Abstract
Objective:
The influence of biological sex on human immunodeficiency virus (HIV) antiretroviral treatment outcome is not well described in HIV–hepatitis C (HCV) coinfection.
Methods:
We assessed patients’ clinical outcomes of HIV–HCV coinfected patients initiating antiretroviral therapy attending the Ottawa Hospital Immunodeficiency Clinic from January 1996 to June 2008.
Results:
We assessed 144 males and 39 females. Although similar in most baseline characteristics, the CD4 count was higher in females (375 vs 290 cells/μL). Fewer females initiated ritonavir-boosted regimens. The median duration on therapy before interruption or change was longer in males (10 versus 4 months) (odds ratio [OR] 1.40 95% confidence interval: 0.95–2.04; P = 0.09). HIV RNA suppression was frequent (74%) and mean CD4 count achieved robust (over 400 cells/μL) at 6 months, irrespective of sex. The primary reasons for therapy interruption in females and males included: gastrointestinal intolerance (25% vs 19%; P = 0.42); poor adherence (22% vs 15%; P = 0.31); neuropsychiatric symptoms (19% vs 5%; P = 0.003); and lost to follow-up (3% vs 13%; P = 0.08). Seven males (5%) and no females discontinued therapy for liver-specific complications. Death rate was higher in females (23% vs 7%; P = 0.003).
Conclusion:
There are subtle differences in the characteristics of female and male HIV–HCV coinfected patients that influence HIV treatment decisions. The reasons for treatment interruption and change differ by biological sex. This knowledge should be considered when starting HIV therapy and in efforts to improve treatment outcomes.
Introduction
The natural history and clinical outcomes in human immunodeficiency virus (HIV) may differ by biological sex.Citation1 When highly-active antiretroviral therapy (HAART) first became available, females were less likely to be on therapy and were observed to have diminished therapeutic outcomes.Citation2–Citation4 Although more recent data suggest that these gaps have diminished in magnitude, concerns related to decreased access and quality of care for females persist.Citation2,Citation4 Although data are conflicting, several studies suggest that women have lower plasma HIV RNA levels than men.Citation1,Citation5 Since this measure influences the timing of HAART initiate, it is plausible that women may start treatment later than men, which may contribute to poorer outcomes.Citation5,Citation6 Women may experience more lactic acidosis, drug toxicity and hypersensitivity reactions on antiretroviral therapy which may contribute to decreased adherence and treatment interruption.Citation1,Citation7 Some studies indicate that women have higher rates of clinical progression to acquired immunodeficiency syndrome (AIDS) and death while others have suggested the opposite.Citation1,Citation2,Citation8 Differential access to care for women between studies may be an important factor explaining these discrepancies.Citation1,Citation2,Citation8 Many women with HIV report high rates of poverty, are often depended on for care of children or family members, and may show a reluctance to take medication in public due to denial or fear of disclosure which may lead to compromised self-care, access to care, and response to treatment.Citation4,Citation9 Depression disproportionally affects women and may contribute to worse outcomes, as depressive symptoms in women are associated with hastened HIV progression.Citation10
Hepatitis C virus (HCV) coinfection in HIV is common and contributes to increased morbidity and mortality.Citation11 Many of the barriers to HIV care access and treatment listed above are more frequent and more severe in those with HIV–HCV coinfection.Citation12,Citation13 Furthermore, HCV itself may negatively influence the natural history of HIV disease and therapeutic response to HAART.Citation11 The interaction between HIV, HCV, and sex is an area that remains largely unexplored. Well-established gender differences between sexes in HIV–HCV coinfected women include greater likelihood of spontaneous clearance of acute HCV infection and higher symptom burden.Citation14,Citation15 There is no literature evaluating differences in HIV-related outcomes in the HIV–HCV coinfected population. To this end, we evaluated the influence of biological sex on HIV antiretroviral clinical, immunological, and virological outcome in a well-described HIV–HCV coinfected population followed in a tertiary care-based, specialized immunodeficiency clinic. Specific data on sex and HIV–HCV coinfection is important, as women are a rapidly growing segment of the overall HIV population.Citation16,Citation17
Methods
We assessed all HAART-treated HCV–HIV coinfected individuals followed at The Ottawa Hospital Immunodeficiency Clinic from January 1996 to June 2008. We identified patients in a prospective cohort of clinic attendees and supplemented this data by chart review. HIV RNA viral load, CD4 lymphocyte counts, liver enzymes and function tests, and HCV serology results were collected. Characteristics including age, history of excess alcohol, history of injection drug use (IDU), treatment start date, HAART composition, and pregnancy status were identified.
Primary outcome measures included duration of therapy before interruption or change, suppression of HIV RNA levels below detection within 6 and 12 months, mean CD4 counts after 6 months of HAART, and cumulative survival. The primary reason for treatment interruption was assessed. Categories included gastrointestinal side effects (eg, nausea, vomiting, abdominal discomfort, diarrhea), neuropsychiatric symptoms (eg, depression, headaches, difficulties with mentation, neuropathy), adherence difficulty, metabolic complications, liver toxicity, renal complications, musculoskeletal side effects, hematologic abnormalities, dermatologic complications, alcohol and/or substance abuse, pill count or dosing frequency issues, virologic failure, and death. Measures of adherence were based on self-report and clinician suspicions of nonadherence.
