Abstract
Clinical practice research provides a unique opportunity to care for a diverse patient population in various health care system settings. Federal study of Adherence to Medications in the Elderly (FAME) was the first prospective observational and randomized controlled trial to implement effective strategies to enhance medication adherence and health outcomes in older patients using polypharmacy. Ten lessons learned from conducting this adherence intervention trial are described: (1) Link the trial to existing clinical work, (2) Begin with a thorough understanding of medication adherence, (3) Ensure that trial highlights individualized intervention, (4) Tailor inclusion criteria and study duration to target population, (5) Employ a range of outcomes linked to meaningful clinical effects, (6) Win the support of the multidisciplinary team and the administration, (7) Promote team work, (8) Consider the potential limitations, (9) Seize the grant opportunities, and (10) Share the findings.
Clinical practice research provides a unique opportunity to care for a diverse patient population in various health care system settings. Such research can lead to strategic discoveries in improving medication delivery and patient safety. The Federal study of Adherence to Medications in the Elderly (FAME)Citation1 was the first clinical trial to adopt effective interventions to enhance medication adherence and health outcomes in older patients, tested via a prospective observational phase followed by a randomized controlled trial. A comprehensive pharmacy care program was developed using three key components: (1) an individualized education provided by clinical pharmacists, (2) an adherence aid (custom blister-packaging of medications), and (3) a serial follow-up schedule. After a six-month exposure to the comprehensive pharmacy care program (study phase 1), the subjects’ medication adherence increased from 61.2% (baseline) to 96.9% (p < 0.001). The increased adherence rate was associated with significant improvements in systolic blood pressure (BP) (133.2 to 129.9 mm Hg; p = 0.02) and low-density lipoprotein cholesterol (LDL-C) (91.7 to 86.8 mg/dL; p = 0.001). At the beginning of the study phase 2, all active patients were randomized to either the continued pharmacy care group or the usual care group. At the end of the six-month randomized trial, the pharmacy care group patients successfully achieved medication persistence (sustained adherence) at 95.5%; however, the usual care group patients’ adherence rate returned to near the baseline (69.1%; p < 0.001). The medication persistence was associated with further reductions in systolic BP in the continued pharmacy care group.Citation1
Many lessons were learned from conducting the FAME trial, while designing the intervention program; recruiting, enrolling, and retaining study patients; coordinating the blister-packing process; working with multidisciplinary providers and departments; obtaining a foundation grant; and presenting the study findings. Ten lessons learned are described (see ) to promote ongoing practice-based research to enhance medication adherence.
Table 1 Ten lessons learned from conducting the Federal study of Adherence to Medications in the Elderly trial
Link the trial to existing clinical work
Your patients’ specific needs, limitations in a health care system, or missing links to an optimal adherence pattern may be recognized in a daily clinical work and fuel research questions. In turn, innovative ways to fulfill the identified needs, to overcome the limitations, or to connect the missing links can serve as your research interventions. For example, after reviewing pharmacy notes from a geriatric clinic, it was evidenced that poor medication adherence was the most frequently identified problem among our elderly patients. Of 308 patient encounters, only 40% of the patients were documented to be adherent. Besides providing pillboxes, our health care system offered no specific solution to nonadherence. At the same time, a literature review revealed 65% of adults 65 years and older having two or more chronic health problemsCitation2 that often led to polypharmacy use, nonadherence, and hospitalization.Citation3 Despite this well known problem in the elderly, there were no adherence interventions proven to be effective or widely in use. The need for adherence improvement in our patients and the lack of adherence strategies for older adults fueled the questions regarding possible interventions to enhance adherence and health care outcomes, which became the FAME research aims.
Begin with a thorough understanding of medication adherence
Before constructing an adherence intervention trial, investigators must first understand the definition of medication adherence and methods used for assessment. Medication adherence is a term describing a patient’s medication-taking behavior, generally defined as the extent to which a patient adheres to an agreed regimen derived by a collaboration between the patient and their health care provider.Citation4–Citation6 The word “adherence” is often preferred over “compliance” because medication compliance implies the patient passively complying to the provider’s medication orders with no attempts made at collaboration.Citation4,Citation6,Citation7
There are numerous methods used to assess medication adherence in practice, including the subjective, direct, and indirect measures.Citation4,Citation8 An array of methods such as patient self-report, pharmacy refill history, pill counts, serum drug level, etc. have been used. A single or a combination of these measures can be used in a trial. Employing a consistent method throughout a trial period is of importance. During FAME, adherence for all chronic medications was assessed using pill counts calculated as the number of pills taken (the number of pills dispensed relative to the number of pills remaining at follow-up).Citation1 Even though pill counts can become labor-intensive with a polypharmacy regimen, using the blister-packs as an adherence aid eased this process in FAME.
