Abstract
Background:
Type 2 diabetes mellitus (T2DM) is a complex disorder in which interactions between environmental and genetic factors result in the development of insulin resistance (in most cases) and progressive pancreatic β-cell failure. The currently available oral anti-diabetes treatments are effective as monotherapy; however, due to the progressive decline in β-cell function, most patients will require the use of combination therapy and eventually insulin to reach glycemic targets. These therapeutic options are not without undesirable side effects such as weight gain and hypoglycemia. Furthermore, T2DM is associated with impaired quality of life (QOL) and poor compliance with treatment. Hence, there is a need for anti-diabetes agents that result in sustained improvements in glycemic control without hypoglycemia or weight gain and have a positive impact on patients QOL and thereby hopefully improve compliance. Incretin-based therapy is the latest addition to anti-diabetes treatments which addresses some of the shortcomings of older treatments.
Aims:
To review the evidence for the use of exenatide once-weekly.
Methods:
We have searched Medline using the terms “exenatide”, “exenatide once-weekly”, and “exenatide LA”.
Results:
Exenatide once-weekly is an incretin mimetic that is currently undergoing phase 3 clinical trials, and has been shown to improve glycemic parameters (HbA1c and fasting and postprandial glucose levels), with low risk of hypoglycemia, causes weight loss, and use was associated with improvements in patient satisfaction which might have a positive impact on treatment compliance.
Conclusions:
Exenatide once-weekly is effective, well tolerated in patients with T2DM and should be a useful addition to the available range of anti-diabetes treatments.
Introduction
Type 2 diabetes mellitus (T2DM) is a global epidemic with an estimated worldwide prevalence of 6% (246 million people) in 2007 that is forecasted to rise to 7.3% (380 million) by 2025.Citation1,Citation2 The health, social, and economic burden of T2DM is great;Citation3–Citation5 it continues to pose a major challenge to healthcare provision around the world.
The development of insulin resistance (IR) and pancreatic β-cell dysfunction due to various environmental and genetic factors results in onset of T2DM.Citation6,Citation7 Despite obesity being the single most important contributor to IR, most obese insulin-resistant individuals do not develop T2DMCitation8,Citation9 because their β-cells are capable of producing sufficient insulin to maintain euglycemia.Citation9–Citation13 This suggests that the failure of β-cells to secrete sufficient insulin to overcome IR is the key step in the development and progression of T2DM.Citation6,Citation7,Citation9,Citation10 Pancreatic α-cell dysfunction manifesting as non-suppressed glucagon secretion in the presence of hyperglycemia is also manifest in patients with T2DM.Citation14
Several pharmacological agents have been developed to treat patients with T2DM. They either improve insulin resistance (biguanides, glitazones), stimulate insulin secretion from the β-cell (sulfonylureas, metaglinides), or decrease glucose absorption from the gut (α-glucosidase inhibitors).Citation15 The initial improvements in glycemic control observed with these agents as monotherapy are not sustained because of the progressive nature of the disease due to the continuing decline in β-cell function.Citation9,Citation16 This often necessitates the use of combination therapy and eventually insulin. Furthermore, current agents may be associated with undesirable side effects including gastrointestinal (metformin, α-glucosidase inhibitors), weight gain (sulfonylureas, metaglinides, glitazones, and insulin), and hypoglycemia (sulfonylureas, metaglinides, and insulin).Citation17 These side effects may contribute to further worsen the already impaired health-related quality of life (QOL) found in patients with T2DMCitation18 and may contribute to poor compliance common in this group of patients.Citation19
Treatment acceptability and adherence are particularly important in the management of T2DM. A systematic review showed that many patients took less than their prescribed dose of insulin and/or oral anti-diabetes medicationsCitation20 and that a substantial proportion of patients had difficulty in dealing with various elements of the chronic disease management, particularly adhering to a strict drug regimen.Citation21,Citation22
Taking the above into account, there is a need for new pharmacological agents that are well tolerated with sustainable impact on glycemic control, and with very low risk of hypoglycemia, cause weight loss (or at least no weight gain) and thereby encourage patient adherence to therapy. Incretin-based therapy is the latest class of anti-diabetes medications to become available and addresses some of the shortcomings of conventional anti-diabetes treatments. Incretin-based therapy can be given either orally (dipeptidyl peptidase-4 (DPP-4) inhibitors) or via a subcutaneous injection (glucagon-like peptide (GLP-1) analogues/mimetics). They improve glycemic control with favorable impact on weight and low risk of hypoglycemia (apart from when used with sulfonylureas).Citation23 In addition, animal studies have shown that some of these agents improve β-cell survival,Citation23 which if true in humans might result in a more sustained impact on glycemic control. GLP-1 analogs/mimetics are given in once- or twice-daily dosing regimes. However, other drugs are in development in this category that require administration once weekly or even less frequently.Citation23 Such a dosing regimen might be highly acceptable to patients and encourage compliance with treatment.
