Abstract
Respiratory syncytial virus (RSV) is a significant cause of morbidity in high-risk infants. Palivizumab is proven to prevent serious RSV disease, but compliance with prophylaxis (monthly doses during the RSV season) is essential to ensure protection. We invited 453 pediatricians to participate in a survey to identify their perspectives of barriers to compliance and interventions to improve compliance with palivizumab prophylaxis schedules. One hundred physicians from five continents completed the survey, identifying caregiver inconvenience, distance to clinic, cost of prophylaxis, and lack of understanding of the severity of RSV as the most common reasons for noncompliance. They recommended provision of educational materials about RSV, reminders from hospital or clinic, and administration of prophylaxis at home to increase compliance. Globally, physicians recognize several obstacles to prophylaxis compliance. This survey suggests that focused proactive interventions such as empowering caregivers with educational materials and reducing caregiver inconvenience may be instrumental to increase compliance.
Adherence to health care interventions is an important part of enhancing health. Nonadherence to intervention, be it pharmaceutical or surgical treatment, physical therapy, dietary changes, lifestyle changes, screening procedures, vaccinations, or any other regimen, has significant consequences for patients in terms of outcome and quality of life, as well as a significant cost and resource burden on the health care system.
The World Health Organization (WHO), in its report on adherence to medications, defined adherence to long-term therapy as: “The extent to which a person’s behaviour ... corresponds with agreed recommendations from a health care provider.”Citation1 The definition recognizes the partnership between patients and providers in making health care choices, in which the patient is an active participant in decisions and not a passive recipient of instructions from the physician or other health care provider. While this definition was adopted in the context of treatment for chronic conditions, it is also applicable to surveillance activities, such as routine screening procedures, or preventive measures, such as vaccination schedules.
The WHO report and much of the literature describe adherence related to chronic disease, but nonadherence to disease prophylaxis regimens is also of concern. The economic burden caused by nonadherence to prophylaxis against many preventable infectious diseases can be estimated, but it is less straightforward to estimate costs associated with partial adherence, for example to some but not all vaccines or to some but not all doses of an individual vaccine.
Respiratory syncytial virus (RSV) infection is the leading cause of lower respiratory tract infection (LRTI) in infants.Citation2 RSV is a seasonal virus in most regions of the world. Epidemics lasting 4–6 months occur during the winter season in temperate climates, with peak infection periods in December and January in the northern United States, Canada, and much of northern Europe.Citation3 By the age of two years, nearly all children have been infected.Citation2 Generally, RSV infection results in an upper respiratory tract infection; however, 25% to 40% of infected children develop a mild-to-moderate LRTI, and about 1% of previously healthy infected children require hospitalization. Risk factors contributing to serious RSV disease and hospitalization include chronic lung disease, congenital heart disease, and premature birth (≤35 weeks gestational age).Citation3,Citation4 GreenoughCitation5 and SampalisCitation6 demonstrated that RSV hospitalization is associated with greater utilization of health care resources in infants ≤35 weeks of gestation. RSV-LRTI and associated hospitalizations pose a significant burden of illness to patients and their families and have an economic impact on the health care system.Citation7 Prevention of RSV-LRTI may thus reduce this burden to families and society in general.
Because no licensed vaccines are currently available to prevent RSV infection, passive immunoprophylaxis with anti-RSV IgG antibody is the only option for preventing RSV disease in high-risk children. Two passive immunoprophylaxis agents are approved for the prevention of RSV infection. Palivizumab (marketed in the US by MedImmune, LLC, Gaithersburg, MD; marketed outside the US by Abbott, Abbott Park, IL) is a humanized immunoglobulin G (IgG) monoclonal antibody approved for use in infants at high risk for severe RSV disease, and is administered monthly by intramuscular injection throughout the RSV season. Intravenous RSV immunoglobulin (RSV-Ig) is prepared from pooled human blood, and is administered monthly by intravenous (IV) infusion during the RSV season. Guideline recommendations for RSV prophylaxis in high-risk infants have been published by the American Academy of Pediatrics,Citation8 the Canadian Paediatric Society,Citation9 and other organizations dedicated to children’s health and disease prevention. Though both palivizumab and RSV-Ig are included in guideline recommendations,Citation8,Citation9 palivizumab is the preferred agent for RSV prophylaxis; not only is it effective, it is also convenient to administer, leading to significantly reduced time costs for patients’ families.Citation10 Intramuscular injection is completed in a matter of minutes, compared with several hours required to complete IV infusion. In addition, IM injection is a simple procedure that can be performed by medical office staff, whereas IV infusion requires specialized training and equipment.