Ensure that trial highlights individualized intervention
When designing an adherence study, seek to implement a creative and individualized intervention strategy to meet the specific needs of the target population. In general, a comprehensive intervention using cognitive and behavioral characteristics is most effective.Citation9 Patient education, regarded as an essential initial step to ensuring adherence, has only a marginal and transient effect on medication adherence when used alone.Citation10–Citation12 Similarly, a meta-analysis of medication packaging alone suggested a slight increase in adherence rate in only half of the studies (all short-term) included.Citation13 In contrast to above studies, two studies that implemented complex intervention programs reported positive results for both the adherence and the clinical outcomes in hypertensive patients.Citation14,Citation15 Individualization of the adherence intervention to the intended trial population is essential. For instance, to serve the community dwelling elderly patients using polypharmacy, we employed a comprehensive intervention strategy. However, younger patients who are at lower risk for medication nonadherence, may not require such intensive program.
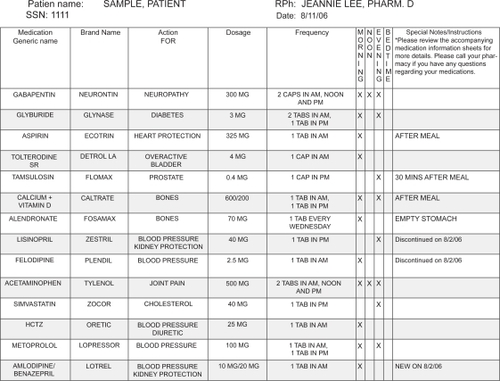
The FAME comprehensive pharmacy care program combined an individualized medication education with a provision of custom blister-packed medications and a serial follow-up with clinical pharmacists every two months.Citation1 The individualized education was performed using a standardized checklist of education component and tools used, in order to minimize the inter-rater variability among the pharmacists. The study pharmacists also tailored the patients’ medication regimen, taking into account their preferences and daily routine, any drug–drug or drug–food interactions, and adverse drug reactions. A personalized medication chart, updated at each study session, was provided to reinforce the teaching and to be used as a reference during all health care visits (). Accordingly, all chronic medications were custom packaged in morning, noon, evening, and/or bedtime blister-packs for convenience, which allowed patients to easily access their medications and visually track adherence.Citation1 The follow-up appointments were scheduled every two months, during which education and blister-packs were provided.
Tailor inclusion criteria and study duration to target population
Efforts were made to preserve generalizability of the study results, and to meet the power needed for valid outcomes when recruiting patients for FAME. A broad set of inclusion criteria, consisting of men and women aged ≥65 years who used polypharmacy (four or more chronic medications), was chosen. A limited exclusion criteria of a presence of any condition that would make one-year survival unlikely was placed.Citation1
Study duration is a balance among sample size, time required to demonstrate an effect of interventions, and risk of subject loss due to prolonged time to endpoint assessment. The randomized phase of the FAME trial provided insight into the required duration of an adherence intervention. Despite receiving six months of comprehensive interventions, the group randomized to resume usual care for six months reverted to near the original level of medication adherence. In contrast, the group randomized to receive continued pharmacy care sustained a high adherence rate, and was found to have further improvements in blood pressure.Citation1 Thus, for a high-risk elderly population, as in FAME, adherence interventions need to continue long-term and perhaps indefinitely. On the other hand, if an education session or an adherence aid alone motivates a low-risk patient group to optimal adherence, then an initial intervention and a follow-up appointment to assess the impact may suffice.
Employ a range of outcomes linked to meaningful clinical effects
Prior to implementing an adherence trial, the focused population’s disease states should be evaluated and measurable outcomes selected. Connecting adherence changes to improved health outcomes is crucial for acceptance of the study results and translation of the results to practice. In FAME, a hypothesis was generated that older patients achieving higher rates of adherence would have better control of their hypertension and hyperlipidemia, the two most prevalent disease states in our patients. Hypertension and hyperlipidemia are also the conditions in which the drug effects are objectively measured and linked to patient outcomes.Citation16,Citation17 The FAME results showed that marked improvements in medication adherence not only equates to a 16-fold increase in proportion of the “adherent” patients (those who took at least 80% of all of their medications), but that these changes were associated with clinically-meaningful reductions in BP and LDL-C.Citation1 Therefore, large gains in adherence are achievable with focused interventions and in turn lead to improved health outcomes.