In this article, we aim to review the available data regarding the once-weekly use of exenatide in the management of T2DM and the potential patient considerations for the use of this drug. Further details regarding incretin-based therapies are not within the scope of this article and can be found elsewhere.Citation24–Citation27
Incretins
Incretins are hormones that are released from the gut in response to ingestion of food.Citation28 The incretin effect was first described in 1964, when it was observed that the insulin response to oral glucose challenge was substantially higher than to an intravenous glucose load.Citation29 The incretin response accounts for at least 50% of insulin secretion in healthy individuals.Citation30
Glucose-dependent insulinotropic polypeptide (GIP) was the first incretin to be isolated and characterized.Citation31,Citation32 It is a 42 amino acid peptide secreted in the bioactive form from the K-cells in duodenum and jejunum in response to ingestion of carbohydrates and lipids.Citation33 The second incretin to be isolated was GLP-1, which is cleaved from pro-glucagon and secreted from the L-cells in the distal ileum and colon.Citation33 GLP-1 levels are reduced in patients with T2DM, unlike GIP levels which are maintained.Citation34
Both GIP and GLP-1 facilitate glucose-dependent insulin secretion through their action on pancreatic β-cells. GLP-1 increases insulin gene transcription as well as all the steps of insulin biosynthesis.Citation35 In addition, GLP-1 results in glucose-dependent glucagon suppression, delays gastric emptying, increases satiety, and possibly reduces insulin resistance.Citation36,Citation37 There is also evidence that GLP-1 increases β-cell mass in animal studies.Citation38
GLP-1 secretion is reduced in patients with T2DM.Citation39 Although there is a blunting of GLP-1 secretory response in these patients, their response to exogenous GLP-1 is intact.Citation23 A continuous 6-hour intravenous infusion of GLP-1 in the fasting state, leading to GLP-1 levels 2–3 times higher than normally seen after meals, resulted in lowering of glucose and glucagon levels, with increases in insulin secretion without any hypoglycemic events in patients with poorly controlled T2DM.Citation40 Subcutaneous GLP-1 was also shown to have a similar glucose-lowering effect when administered pre-meal in patients with T2DM.Citation41
Incretins are rapidly metabolized by the enzyme DPP-4, and thus have extremely short half-lives (GIP < 2 minutes and GLP-1 5–7 minutes).Citation42,Citation43 The short half-life of these naturally occurring incretins limited their clinical use. This led to the development of various modifications of the amino acids of GLP-1, rendering them DPP-4 resistant. Exenatide (Byetta®; Eli Lilly), a synthetic analog of exendin-4, was the first-in-class incretin mimetic. Liraglutide (Victoza®; Novo Nordisk), an analog of human GLP-1, is a fatty acid derivative of GLP-1 that has been approved for clinical use more recently. There are several long-acting once-weekly preparations currently in phase 3 clinical trials – exenatide once-weekly (Byetta®; Eli Lilly), albiglutide (GlaxoSmithKline) and taspoglutide (Ipsen and Roche), all of which show promising results. Research has also targeted developing inhibitors of the DPP-4 enzyme. The currently available DPP-4 inhibitors are sitagliptin (Januvia®; Merck & Co), vildagliptin (Galvus®; Novartis) and saxagliptin (Onglyza®; Bristol-Myers Squibb and Astra-Zeneca). Alogliptin (Takeda) and linagliptin (Ondero®; Boehringer Ingelheim) are currently undergoing phase 3 clinical trials.
Exenatide, a synthetic version of the naturally occurring salivary peptide isolated from the Gila monster (Heloderma suspectum), is a partial structural analog of human GLP-1 and has 53% amino acid sequence homology with human GLP-1.Citation44 It contains a glycine at position 2, in contrast to human GLP-1, which has an alanine at position 2, thus making the molecule DPP-4 resistant, in turn conferring a longer half-life.Citation44 Exenatide has a half-life of 3.3–4.0 hours and clinical effects lasting for up to 8 hours.Citation45–Citation47 Exenatide treatment results in significant reductions in fasting plasma glucose (FPG) and post-prandial glucose (PPG) in patients with T2DM.Citation48–Citation51 In addition, it results in slowing of gastric emptying (which contributes to the reductions in PPG),Citation52 appetite suppressionCitation53 and weight loss.Citation54
The AC2993 Diabetes Management for Improving Glucose Outcomes (AMIGO) trials were three 30-week randomized, triple-blind, placebo-controlled, multicenter trials that had similar design and examined the impact of exenatide treatment on glycemic control in patients with T2DM. Citation48,Citation55,Citation56 They enrolled subjects aged 16–75 years who were poorly controlled on metformin and/or sulfonylurea with HbA1c 7.5%–11%. In the AMIGO trials, patients were randomized to placebo, exenatide 5 μg or exenatide 10 μg while continuing metformin and/or sulfonylurea. By week 30, exenatide 10 μg resulted in mean HbA1c reduction of −0.8% ± 0.1% to −0.9 ± 0.1% compared with a −0.16% ± 0.1% to 0.08% ± 0.1% in placebo.Citation55 The effects of exenatide on glycemic control appeared to be sustainable as reductions achieved at 30 weeks (−1.0% ± 0.1%) were maintained at 82 weeksCitation57 and 3 yearsCitation58 in the open-label extensions of the AMIGO trials.