Palivizumab is not a vaccine. It provides passive immunization rather than eliciting an active immune response against RSV,Citation4 and must be administered monthly during the RSV season in order to be effective. Palivizumab prophylaxis depends upon full compliance with the monthly dosing schedule. However, full compliance rates vary in different regions studied. In developed countries, rates between 36% and 98% have been reported,Citation11–Citation13 with differences depending not only on the region studied but also on where the doses are delivered (eg, central hospital, pediatrician’s office, or at a patient’s home).
As suggested by the widely varying rates reported, there are many barriers to full compliance with palivizumab prophylaxis. For example, LangkampCitation14 surveyed parents at a single center in the United States to identify barriers to full compliance and discovered that a key factor in compliance was parental belief in the benefit of palivizumab. Difficulty in transportation to the hospital to receive injections also was identified as a compliance barrier in that survey. Though not a significant factor in the Langkamp report, many parents were concerned about out-of-pocket costs of the drug and other indirect costs (eg, time spent negotiating with insurers). Bracht and colleaguesCitation11 established an RSV prevention program at three tertiary centers and the surrounding areas in Canada. Education of parents about risks of RSV disease and benefits of prophylaxis with palivizumab were key components of the program, which achieved 98% compliance. Cost of the treatment was not a factor for this Canadian population, but the researchers identified language barriers and educational limitations in some families as obstacles to the full understanding of the instructional materials provided. Similarly, PignottiCitation15 identified language barriers which caused difficulty in communicating the severity of RSV and the proposed prophylactic schedule as being a key component of nonadherence to the full palivizumab dosing schedule in Italy. Singleton and colleaguesCitation16 reported that transportation delays from adverse weather conditions contribute to low compliance with recommended palivizumab administration in an Alaskan Native population. Compliance increased when palivizumab was delivered by a local trained health aide.Citation16 Thus, previous work has revealed that a lack of parental understanding of RSV disease and prevention, communication obstacles, and transportation difficulties are important barriers to achieving full compliance with palivizumab prophylaxis.
We surveyed physicians to discover if their perceptions of barriers to full compliance with palivizumab prophylaxis matched those reported by patients’ families and in the literature. Pediatricians from countries in which palivizumab is approved for use were identified by Abbott medical directors and staff and invited by electronic mail to participate anonymously in an internet-based survey (http://www.markettools.com/). Physicians attending an invitational global medical conference were invited to participate in the survey at on-site computer terminals. There was no duplication of invitations; physicians who attended the conference were not contacted by electronic mail to complete the survey. In all, 453 physicians were invited to respond to the survey questions. Some invited physicians prescribed palivizumab and some did not. The survey was provided in English and contained 29 questions to assess physician practice habits, perceptions of obstacles to compliance, and measures to enhance compliance with palivizumab prophylaxis (). One hundred physicians (response rate = 22%) completed the survey from Europe (n = 60), Asia (n = 15), North America (n = 13; Canada and Mexico only), South America (n =7), and Africa (n = 5). Information about the responding physicians was based on their answers to survey questions. No information about nonresponders was collected.