Win the support of the multidisciplinary team and the administration
When conducting an adherence intervention trial within a health care system, it is vital to gain the support of the multidisciplinary team and the administration. A targeted education to providers who are in position to recognize nonadherence can accelerate study enrollment. Prior to and during the FAME recruitment, several in-services were given to inform the various providers about the patient inclusion criteria and the referral process. The majority of the FAME subjects were referred by their primary care providers, but professionals including social workers, nurses, psychologists, and nutritionists who attended the FAME in-service also referred patients to the program.
Winning the support of the administration is critical and influences the successful conduction of an adherence intervention trial. For the highly labor-intensive blister-packing process and appointment booking for the study, the department of pharmacy provided us the space, durable equipments, and technician support as required. The facility’s laboratory performed the blood draws, analyzed the results, and reported LDL-C for the study subjects with an approval of the impact statement from the Department of Pathology and Laboratory Services. Do ensure that your objectives meet the overall mission of the institution in order to secure the support needed for a successful trial.
Promote team work
Although study pharmacists served an essential role in the FAME trial, the need for a teamwork approach to conquering nonadherence was strongly underscored. A couple of key partnerships were critical in carrying out the study. The coordination of the FAME project prompted a paradigm shift in the outpatient pharmacy from a dispensing operation to a site of clinical pharmacy care. The blister-packing was conducted at the outpatient pharmacy, where the blister-packs were filled by the pharmacy technicians using a commercially available manual fill system and checked by the clinical pharmacists. Thus, the ongoing collaboration sustained this operation. Once the FAME program was initiated, the multidisciplinary clinicians gradually adopted and supported the program. Clear and open communications about the patients’ medication regimen and reconciliation facilitated this partnership.
Consider the potential limitations
There are several practical limitations to a wide-scale implementation of an adherence intervention trial that must be recognized and overcome, if possible, to ensure success.
In FAME, the study pharmacists were tasked with highly time-intensive duties including patient recruitment and consent, patient and provider education, medication regimen tailoring, data collection (pill counts, BP measuring, LDL-C ordering), and blister-packing oversight. The blister-packing process was particularly time-consuming due to the manual process. Future programs should consider using a technological automation, now available, to ease this task.
The FAME patients received all medications free of charge as part of their military health care benefit.Citation1 Ways to alleviate financial burden should be explored for patients with minimal to no health care coverage. Medication provision through Medicare and patient assistance programs, and generic prescribing should be sought for such population.
Because of the nature of the interventions, blinding of study subjects or research personnel for adherence trials is often difficult. However, concealed allocation during the randomization can be done via a central control of the randomization sequence. Also, subjects can be assigned using the block randomization method to ensure an even allocation of the pivotal patient characteristics, such as the level of baseline adherence, which was done in FAME.Citation1,Citation18
Seize the grant opportunities
The spark for FAME ideas was a competitive junior investigator grant awarded by the American Society of Health-System Pharmacists (ASHP) Research and Education Foundation. This grant program provided an opportunity for a junior pharmacy investigator to work with a highly experienced senior researcher. The steps involved in grant writing, protocol approval, use of funds, trial implementation, publishing the findings, and most importantly caring for the study patients were learned through the process. Submission of a quarterly research report was required by the granting body, which gave us a frequent chance to evaluate the study progression. The accountability supplied by a grant source can strengthen a study through the review process and the progress report requirement, and prevent projects from becoming unfinished.
There are several annual grant opportunities that can help stimulate adherence intervention trials. Following are examples of such opportunities geared towards new investigators and the related links:
ASHP Foundation: Junior Investigator Research Grant; Federal Services Junior Investigator Research Grant; Pharmacy Resident Practice-Based Research Grant. See http://www.ashpfoundation.org/MainMenuCategories/ResearchResourceCenter/FosteringYoungInvestigators/NewInvestigatorGrantPrograms.aspx.
American Pharmacists Association Foundation: Incentive Grants. See http://www.aphafoundation.org/programs/Incentive_Grants/.