The open-label extensions of the AMIGO trial also showed that exenatide treatment promotes progressive weight loss up to 82 weeks (−2.1 ± 0.3 kg versus −4.0 ± 0.3 kg for exenatide 10 μg week 30 versus week 82 respectively).Citation57,Citation59 Furthermore, a subset of patients who had 3.5 years of exenatide exposure had reductions in triglycerides of 12% (P = 0.0003); LDL-C decreased by 6% (P < 0.0001), and HDL-C increased by 24% (P < 0.0001).Citation59
Exenatide is generally well tolerated long term, but the most commonly reported adverse events (AEs) (mostly in the first few weeks of treatment) are nausea, vomiting, diarrhea, headache, dizziness, and dyspepsia.Citation60 In a recent meta-analysis, exenatide was associated with a significant increase in the proportion of patients experiencing hypoglycemia in placebo-controlled trials (OR: 2.92 (1.49–5.75), P = 0.002). This excess, however, was only observed when exenatide was combined with sulfonylureas.Citation61 Concerns about acute pancreatitis have been raised in patients using exenatide. However, a 1-year follow-up study of patients who were initiated on exenatide, sitagliptin, glyburide, or metformin showed the risk of acute pancreatitis to be comparable between the cohorts.Citation62 Nonetheless, the FDA has changed the labeling on the drug to warn about possibility of acute pancreatitis particularly in susceptible patients, based on post-marketing analysis showing 30 reported cases of pancreatitis in 2007 and 6 cases of necrotizing hemorrhagic pancreatitis in 2008.Citation60 The FDA also warns that exenatide should not be used in patients with severe renal impairment (creatinine clearance < 30 mL/min) or end-stage renal failure, and should be used with caution in those with renal transplant or moderate renal impairment (creatinine clearance 30–50 mL/min).Citation60
Although exenatide is relatively well tolerated and effective in improving glycemic control with favorable impact on weight and low risk of hypoglycemia, the main drawback is that it needs to be administered by twice-daily injections. As a result, the development of exenatide once-weekly is now in progress.
Exenatide once-weekly
Chemistry
Exenatide once-weekly uses a sustained release drug delivery system. Molecules of exenatide are encapsulated in injectable microspheres of poly (D, L lactic-co-glycolic acid), a biodegradable polymeric matrix commonly used in extended release preparations.Citation63 This poly-lactide-glycolide and exenatide microsphere suspension allows gradual drug delivery at a controlled rate by diffusion and erosion of the microspheres.Citation63,Citation64
Pharmacokinetics
Mean plasma concentration of exenatide once-weekly (0.8 or 2 mg) reached clinically significant levels (at which exenatide lowers blood glucose) by week 2 in a 15-week phase 2 study of 45 adults (60% men, 60% Caucasians) whom glycemic control was suboptimal (HbA1c 8.5% ± 1.2%) with metformin and/or life-style changes.Citation64,Citation65 By week 6, exenatide once-weekly attained a maximum concentration higher than that attained by a single injection of exenatide 10 μg (a steady state concentration of 232 pg/mL versus 211 pg/mL).Citation59 Six weeks after stopping treatment, the serum concentration of exenatide once-weekly declined steadily to insignificant levels.Citation64
In a randomized, double-blind, parallel study in Japanese patients with T2DM (59% men, aged 58 ± 9 years), the AUC (0–8 hours) of exenatide once-weekly on day 1 was 187.6 (133.7–263.3) pg * h/mL and 405.6 (278.4–590.8) pg * h/mL for 0.8 mg and 2 mg respectively.Citation66 The Cmax on day 1 was 64.3 (38.3–107.8) pg/mL for 0.8 mg and 137.3 (74.6–252.6) pg/mL for 2 mg of exenatide once-weekly. Geometric mean (90% CI) steady-state plasma concentrations were 81.2 (68.3–96.4) pg/mL and 344.5 (256.5–462.7) pg/mL with 0.8 mg and 2.0 mg respectively ().Citation66
Figure 1 Mean (±SD) plasma exenatide trough concentration-versus-time profiles in pharmacokinetic evaluable patients receiving exenatide once weekly 0.8 mg (closed triangles) (n = 8) or exenatide once weekly 2.0 mg (closed circles) (n = 6). Reproduced with permission from Iwomoto K, et al. Endocr J. 2009;56(8):951–962.Citation66
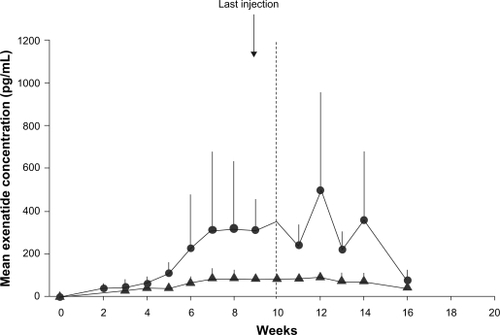
The diabetes therapy utilization researching changes in A1c, weight, and other factors through intervention with Exenatide once-weekly (DURATION)-1 study (described below) showed that plateau concentrations of exenatide were achieved after 6–10 weeks of exenatide once-weekly with a geometric mean steady state plasma concentration of 71.7 pmol/L.Citation67
Clinical efficacy
Impact on glycemic parameters
There are 3 published randomized controlled trials that assessed the impact of exenatide once-weekly on glycemic parameters (). Exenatide once-weekly produced significant reductions in HbA1c, FPG, and PPG when used in drug-naïve patients or patients treated with one or more oral anti-diabetes therapy.Citation64,Citation66,Citation67
Table 1 Designs and clinical outcomes of the published exenatide once-weekly studies
The DURATION-1 study was a randomized, open-label, non-inferiority study that compared exenatide 2.0 mg weekly to exenatide 10 μg twice daily in patients with T2DM. 303 patients were enrolled and 295 (53% men, 78% Caucasians) were randomized.Citation67 All patients underwent a 3-day lead-in period with exenatide 5 μg twice daily, after which they were randomized to either exenatide 2.0 mg once-weekly or exenatide 5 μg twice daily for 28 days, followed by exenatide 10 μg twice daily. Participants had a mean age of 55 ± 10 years with a mean BMI of 35 ± 5 kg/m2. The baseline anti-diabetes treatment included metformin (73%), sulfonylurea (37%), and thiazolidinediones (16%) alone or in combination.Citation67 By week 10, there were significantly greater reductions in HbA1c in the once-weekly group compared with the twice-daily group, which continued to be the case until study end (week 30) (). A greater proportion of patients randomized to exenatide once-weekly achieved target HbA1c ≤ 7.0% (77% versus 61%; P = 0.0039). Exenatide once-weekly also resulted in greater reductions of FPG and 2-hour PPG (measured during a mixed meal tolerance test) ().Citation67
In another randomized, placebo-controlled, phase 2 trial, 45 (60% male) drug-naïve or metformin-treated patients with T2DM were randomized to 2.0 mg or 0.8 mg exenatide once-weekly or placebo.Citation64 HbA1c reductions were apparent in both the exenatide once-weekly groups from week 3 onwards and continued to improve until study end (). 86% of the 2.0 mg group and 36% of the 0.8 mg group achieved target HbA1c 7.0% or less compared with 0% in the placebo group.Citation64 In addition, by week 15, exenatide once-weekly (2.0 mg or 0.8 mg) resulted in significant reductions in FPG and PPG (based on self monitored blood glucose profiles) compared with placebo.