Table 1 Survey questions
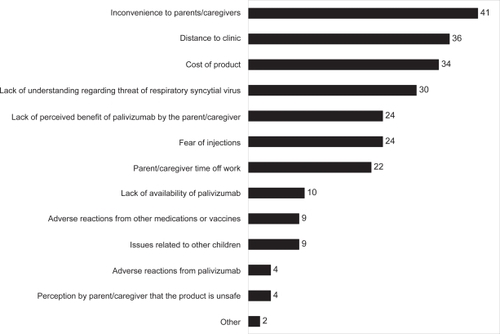
The physicians responding to our survey identified inconvenience to parents and distance to the clinic, cost of palivizumab, and a lack of understanding of the threat of RSV as the primary barriers to adherence to the full dosing schedule (). The most frequent barriers identified differed when the responses were stratified by region. For example, European physicians (n = 60) believed that inconvenience and distance to the clinic were the most important barriers to full compliance, whereas cost of prophylaxis was less important. By contrast, non-European physicians (n = 40) ranked cost as the primary barrier to full compliance with palivizumab prophylaxis, though inconvenience to caregivers was also thought to be important. Our survey asked physicians to speculate about the reasons their patients’ parents might be noncompliant with palivizumab dosing schedules, in contrast to other surveys in which parents were asked directly. It is interesting that the physicians in our survey identified barriers to adherence similar to those that have been reported from the perspectives of patients’ parents.
Figure 1 Barriers to full compliance. Physicians were asked what they believed to be the top three factors that contribute most to noncompliance with recommended palivizumab dosing schedules.
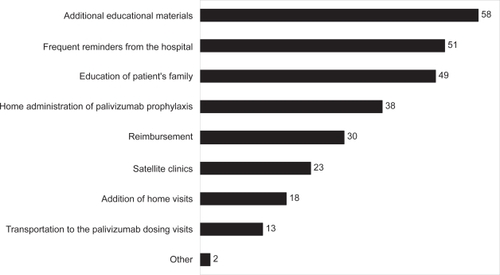
In our survey, physicians recommended additional educational materials and education of patients’ families about the threat from severe RSV disease, frequent reminders from the hospital, and administration of palivizumab in patients’ homes as key drivers of full compliance (). The same drivers were identified when the responses were stratified by region (Europe versus non-Europe). Thus, our survey results emphasize the universal importance of educating families about RSV disease and palivizumab prophylaxis. In addition, the results support home administration of palivizumab as a driver for full compliance with the monthly dosing schedule, in agreement with previous work that has demonstrated higher compliance rates with home or local administration.Citation12,Citation16
Figure 2 Recommended interventions. Physicians were asked to choose the top three interventions they would recommend to increase full compliance with palivizumab prophylaxis.
Of the physicians responding to our survey, 68% believe that their patients’ parents think that palivizumab is a vaccine. In fact, 38% of the responding physicians believe that palivizumab is a vaccine. Thus, our survey identified a need for further education not only of patients’ families, but also in some cases of physicians themselves. Unlike most vaccines, palivizumab has a strict dosing schedule that must be followed to achieve efficacy throughout the RSV season. Equating palivizumab to a vaccine may cause caregivers or even physicians to falsely believe that a single injection will protect at-risk children. In our survey, 86% of the respondents provide information to their patients’ caregivers about RSV and prophylaxis. Physicians need to be fully educated about the action of palivizumab and the necessity of multiple doses on a strict schedule in order to effectively communicate the importance of adherence to their patients’ families.
Though the survey invited a large number of physicians to participate, the results are limited by bias in the selection process and the low rate of response. Invitations to participate were not random, because contact information for a random sample of pediatricians in each country was unavailable. In addition, responders were more likely to be fluent in English than nonresponders, since the survey was provided in English. On the other hand, physicians practicing in developed as well as developing countries participated in the survey, leading to a broad spectrum of responses. Furthermore, the anonymity of the survey may have encouraged responders to answer the questions candidly, contributing some strength to the results. Indeed, the barriers to compliance and the recommendations to increase compliance identified in this survey are similar to those previously reported in the literature, suggesting that the results may be generally applicable in a larger and more randomly selected population of pediatricians.
