermal EE/NGMN contraceptive patch, confirming that it can provide a convenient form of reversible contraception (CitationLogsdon et al 2004). Its ease of use and the fact that it does not require daily attention are well acknowledged by adolescents who have used it (CitationRubinstein et al 2004).
The finding that users of the transdermal EE/NGMN contraceptive patch generally experience minimal changes in body weight could be relevant to adolescent use, because weight gain is a significant predictor of early discontinuation of combined hormonal contraceptives in this age subgroup population (CitationHarel et al 2005).
Improvement of facial acne, relief of dysmenorrheal symptoms, and reduction of heavy periods are further benefits. In a recent study of Thai adolescent women, participants reported a decrease in dysmenorrhea, shorter duration of bleeding, and an improvement of facial acne (CitationPiyasirisilp and Taneepanichskul 2008). These data confirm the previously reported findings of Harel’s study, that is, an improvement in the facial acne of one third of adolescents, and favorable results in a preliminary study of patch use among women suffering from papulo-pustular inflammatory acne. Clinical improvement started from the fourth month, with lesions almost disappearing in some patients (CitationCaputo et al 2005). Furthermore, in women with acne vulgaris, an oral contraceptive containing norgestimate has been shown to be effective in normalizing skin-surface lipids in seborrheic areas, while skin hydration did not undergo any important changes (CitationSator et al 2003).
These therapeutic effects have been ascribed to the progestin component of the patch, which interacts selectively with the progesterone receptor and, therefore, does not stimulate androgen receptors (CitationWhite et al 2005). Norgestimate, which is metabolized to norelgestromin, has negligible binding affinities for the androgen receptor and for the sex hormone-binding globulin (SHBG), reflecting the low androgenicity of this progestin, which is a desirable property particularly when signs of skin hyperandrogenism such as acne appear. In addition, the antiandrogenic activity of norgestimate and of norelgestromin has recently been demonstrated, using a human androgen-dependent stable-transfected cell line (CitationParis et al 2007). Finally, in skin tissue, norgestimate is a potent inhibitor of 5α-reductase, the enzyme responsible for transforming testosterone in the more potent 5α-dihydrotestosterone (CitationRabe et al 2000). Given these findings, the contraceptive EE/NGMN patch could be useful in women with disorders of androgen excess (CitationWhite et al 2005).
Adult women
Some evidence indicates that the patch is particularly beneficial for emotional and physical well-being, and for premenstrual syndrome, which is more common in women over the age of 30 (CitationWarner and Bancroft 1990).
Women who use the transdermal contraceptive patch have been reported to give higher ratings than oral contraceptive users when questioned on their emotional and physical well-being, and on improvements in premenstrual symptoms. In a randomized study comparing the patch with an oral contraceptive containing desogestrel and ethinyl estradiol, emotional and physical well-being were significantly higher with the patch than with the oral contraceptive, the difference being clustered in women aged 34 years and over. The same difference in improvement of premenstrual symptoms (p < 0.01) favoring the contraceptive patch was found, once again especially in women aged 34 years and over (CitationUrdl et al 2005).
These non-contraceptive beneficial effects may, at least in part, explain the high level of satisfaction reported by users of the transdermal patch and may contribute to their adherence to treatment.
The perimenopause
The perimenopause is a period lasting up to 5 to 6 years during which women experience menstrual cycle changes and may also experience typical menopausal signs and symptoms, such as bone mineral density loss, vasomotor instability, and joint pain. Some women erroneously believe that they no longer need contraception, although statistics show that up to 80% of women aged 40 to 44 years can conceive (CitationSchmidt-Sarosi 1998). For these women, hormonal contraception, which offers protection against undesired pregnancy as well as correction of hormonal imbalance, is more suitable than hormone replacement therapy, which offers only the latter (CitationKaunitz 2001).
In hormonal combinations, contraceptives containing norgestimate have demonstrated prominent changes in bone resorption and formation markers in patients with hypothalamic amenorrhea, a young population experiencing significant bone loss (CitationGrinspoon et al 2003). These findings suggest that suitable hormonal combinations can decrease the rate of bone turnover and attenuate bone loss in at risk populations, such as perimenopausal women (appropriate calcium and vitamin D daily intake must be checked and integrated if necessary).
In conclusion, in a mutual decision making process with their physician, healthy perimenopausal women can be assured that the transdermal patch is a useful and reliable contraceptive method, and an alternative to oral formulations, which can improve perimenopausal symptoms, reduce some long-term health risks, and enhance quality of life (CitationKaunitz 2001).