Exenatide once-weekly was also examined in a Japanese population in a 10-week randomized, placebo-controlled, double-blind, parallel study in patients with T2DM suboptimally controlled by life style and/or biguanide, sulfonylurea, thiazolidinedione, or combinations of these agents.Citation66 Patients continued their baseline medications during this study. Patients were randomized in a 1:1:1 ratio to subcutaneous placebo once weekly, exenatide once weekly 0.8 mg, or exenatide once weekly 2.0 mg.Citation66 At week 10, there was significant reduction in HbA1c in the exenatide once-weekly groups compared with placebo (). Similarly, FPG and PPG concentrations showed clinically relevant reductions in the exenatide once-weekly groups compared with placebo ().
The impact of exenatide once weekly seems to be sustainable up to 2 years following initiating treatment.Citation68,Citation69 In the open-label extension of the DURATION-1 trial, 258 patients entered the 22-week open-ended assessment phase (n = 128 exenatide once weekly only; n = 130 switched from daily to once weekly exenatide).Citation68 Exenatide once weekly maintained the HbA1c improvements through the 52 weeks (−2.0% [−2.1% to −1.8%]). Patients switching from daily to weekly exenatide achieved further HbA1c improvements, but both groups had a mean HbA1c of 6.6% at study-end. At week 52, 71% and 54% of all patients achieved an A1c < 7.0% and ≤6.5%, respectively.Citation68 This glycemic improvement was achieved without any major hypoglycemia. A further open-label extension of the DURATION-1 trial involving 135 patients who have completed 2 years treatment with 2 mg exenatide once weekly showed that the initial improvements in HbA1c were maintained at 2 years with 66% and 42% of patients achieving an HbA1c ≤7.0% and ≤6.5%, respectively.Citation69
Impact on weight
Similar to exenatide twice-daily treatment, exenatide once weekly results in significant weight loss (). In the study by Kim et al exenatide once weekly 2 mg resulted in a weight loss of 3.8 ± 1.4 kg (mean ± SE) from baseline by week 15 (P < 0.05, compared with the placebo).Citation64 In the DURATION-1 trial, the weekly exenatide treatment group had a weight change of −3.7 kg (SE 0.5) at week 30, which was comparable to the twice-daily exenatide treatment (−3.6 kg [SE 0.5]).Citation67
However, in the Japanese study, exenatide once weekly resulted in a weight neutral effect, while the placebo group lost 1.6 kg ().Citation66 This effect was seen in earlier exenatide twice daily studies in Japanese subjects.Citation70 The authors hypothesize that the leanness of the Japanese cohort could contribute to this apparent neutral effect on weight.
The impact of exenatide once weekly on weight seems to be sustainable. At 52 weeks, body weight was reduced by >4 kg.Citation68 In the 2-year open-label extension of the DURATION-1 study, there was significant reduction in body weight from baseline (−3.6 ± 0.6 kg; 95% CI: −4.8 to −2.3 kg) by 2 years.Citation69
Impact on cardiovascular risk factors
Exenatide once weekly resulted in significant reductions in lipids and blood pressure ().Citation67
Table 2 Comparison of cardiovascular parameters in exenatide once weekly (30-week original study and 2-year open-label extension) and twice daily
The 2-year open-label extension of the DURATION-1 study reported that exenatide once weekly improved serum lipids (triglycerides: −18%, 95% CI −24% to −12%; total cholesterol: −0.25 ± 0.09 mmol/L, 95% CI −0.42 to −0.07 mmol/L). These subjects were also able to maintain a significant reduction in systolic blood pressure (−3.2 ± 1.2 mmHg; 95% CI −5.5 to −0.8 mmHg) throughout the treatment period.Citation69
Impact on glucagon and other incretin-related effects
Exenatide once weekly resulted in more glucagon suppression than exenatide twice daily.Citation67 In the DURATION-1 study, glucagon levels changed by −18·0 (SE: 2·9) ng/L (from a baseline of 103 (3·1) ng/L) and −6·4 (2·9) ng/L (from a baseline of 99·0 (3·0) ng/L) for exenatide once weekly and exenatide twice daily respectively (P < 0.05).Citation67 The impact of exenatide once weekly on satiety and gastric emptying has not been examined.