Infants are a special subpopulation in whom compliance to any medical regimen is dependent on parental decisions. Serious RSV disease resulting in hospitalization places a burden on families and on the health care system. Palivizumab prophylaxis has been shown to reduce the incidence of RSV-LRTI and hospitalization in at-risk infants,Citation4,Citation17 but effective prophylaxis requires full compliance with the monthly dosing schedule. While several approaches to increasing full compliance with RSV prophylaxis may be beneficial, including cost reductions (in some areas of the world), frequent reminders, home or local administration, and assistance with transportation to clinics, it is critically important that parents of children at high risk for severe RSV disease be empowered with clear information about the threat from RSV and the benefits of palivizumab prophylaxis to enable them to make informed choices. Physicians and other health professionals are primary sources of information for patients or their caregivers, and thus play a key role in guiding families in their choices about RSV prophylaxis. The physicians we surveyed recognized both the importance of educating families about RSV and prophylaxis, and their own roles in providing this information.
Acknowledgements
The authors would like to thank Laurinda Cooker, PhD, of Abbott, for her assistance in the writing and preparation of this manuscript. The authors report no conflicts of interest in this work.
References
- World Health OrganizationAdherence to long-term therapies Evidence for actionGeneva, SwitzerlandWorld Health Organization2003
- GlezenWPTaberLHFrankALKaselJARisk of primary infection and reinfection with respiratory syncytial virusAm J Dis Child198614065435463706232
- LawBJCarbonell-EstranyXSimoesEAAn update on respiratory syncytial virus epidemiology: a developed country perspectiveRespir Med200296Suppl BS1S711996399
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study GroupPediatrics1998102(3 Pt 1):531537
- GreenoughACoxSAlexanderJHealth care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infectionArch Dis Child200185646346811719328
- SampalisJSMorbidity and mortality after RSV-associated hospitalizations among premature Canadian infantsJ Pediatr2003143Suppl 5S150S15614615714
- PelletierAJMansbachJMCamargoCAJrDirect medical costs of bronchiolitis hospitalizations in the United StatesPediatrics200611862418242317142527
- American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infectionsPediatrics20031126 Pt 11442144614654627
- Canadian Paediatric SocietyPalivizumab and respiratory syncytial virus globulin intravenous for the prophylaxis of respiratory syncytial virus infection in high risk infants. Infectious Diseases and Immunization Committee and Fetus and Newborn CommitteePaediatr Child Health199944744480
- RobbinsJMTilfordJMGillaspySRParental emotional and time costs predict compliance with respiratory syncytial virus prophylaxisAmbul Pediatr20022644444812437390
- BrachtMHefferMO’BrienKDevelopment, implementation, and evaluation of a community- and hospital-based respiratory syncytial virus prophylaxis programAdv Neonatal Care200551394915685161
- GolombekSGBerningFLagammaEFCompliance with prophylaxis for respiratory syncytial virus infection in a home settingPediatr Infect Dis J200423431832215071285
- PaulDALeefKHChidekelAHome delivery of palivizumab: outcomes and compliance in regional preterm infantsDel Med J2002741111511838265
- LangkampDLHlavinSMFactors predicting compliance with palivizumab in high-risk infantsAm J Perinatol200118634535211607852
- PignottiMSCatarziSDonzelliGA 4-year survey on palivizumab respiratory syncytial virus (RSV)-prophylaxis: how can compliance be improved?J Matern Fetal Neonatal Med200619422122416854695
- SingletonRJBrudenDBrooksLCloser to home: local care improves compliance with RSV prophylaxis in high-risk infantsInt J Circumpolar Health20066514716544642
- OhPILanctôtKLYoonAPalivizumab prophylaxis for respiratory syncytial virus in Canada: utilization and outcomesPediatr Infect Dis J200221651251812182374