Satisfaction with the transdermal patch
Because human behavior tends to be repeated when rewarded, satisfaction with a contraceptive method is essential for long-term adherence, and depends mainly on selecting the optimal contraceptive for the couple ().
A large European study compared patient attitudes (satisfaction and preference) toward the transdermal contraceptive patch and toward previous contraceptive methods. Of all the women surveyed, 88% said that they were satisfied or very satisfied with the patch and 70.1% preferred the patch to their previous method of contraception, which in 72.4% had been an oral contraceptive. The main reasons for this preference were: convenience (40.9%), ease of use/simplicity (31.5%), and fewer side effects (19.3%) (CitationJakimiuk et al 2006b).
In another similar study 74.9% of women preferred the patch, mainly because of its convenience (50.2%) and simplicity (32.5%); 91% were satisfied or very satisfied with the patch (CitationWeisberg et al 2005).
Another clinical study showed that patch users were significantly more satisfied with their contraceptive than users of oral contraceptives (p = 0.001), and that satisfaction was associated with duration of use and mental well-being (CitationWan et al 2007).
Patch users sometimes refer to inconveniences, which can depend either on the transdermal route of administration or on the hormonal mode of action. These include application site reactions or pruritus, mainly in subjects with irritated or sensitive skin (but <2% of participants discontinued treatment for this reason), incomplete adhesive reliability (although only a minimal proportion of patches requires replacement), patch visibility (which is a problem for only a limited subset of subjects; applying the patch to the buttock or to lower abdomen guarantees discretion), appearance of a dark ring around the patch (probably due to adhesive components; washing the area normally where the patch is attached could resolve this inconvenience), unpredictable vaginal bleeding (an adverse event, common even with oral contraceptives, which generally decreased over time) (CitationAudet et al 2001; CitationSibai et al 2002). Therefore, a further optimization of structural support and hormones of the patch could help improve esthetics and cutaneous tolerability, and reduce breakthrough bleeding or spotting.
Conclusions
The transdermal EE/NGMN contraceptive patch is an excellent choice for women of any age who desire convenient, easy-to-use, reversible, hormonal contraception. The contraceptive efficacy of the patch is comparable with that of oral contraceptives while adherence and persistence of use are consistently better for the patch in all age groups. The patch enables a contraceptive to be tailored to suit the needs of women of all ages and characteristics. Combined with a valuable doctor-patient relationship which permits the development of a therapeutic alliance, the patch has received higher satisfaction ratings in addition to ensuring effectiveness and safety.
Disclosures
Dr Graziottin has served on the Speakers’ Bureau for Bayer-Schering Healthcare, Boeringher-Ingelheim, Janssen-Cilag, and Procter and Gamble, and as a consultant for Theramex.
References
- AbramsLSSkeeDMNatarajanJ2001Pharmacokinetics of norelgestromin and ethinyl estradiol delivered by a contraceptive patch (Ortho Evra™/Evra™) under conditions of heat, humidity and exerciseJ Clin Pharmacol411301911762557
- ArcherDFBigriggASmallwoodGH2002Assessment of compliance with a weekly contraceptive patch (Ortho Evra™/Evra™) among North American womenFertil Steril772, Suppl 2S27S3011849633
- ArcherDFCullinsVCreasyGW2004The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra®) on contraceptive efficacyContraception691899514969665
- AudetMCMoreauMKoltunWD2001Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive. A randomized controlled trialJAMA28523475411343482
- BurkmanRT2007Transdermal hormonal contraception: benefits and risksAm J Obstet Gynecol197134e1617689623
- CaputoRBarbareschiMLunardonL2005A patch-released norelgestromin and ethinyl estradiol association in the treatment of papulo-pustular inflammatory acneG Ital Dermatol Venereol14072732
- ColeJANormanHDohertyM2007Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system usersObstet Gynecol1093394617267834
- Collaborative Group on Epidemiological Studies of Ovarian Cancer2008Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controlsLancet3713031418294997