Adverse events
Hypoglycemia
There were no significant events of hypoglycemia reported with exenatide once weekly in the DURATION-1 study or its open-label extensions.Citation67–Citation69 In the Japanese study, patient-reported hypoglycemia was reported in 2 patients. Both of these patients were on concomitant sulfonylurea therapy.Citation66
In the earlier study by Kim et al patient-reported hypoglycemia was reported in 25% with exenatide once weekly 0.8 mg, of which only one was confirmed with a blood glucose level of 3.1 mmol/L and all were mild, and 0% in the 2.0 mg group; patients in the placebo arm had no hypoglycemia events.Citation64
Other AEs
The common AEs are summarized in . Nausea was the most commonly reported side effect. Other AEs included vomiting, diarrhea, injection-site pruritus, and bruising. Elevated blood amylase levels were reported in 2 patients in the exenatide 0.8 mg once-weekly group, but these were not associated with any clinical symptoms of pancreatitis. Baseline to week 10 elevations in amylase levels did not reach significance in any of the groups, and no cases of pancreatitis were reported. There were no clinically relevant AEs relating to vital signs, ECG, or blood results. There were no withdrawals from the study due to AEs.
Table 3 Most common AEs from the 3 published studies on exenatide once weekly
In the 2-year open-label extension of the DURATION-1 study, mild nausea diminished over time, occurring in only 8% of patients during the open-ended treatment period compared with 26.4% patients during the 30-week study period.Citation69
Overall, exenatide once weekly was well tolerated with no serious patient-reported AEs in any of the 3 published studies. Mild nausea and injection-site pruritus were commonly encountered. Incidences of hypoglycemia were mild. So far, no cases of acute pancreatitis have been reported in any of the studies.
Anti-exenatide antibodies
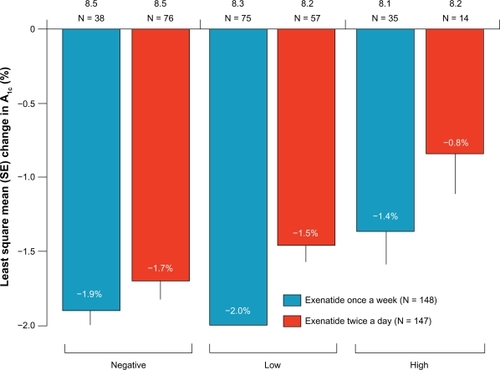
The development or presence of antibodies did not have any clinical effect on the incidence of hypoglycemia or change in HbA1c in any of the available studies.Citation64,Citation66,Citation67 Kim et al reported that 67% of subjects receiving exenatide once weekly had anti-exenatide antibodies at week 15, but no association could be found with safety or efficacy in individual patient profile.Citation64 DURATION-1 reported that anti-exenatide antibody levels were higher with exenatide once a week compared with twice-daily exenatide (P = 0·0002); however, there were significant reductions in mean HbA1c over 30 weeks in patients with negative, low titer (1/25 to 1/125), and high titer (>1/625) antibodies in the exenatide once-weekly group compared with twice daily ().Citation67 Anti-exenatide antibodies were present in 60.0% (6/10) and 77.8% (7/9) of patients in the exenatide once-weekly 0.8 mg and exenatide once-weekly 2.0 mg groups, respectively, at any point during the Japanese study. But this did not have any clinical effect on hypoglycemia or HbA1c change (data not available).Citation66
Figure 2 Intention-to-treat subanalysis (N = 295) of change in HbA1c (least square mean (SE) by antibody status). Negative antibodies were not detectable in repeated analyses throughout the 30 weeks; low titer (≤1/625) at any point during the 30 weeks; and high titer (≥1/625) at any point during the 30 weeks; HbA1c reductions of −1.4% were observed in patients treated once a week in the high titer group. Reprinted from Drucker DJ, Buse JB, Taylor K. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. The Lancet. 372:1240–1250, Copyright © 2008, with permission from Elsevier.