American College of Clinical Pharmacy Research Institute: Frontiers Career Development Research Award; Investigator Development Research Awards. See http://www.accp.com/frontiers/research.php.
Share the findings
Compared to the vast and expanding literature on the effectiveness of novel drugs, only a few prospective trials enhancing adherence to them has published to date. There is a lack of evidence from randomized trials.Citation9 Following the initial presentation at the 2006 American Heart Association Scientific Sessions and the publication in the Journal of American Medical Association (JAMA),Citation1 the impact of the FAME findings was immediate. The work received broad attention in the national media including USA Today, Wall Street Journal and Reuters, in addition to over 400 print and online articles. A media presentation (filmed by the JAMAVISION, the video version of JAMA) sent to news outlets around the country was believed to have reached approximately 4.5 million viewers and countless Internet views. FAME was one of the Medscape’s Top Ten Pharmacy News that year, and we received inquiries about implementation of similar programs from practitioners in health care systems around the world. The lesson here is that the success stories of adherence intervention trials impact, not only the targeted patients in the program, but also the multidisciplinary professionals and their patients globally, if they are shared.
Conclusion
Medication nonadherence, a prevalent and penetrating problem in today’s growing older population, influences health care providers, health care systems, third party payers, governmental agencies, and policy makers who converge on the issue of medication provision.Citation19 Using the ten lessons described, pharmacists and other health care providers should develop and implement intervention trials to promote medication adherence. These trials must advance current knowledge regarding adherence through practical innovations, while maintaining the ultimate focus on the optimal patient care. When JAMAVISION came to film the FAME pharmacy care program, a 92-year-old patient who took part in FAME was interviewed. She exclaimed without any hesitation, “I loved the FAME program … it made my life easy!”
Disclosure
The opinions or assertions herein are the private views of the authors and are not to be construed as reflecting the views of the University of Arizona, the Department of the Army, or the Department of Defense. The authors report no conflicts of interest in this work.
References
- LeeJKGraceKATaylorAJEffect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trialJAMA20062962563257117101639
- WolffJLStarfieldBAndersonGPrevalence, expenditures, and complications of multiple chronic conditions in the elderlyArch Intern Med20021622269227612418941
- FlahertyJHPerryHMIIILynchardGSMorleyJEPolypharmacy and hospitalization among older home care patientsJ Gerontol A Biol Sci Med Sci200055M554M55911034227
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med200535348749716079372
- SabateEAdherence to long-term therapies: evidence for actionGenevaWorld Health Organization20031211
- LutfeyKEWishnerWJBeyond “compliance” is “adherence”Diabetes Care19992263563910189544
- BarberNShould we consider noncompliance a medical errorQual Saf Health Care200211818412078377
- Chisholm-BurnsMASpiveyCAPharmacoadherence: A new term for a significant problemAm J Health-Syst Pharm20086566166718359976
- RoterDLHallJAMeriscaRNordstromBCretinDSvarstadBEffectiveness of interventions to improve patient compliance: a meta-analysisMed Care199836113811619708588
- PetersonAMTakiyaLFinleyRMeta-analysis of trials of interventions to improve medication adherenceAm J Health Syst Pharm20036065766512701547
- TakiyaLNPetersonAMFinleyRSMeta-analysis of interventions for medication adherence to antihypertensivesAnn Pharmacother2004381617162415304624
- HaynesRBYaoXDeganiAKripalaniSGargAMcDonaldHPInterventions to enhance medication adherenceCochrane Database Syst Rev20054CD00001116235271
- ConnorJRafterNRodgersADo fixed-dose combination pills or unit-of-use packaging improve adherence? A systematic reviewBull World Health Organ20048293593915654408
- HaynesRBSackettDLGibsonESImprovement of medication compliance in uncontrolled hypertensionLancet197611265126873694
- LoganAGMilneBJAchberCCampbellWPHaynesRBWork-site treatment of hypertension by specially trained nurses. A controlled trialLancet197921175117891901
- McDonaldHPGargAXHaynesRBInterventions to enhance patient adherence to medication prescriptions: scientific reviewJAMA20022882868287912472329
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation20021063143342112485966
- BotelhoRJDudrakRHome assessment of adherence to long-term medication in the elderlyJ Fam Pract19923561651613477
- MojtabaiROlfsonMMedication costs, adherence, and health outcomes among Medicare beneficiariesHealth Aff (Millwood)20032222022912889771