- CramerJARoyABurrellA2008Medication compliance and persistence: terminology and definitionsValue Health1144718237359
- CreasyGWAbramsLSFisherAC2001Transdermal contraceptionSemin Reprod Med193738011727179
- DittrichRParkerLRosenJB2002Transdermal contraception: evaluation of three transdermal norelgestromin/ethinyl estradiol doses in a ranmdomised, multicenter, dose-response studyAm J Obstet Gynecol186152011810078
- D’SouzaREGuillebaudJ2002Risks and benefits of oral contraceptive pillsBest Pract Res Clin Obstet Gynecol1613354
- FrenchL2008Dysmenorrhea in adolescents: diagnosis and treatmentPaediatr Drugs101718162003
- GalloMFLopezLMGrimesDA2006Combination contraceptives: effects on weightCochrane Database of Systematic ReviewsIssue 1 Art. No: CD00398710.1002/14651858.CD003987.pub2
- GrinspoonSKFriedmanAJMillerKK2003Effects of a triphasic combination oral contraceptive containing norgestimate/ethinyl estradiol on biochemical markers of bone metabolism in young women with osteopenia secondary to hypothalamic amenorrheaJ Clin Endocrinol Metab883651612915650
- HannafordPCSelvarajSElliottAM2007Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception studyBMJ335651917855280
- HarelZRiggsSVazR2005Adolescents’ experience with the combined estrogen and progestin transdermal contraceptive method Ortho EvraJ Pediatr Adolesc Gynecol18859015897103
- HedonBHelmerhorstFMCronjeHS2000Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs an oral contraceptiveInt J Gynecol Obstet70Suppl 178
- JakimiukAMadelenetPChernevT2006aEfficacy and tolerability of transdermal hormonal contraception over 6 monthsEur J Contracept Reprod Health Care11S1P024,667
- JakimiukAMadelenetPChernevT2006bTransdermal contraception is associated with high levels of patient satisfaction and complianceEur J Contracept Reprod Health Care11S1P028,689
- JensenJTSperoffL2000Health benefits of oral contraceptivesObstet Gynecol Clin North Am277052111091985
- JickSSJickH2006Cerebral venous sinus thrombosis in users of four hormonal contraceptives: levonorgestrel-containing oral contraceptives, norgestimate-containing oral contraceptives, desogestrel-containing oral contraceptives and the contraceptive patchContraception74290216982227
- JickSSKayeJARussmannS2006Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 mcg of ethinyl estradiolContraception73223816472560
- JickSSJickH2007The contraceptive patch in relation to ischemic stroke and acute myocardial infarctionPharmacotherapy272182017253912
- JickSKayeJALiL2007Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 mcg of ethinyl estradiolContraception764717586129
- JohnsonJVLowellJBadgerGJ2008Effects of oral and transdermal hormonal contraception on vascular risk markersObstet Gynecol111270283
- KaunitzAM2001Oral contraceptive use in perimenopauseAm J Obstet Gynecol185S32711521120
- KluftCMeijerPLaGuardiaKD2008Comparison of a transdermal contraceptive patch vs oral contraceptives on hemostasis variablesContraception77778318226669
- LeteIDovalJLPérez-CamposE2007Factors affecting women’s selection of a combined hormonal contraceptive method: the TEAM-06 Spanish cross-sectional studyContraception76778317656174
- LippmanJSShangoldGA1997A review of post-marketing safety and surveillance data for oral contraceptives containing norgestimate and ethinyl estradiolInt J Fertil422309
- LogsdonSRichardsJOmarHA2004Long-term evaluation of the use of the transdermal contraceptive patch in adolescentsThe Scientific World45126
- LopezLMGrimesDAGalloMF2008Skin patch and vaginal ring versus combined oral contraceptives for contraceptionCochrane Database of Systematic ReviewsIssue 1 Art. No: CD00355210.1002/14651858.CD003552.pub2
- MillerWB1986Why some women fail to use their contraceptive method: a psychological investigationFam Plann Perspect1827323803546
- O’ConnellKBurkmanRT2007The transdermal contraceptive patch: an updated review of the literatureClin Obstet Gynecol509182617982334
- ParisFRabeolinaFBalaguerP2007Antiandrogenic activity of norgestimate in a human androgen-dependent stable transfected cell lineGynecol Endocrinol23193717505938
- PetittiDB2003Combination estrogen-progestin oral contraceptivesN Engl J Med34944350
- PiersonRAArcherDFMoreauM2003Ortho Evra™/Evra™ versus oral contraceptives: follicular development and ovulation in normal cycles and after an intentional dosing errorFertil Steril80344212849799