Ongoing clinical trials
DURATION-2, a head-to-head comparative study of exenatide once weekly against sitagliptin or pioglitazone is a 26-week, double-blinded, phase 3, superiority study involving 491 patients whose diabetes was suboptimally controlled on metformin. Preliminary results were presented at the European Association for the Study of Diabetes annual meeting in 2008 and American Diabetes Association conference in 2009, yet to be published.Citation71,Citation72 HbA1c reduction and weight loss was superior to the comparator arms; at 26 weeks the change in HbA1c was −1.55%, −0.92% and −1.23% for exenatide once weekly, sitagliptin 100 mg daily and pioglitazone 45 mg daily, respectively. Weight change was −2.7 kg, −0.9 kg, and +3.2 kg respectively.Citation71,Citation72
DURATION-3 was another phase 3, 26-week, open-label, superiority study involving 467 patients with T2DM who were suboptimally controlled on metformin and/or a sulfonylurea, comparing exenatide once weekly with insulin glargine. The results are still awaited, but a press release reports a greater reduction in HbA1c, weight loss, and fewer hypoglycemic episodes in the exenatide once-weekly group compared with insulin glargine.Citation73
DURATION-4 is currently in progress, comparing exenatide once weekly to metformin, sitagliptin, and pioglitazone; DURATION-5 is also ongoing, comparing exenatide once weekly to exenatide twice daily. Several other comparative trials are currently underway; details can be accessed from www.clinicaltrials.gov.Citation74
Patient considerations
Diabetes imposes significantly on the patient’s and carer’s QOL, particularly considering the chronic and progressive nature of the disease, increasing treatment burden with time and multiple long-term complications and its attendant consequences, both financially and socially.Citation5 Poor medication compliance is one of the major challenges for the success of treatment of conditions like diabetes and hypertension.Citation75 Patient satisfaction and tolerability may have a decisive role in the use and choice of these new diabetes treatment options that will soon be available.Citation76
The DURATION-1 study examined treatment satisfaction using Diabetes Treatment Satisfaction Questionnaire (DTSQ). The researchers found that patients treated with exenatide once weekly reported a significant increase in treatment satisfaction from baseline compared with exenatide twice daily, despite having similar compliance in the 2 groups (injections received/injections planned (98%)).Citation67
The treatment satisfaction and weight-related QOL in patients treated with exenatide once weekly was assessed in a randomized, multicenter, open-label study and involved 295 patients randomized to exenatide once weekly or exenatide twice daily for 30 weeks.Citation76 At study-end, patients receiving exenatide twice daily were switched to exenatide once weekly for a further 22 weeks. DTSQ and Impact of Weight on Quality of Life – Lite (IWQOL-Lite) were assessed at baseline, 30 weeks, and 52 weeks. Both groups showed statistically significant improvements in DTSQ measures from baseline to week 30. At week 30, between-group differences from baseline in DTSQs total scores were not statistically significant (5.17 ± 0.54 versus 3.97 ± 0.53; P = 0.09), but treatment satisfaction did improve more in the exenatide once-weekly arm for perceived hypoglycemia frequency (from 1.86 ± 0.15 to 1.42 ± 0.15; P = 0.03) and willingness to continue current treatment (from 1.43 ± 0.13 to 0.99 ± 0.12; P = 0.01).Citation76
In the group who switched from exenatide twice daily to exenatide once-weekly at 30 weeks, DTSQ total score improved significantly (1.16 ± 6.1; P = 0.037), as did treatment convenience (0.42 ± 1.6; P = 0.003), treatment flexibility (0.39 ± 1.7; P = 0.012) and satisfaction with continuing treatment (0.24 ± 1.3; P = 0.048) by week 52.
There were no statistically significant differences in weight-related QOL (IWQOL-Lite) between the 2 treatment arms at week 30. Treatment satisfaction and QOL improved significantly from weeks 30 to 52 in those who switched the regimen at week 30, reporting further significant improvement in physical function (2.13 ± 11.5; P = 0.04) and public distress (5.04 ± 11.2; P < 0.001) domains. Patients who continued on once-weekly treatment improved significantly from week 30 to week 52 for public distress (6.96 ± 13.2; P < 0.001).Citation76
This study has several important highlights. Both exenatide twice daily and once weekly improved treatment satisfaction and weight-related QOL significantly. The fact that the effect was maintained over 52 weeks is encouraging and suggests that these effects may be durable and patients could benefit from the sustained clinical effects of the drug. There is some evidence that exenatide once-weekly therapy resulted in greater treatment satisfaction compared with exenatide twice daily, and patients who were on exenatide once weekly were more willing to continue treatment. This suggests that acceptance of exenatide once weekly may be more than for exenatide twice daily. This might be due to the ease of use and reduced frequency of injections.Citation76 Furthermore, the efficacy of exenatide once weekly compared to exenatide twice daily and the reduction in the perceived frequency of hyperglycemia could also have contributed to the impact on treatment satisfaction.Citation67
In the DURATION-2 study (described above), weight-related QOL, psychological general wellbeing, diabetes treatment satisfaction, and general health status were assessed using the IWQOL-Lite, Psychological General Well-being (PGWB) index, DTSQ, and EQ-5D at baseline and week 26.Citation77 The exenatide once-weekly group experienced significant improvement in physical function, self-esteem, sexual life, public distress, work, and IWQOL total score compared with pioglitazone; however, there were no significant differences between exenatide once weekly and sitagliptin. Overall treatment satisfaction improved significantly in the exenatide once-weekly group versus sitagliptin. Both exenatide once-weekly and sitagliptin groups experienced significant improvements in overall health status.
It is relevant to note that the recognised AEs of exenatide, both twice daily and once weekly, nausea and injection-site reactions did not deter patients from continuing with treatment, nor did it affect their QOL.Citation76 This suggests that these issues may not be significant obstacles to improving patient acceptance of this new modality of treatment.
Conclusion
Exenatide once weekly is a new agent for the treatment of patients with T2DM and is currently going through phase 3 trials. Exenatide once weekly produce significant reduction in weight and glycemic parameters when compared with placebo or exenatide twice daily. These improvements were achieved with low risk of hypoglycemia and were sustainable up to 2 years in extension trials. Furthermore, exenatide once-weekly treatment has been shown to be associated with improvements in patient satisfaction and QOL, which might have a positive impact on patient adherence and compliance with treatment, which needs further testing in real-world situations. The results of the other phase 3 trials are awaited with interest.
Disclosure
Dr Abd Tahrani is a research training fellow supported by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. AT has also won research grants from sanofi-aventis and Novo Nordisk UK Research Foundation. Prof. Anthony Barnett has received honoraria for lectures and advisory work and research funding from Servier, MSD, Novartis, Takeda, GlaxoSmithKline, BMS/Astra-Zeneca, Eli Lilly, Novo Nordisk, Roche and sanofi-aventis.
References
- MokdadAHFordESBowmanBAPrevalence of obesity, diabetes, and obesity-related health risk factorsJ Am Med Assoc20032897679
- IDFThe Diabetes Atlas IDF [serial online]2006
- de GrootMAndersonRFreedlandKEClouseRELustmanPJAssociation of depression and diabetes complications: a meta-analysisPsychosom Med20016361963011485116
- WanlessDSecuring our future health: taking a long-term view [April 2002] Available from: http://www.hm-treasury.gov.uk/consultations_and_legislation/wanless/consult_wanless_final.cfm. Accessed Feb 12, 2010.
- JacobsonAMImpact of improved glycemic control on quality of life in patients with diabetesEndocr Pract20041050250816033724
- FacchiniFSHuaNAbbasiFReavenGMInsulin resistance as a predictor of age-related diseasesJ Clin Endocrinol Metab2001863574357811502781
- StumvollMGoldsteinBJvan HaeftenTWType 2 diabetes: principles of pathogenesis and therapyLancet20053651333134615823385
- ReavenGMRole of insulin resistance in human diseaseDiabetes198837159516073056758
- KahnSEHullRLUtzschneiderKMMechanisms linking obesity to insulin resistance and type 2 diabetesNature198844484084617167471
- KahnSEThe importance of [beta]-cell failure in the development and progression of type 2 diabetesJ Clin Endocrinol Metab2001864047405811549624
- KahnSEQuantification of the relationship between insulin sensitivity and B-cell function in human subjects. Evidence for a hyperbolic functionDiabetes199342166316728405710
- PerleyMKipnisDMPlasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjectsDiabetes1966158678745957477
- PolonskyKSGivenBDvan CauterETwenty-four-hour profiles and patterns of insulin secretion in normal and obese subjectsJ Clin Invest1988814424483276730
- BurcelinRKnaufCCaniPDPancreatic alpha-cell dysfunction in diabetesDiabetes Metab200834Suppl 2S49S5518640586
- SalvatoreTGiuglianoDPharmacokinetic-pharmacodynamic relationships of AcarboseClin Pharmacokinet199630941068906894
- DelPSBianchiCMarchettiPBeta-cell function and anti-diabetic pharmacotherapyDiabetes Metab Res Rev20072351852717883249
- PiyaMKTahraniAABarnettAHLiraglutide: a new option in the management of type 2 diabetesFuture Prescriber20089612
- RedekkopWKKoopmanschapMAStolkRPRuttenGEHNiessenLWHealth-related quality of life and treatment satisfaction in Dutch patients with type diabetesDiabetes Care20022545846311874930
- UK Prospective Diabetes Study GroupIntensive blood-glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet19983528378539742976
- CramerJAA systematic review of adherence with medications for diabetesDiabetes Care2004271218122415111553
- GlasgowRECompliance to diabetes regimens: conceptualization, complexity, and determinantsCramerJASpilkerBPatient Compliance in Medical Practice and Clinical TrialsNew YorkRaven Press1991209224
- PughMJAndersonJPogachLMBerlowitzDRDifferential adoption of pharmacotherapy recommendations for type 2 diabetes by generalists and specialistsMed Care Res Rev20036017820012800683
- TahraniAAPiyaMKKennedyABarnettAHGlycaemic control in type 2 diabetes: targets and new therapiesPharmacol Ther20102125232836119931305
- PalalauAITahraniAAPiyaMKBarnettAHDPP-4 inhibitors in clinical practicePostgrad Med200912167010019940419
- TahraniAAPiyaMKBarnettAHSaxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitusAdv Ther20092624926219330494
- PetersAIncretin-based therapies: review of current clinical trial dataAm J Med2010123Suppl 3S28S3720206729
- TahraniAAPiyaMKBarnettAHDrug evaluation: vildagliptin-metformin single-tablet combinationAdv Ther2009226213815419288260
- AhrenBGut peptides and type 2 diabetes mellitus treatmentCurr Diab Rep2003336537212975025
- ElrickHStimmlerLHladCJJrRaiYPlasma insulin response to oral and intravenous glucose administrationJ Clin Endocrinol Metab1964241076108214228531
- NauckMAHombergerESiegelEGIncretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responsesJ Clin Endocrinol Metab1986634924983522621
- BrownJCDryburghJRA gastric inhibitory polypeptide. II. The complete amino acid sequenceCan J Biochem1971498678725120249
- DupreJRossSAWatsonDBrownJCStimulation of insulin secretion by gastric inhibitory polypeptide in manJ Clin Endocrinol Metab1973378268284749457
- BaggioLLDruckerDJBiology of incretins: GLP-1 and GIPGastroenterology20071322131215717498508
- KimWEganJMThe role of incretins in glucose homeostasis and diabetes treatmentPharmacol Rev200860447051219074620
- DruckerDJPhilippeJMojsovSChickWLHabenerJFGlucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell lineProc Natl Acad Sci U S A198784343434383033647
- NauckMAHombergerESiegelEGIncretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responsesJ Clin Endocrinol Metab1986634924983522621
- AhrenBGut peptides and type 2 diabetes mellitus treatmentCurr Diab Rep2003336537212975025
- DruckerDJGlucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosisEndocrinology20031445145514814645210
- Toft-NielsenMBDamholtMBMadsbadSDeterminants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patientsJ Clin Endocrinol Metab2001863717372311502801
- NauckMAKleineNOrskovCHolstJJWillmsBCreutzfeldtWNormalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patientsDiabetologia1993367417448405741
- GutniakMKLindeBHolstJJEfendicSSubcutaneous injection of the incretin hormone glucagon-like peptide 1 abolishes postprandial glycemia in NIDDMDiabetes Care199417103910447988303
- DeaconCFJohnsenAHHolstJJDegradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivoJ Clin Endocrinol Metab1995809529577883856
- MentleinRDipeptidyl-peptidase IV (CD26) – role in the inactivation of regulatory peptidesRegul Pept19998592410588446
- BarnettAExenatideExpert Opin Pharmacother200782593260817931093
- BYETTA prescribing information [article on the Internet]. Available from: http://pi.lilly.com/us/byetta-pi.pdf. Accessed Feb 16, 2010.