- PiyasirisilpRTaneepanichskulS2008A clinical study of transdermal contraceptive patch in Thai adolescence womenJ Med Assoc Thai911374118389975
- PonsJEHormonal contraception compliance in teenagers2006Pediatr Endocrinol Rev3Suppl 1164616641852
- PotterLOakleyDDe Leon-WongE1996Measuring compliance among oral contraceptive usersFam Plann Perspect2815488853280
- ProbstfieldJLCramerJASpilkerBThe clinical trial pre-randomization compliance (adherence) screen1991Patients compliance in medical practice and clinical trialsNew YorkRaven Press32334
- PrausnitzMRMitragotriSLangerR2004Current status and future potential of transdermal drug deliveryNature Rev Drug Discov31152415040576
- RabeTKowaldAOrtmannJ2000Inhibition of skin 5 alpha-reductase by oral contraceptive progestins in vitroGynecol Endocrinol142233011075290
- RamaRaoSLacuestaMCostelloM2003The link between quality of care and contraceptive useInt Fam Plan Perspect29768312783771
- RaschVKnudsenLBGammeltoftT2007Contraceptive attitudes and contraceptive failure among women requesting induced abortion in DenmarkHum Reprod221320617296620
- RodgersAKFalconeT2008Treatment strategies for endometriosisExpert Opin Pharmacother92435518201147
- RosenbergMJWaughMSMeehanTE1995Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuationContraception5128387628201
- RosenbergMJWaughMSBurnhillMS1998Compliance, counseling and satisfaction with oral contraceptives: a prospective evaluationFam Plann Perspect30289929561874
- RubinsteinMLHalpern-FelsherBLIrwinCE2004An evaluation of the use of the transdermal contraceptive patch in adolescentsJ Adolesc Health3439540115093794
- SackettDLSackettDLHaynesRBIntroduction1976Compliance with therapeutic regimensBaltimoreJohns Hopkins University Press
- SatorPGSchmidtJBHonigsmannH2003Clinical evidence of the endocrinological influence of a triphasic oral contraceptive containing norgestimate and ethinyl estradiol in treating women with acne vulgarisDermatology206241812673082
- Schmidt-SarosiC1998Infertility in the older womanClin Obstet Gynecol41940509917949
- SibaiBMOdlindVMeadorML2002A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra™/Evra™)Fertil Steril77Suppl 2S19S2611849632
- SmallwoodGHMeadorMLLenihanJP2001Efficacy and safety of a transdermal contraceptive systemObstet Gynecol9879980511704172
- SmithJDOakleyD2005Why do women miss oral contraceptive pills? An analysis of women’s self-described reasons for missed pillsJ Midwifery Womens Health50380516154064
- SonnenbergFABurkmanRTSperoffL2005Cost-effectiveness and contraceptive effectiveness of the transdermal contraceptive patchAm J Obstet Gynecol1921915671994
- TerefeALarsonCP1993Modern contraception use in Ethiopia: does involving husbands make a difference?Am J Public Health831567718238680
- TrussellJVaughanB1999Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family GrowthFam Plann Perspect31647210224544
- UrdlWApterDAlpersteinA2005Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once-weekly transdermal compared with oral contraceptionEur J Obstet Gynecol Reprod Biol1212021016054963
- WalsemannKMPerezAD2006Anxiety’s relationship to inconsistent use of oral contraceptivesHealth Educ Behav3319721416531513
- WanGJBarnowskiCEAmbegaonkarBM2007Treatment satisfaction with a transdermal contraceptive patch or oral contraceptivesContraception75281417362706
- WarnerPBancroftJ1990Factors related to self-reporting of the premenstrual syndromeBr J Psychiatry157249602224376
- WeisbergFBouchardCMoreauM2005Preference for and satisfaction of Canadian women with the transdermal contraceptive patch versus previous contraceptive method: an open-label, multicenter studyJ Obstet Gynaecol Can27350915937609
- WesthoffCLHeartwellSEdwardsS2007Oral contraceptive discontinuation: do side effects matter?Am J Obstet Gynecol196412e1717403440
- WhiteTJainJKStanczykFZ2005Effect of oral versus transdermal steroidal contraceptives on androgenic markersAm J Obstet Gynecol192200559
- WHOAdherence to long-term therapies2003Evidence for actionGeneva, Switzerland
- YildizBO2008Oral contraceptives in polycystic ovary syndrome: risk-benefit assessmentSemin Reprod Med261112018181089
- ZacurHAHedonBMansourD2002Integrated summary of Ortho Evra™/Evra™ contraceptive patch adhesion in varied climates and conditionsFertil Steril77S32511849634
- ZiemanMGuillebaudJWeisbergE2002Contraceptive efficacy and cycle control with the Ortho Evra™/Evra™ transdermal system: the analysis of pooled dataFertil Steril77S13811849631