- TriplittCDeFronzoRAExenatide: first-in-class incretin mimetic for the treatment of type 2 diabetes mellitusExpert Rev Endocrinol Metab20061329341
- KoltermanOGKimDDShenLPharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitusAm J Health Syst Pharm20056217318115700891
- DeFronzoRARatnerREHanJKimDDFinemanMSBaronADEffects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetesDiabetes Care2005281092110015855572
- BarnettAHBurgerJJohnsDTolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonyulurea: a multinational, randomized, open-label, two-period, crossover noninferiority trialClin Ther200729112333234818158075
- PoonTNelsonPShenLExenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a doseranging studyDiabetes Technol Ther2005746747715929678
- KoltermanOGBuseJBFinemanMSSynthetic Exendin-4 (exenatide) significantly reduces post-prandial and fasting plasma glucose in subjects with Type 2 diabetesJ Clin Endocrinol Metab20078873082308912843147
- EdwardsCMStanleySADavisRExendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteersAm J Physiol Endocrinol Metab20017281E155E161
- Toft-NielsenMBMadsbadSHolstJJContinuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patientsDiabetes Care199972271137114310388979
- RatnerREMaggsDNielsenLLLong-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitusDiabetes Obes Metab20068441942816776749
- BuseJBHenryRRHanJEffects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetesDiabetes Care2004272628263515504997
- KendallDMRiddleMCRosenstockJEffects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylureaDiabetes Care2005281083109115855571
- RiddleMCHenryRRPoonTHExenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metforminDiabetes Metab Res Rev20062248349116634116
- KlonoffDCBuseJBNielsenLLExenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 yearsCurr Med Res Opin20082427528618053320
- BuseJBKlonoffDCNielsenLLMetabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo controlled trialsClin Ther20072913915317379054
- FDA prescribing information for exenatide Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021773s012lbl.pdf. Accessed Mar 18, 2010.
- MonamiMMarchionniNMannucciEGlucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trialsEur J Endocrinol200916090991719318378
- DoreDDSeegerJDArnoldCKUse of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburideCurr Med Res Opin2009251019102719278373
- TracyMAWardKLFirouzabadianLFactors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitroBiomaterials1999201057106210378806
- KimDMacConellLZhuangDEffects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetesDiabetes Care2007301487149317353504
- TaylorKKimDNielsenLLAispornaMBaronADFinemanMSDay-long subcutaneous infusion of exenatide lowers glycemia in patients with type 2 diabetesHorm Metab Res20053762763216278786
- IwamotoKNasuRYamamuraASafety, tolerability, pharmacokinetics, and pharmacodynamics of exenatide once weekly in Japanese patients with type 2 diabetesEndocr J200956895196219706990
- DruckerDJBuseJBTaylorKExenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority studyLancet20083721240125018782641
- BuseJBDruckerDJTaylorKDURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeksDiabetes Care20103361255126120215461
- TrautmannMWilhelmKTaylorKKimTZhuangDPorterLExenatide once-weekly treatment elicits sustained glycaemic control and weight loss over 2 yearsDiabetologia200952Suppl 1S286
- KadowakiTNambaMYamamuraASowaHWolkaAMBrodowsRGExenatide exhibits dose-dependent effects on glycemic control over 12 weeks in Japanese patients with suboptimally controlled type 2diabetesEndocr J20095641542419194050
- WyshamCBergenstalRYanPMacConellLMalloyJPorterLDURATION-2: exenatide once weekly demonstrated superior glycaemic control and weight reduction compared to sitagliptin or pioglitazone after 26 weeks of treatmentDiabetologia200952Suppl 1S290
- BergenstalRWyshamCYanPMacconellLMalloyJPorterLDURATION-2: exenatide once weekly demonstrated superior glycemic control and weight reduction compared to sitagliptin or pioglitazone after 26 weeks of treatmentPresented at ADA 2009San Diego, CA2009
- Press Release. Amylin, Lilly and Alkermes companies announce exenatide once weekly provided superior glucose control compared to Lantus in head-to-head DURATION-3 studySan Diego, CAAmylin Pharmaceuticals2009 Available from: http://files.shareholder.com/downloads/LLY/0x0x307776/675c0e3a-b736-4a08-a30c-bab4ec187b39/LLY_News_2009_7_20_Product.pdf. Accessed February 27, 2010.
- Current trials involving exenatide LAR Available from: http://clinicaltrials.gov|~http://clinicaltrials.gov/. Accessed Mar 18, 2010.
- DiMatteoMRGiordaniPJLepperHSGroghanTWPatient adherence and medical outcomes: a meta-analysisMed Care20024079481112218770
- BestJHBoyeKSRubinRRCaoDKimTHPeyrotMImproved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice dailyDiabet Med200926772272819573122
- BestJHYanPMalloyJDURATION 2: weight-related quality of life, psychological well-being, and satisfaction with exenatide once weekly compared to sitagliptin or piaglitazone after 26 weeks of treatmentDiabetologia200952Suppl 1S292