We assessed baseline characteristics using parametric testing (t-tests and Chi-squared). Our primary outcome measures were analyzed using time to event analysis with a step-wise multivariable analysis. We consider a P-value of less than 0.05 as significant and all P-values are two-sided. Collection and analysis of this data was approved by the Ottawa Hospital Research Ethics Board.
Results
A total of 183 HIV–HCV coinfected HAART-treated patients followed from January 1996 to June 2008 were assessed (). Females made up 21% of the evaluated population. The majority were White males. A high level of excess alcohol use and history of IDU was noted in both sexes. A trend toward lower baseline HIV RNA levels and higher CD4 counts was noted in women. Four women (10%) initiated HAART specifically for pregnancy. There was no difference in baseline CD4 counts between these four patients and the other 35 females who started HAART. None of these four women or two others who became pregnancy while on HAART was exposed to efavirenz.
Table 1 Baseline characteristics of HIV–HCV coinfected patients
There were subtle differences in specific drugs chosen when assessed by sex (). A higher proportion of men initiated high-dose ritonavir and low-dose ritonavir-boosted containing therapy. More females initiated ritonavir-sparing (ie, nelfinavir, atazanavir) treatment. Women were less likely to initiate efavirenz which may reflect concerns related to teratogenicity risk in women of child-bearing age. Because the median start date for HAART was similar by sex (August 1999 for men; December 1998 for women), we are confident that these differences in antiretroviral composition by sex are not a consequence of a treatment era effect bias. Median duration of follow-up after the initiation of therapy was 4.7 years for men and 5.1 years for women (P = 0.55). The median duration on therapy was 4 months for women (range: 1 to 10 months) and 10 months for men (range: 4 to 24 months) (P = 0.01) (). The duration on therapy did not differ for women started on HAART for pregnancy compared to women initiated on treatment for other indications (data not shown). At last assessment, only 9% of the men and 8% of the women were on the same treatment regimen as the one started revealing a high turnover rate of antiretrovirals. Twelve percent of women and 15% of men stopped antiretroviral therapy without resumption, 35% of women and 48% of men immediately changed to a second line regimen without interruption, and 53% of women and 37% of men resumed therapy but only after an interruption in HAART (P = 0.24).
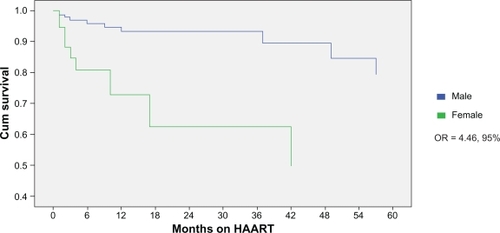
Figure 1 Months on HAART by sex prior to interruption or treatment change.
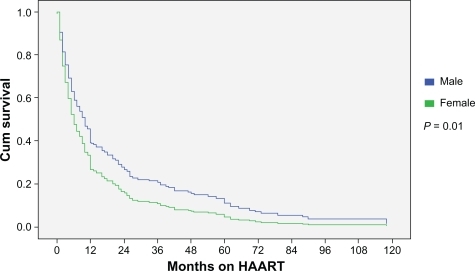
Table 2 HAART composition for HIV–HCV patients starting treatment
Mean CD4 counts increased to 413 cells/μL in women and 400 cells/μL in men after 6 months of HAART and did not differ by sex. The absolute increase in CD4 cells from baseline was greater in males (102 versus 11 cells/μL; P = 0.04). The proportion achieving virological suppression below the lower limit of detection were similar at 6 months (women: 77%, men 74%; P = 0.73) and 12 months (women: 82%, men: 81%; P = 0.87) of therapy in those remaining on HAART at these time points.
Both women (25%) and men (19%) (P = 0.42) cited gastrointestinal complications as the primary reason for HAART interruption (). Adherence difficulties were a common reason for discontinuation in women (22%) and men (15%) (P = 0.31). Women were more likely to stop treatment due to neuropsychiatric issues (P = 0.003). The use of efavirenz was similar in those interrupting therapy for neuropsychiatric complications (23%) and those not (15%; P = 0.46). Two of six men and one of seven women on efavirenz who stopped treatment for neuropsychiatric reasons did so specifically due to efavirenz-attributed side effects. Men were more likely to be lost to follow-up (P = 0.08). Liver complications were an infrequent cause for HAART change or interruption, irrespective of sex. Seven males (5%) and no females discontinued therapy for liver-specific complications.
Table 3 Primary reasons for therapy interruption by sex
Mortality analysis revealed an association between female sex and reduced cumulative survival (odds ratio [OR] = 4.46, 95% confidence intervals [CI]: 1.8–11.0; P = 0.001) (). When controlled for IDU, race, and baseline CD4, this association remained significant. Twenty-three percent of women and 7% of men died during the period of evaluation (P = 0.003). Sixty percent of deaths were end-stage liver disease-related and did not differ by sex.
Discussion
There is evidence that biological sex may influence HIV progression and treatment response.Citation1,Citation7 These differences are of ever-increasing importance given that women comprise a rapidly growing segment of the HIV population.Citation16,Citation17 Little is known as to how HCV coinfection influences the interaction between HIV and biological sex. Our findings indicate that HIV–HCV coinfected women are more likely to interrupt or change treatment before men, differ in reasons for therapy interruption, and may have a lower cumulative survival. These differences are not explained by the subtle baseline differences in characteristics.
When comparing duration on HAART prior to interruption or change in antiretroviral(s), differences between men and women were most apparent during the first 12 months of therapy (). Thereafter, the rates were similar. The reason for differences in duration of therapy before interruption or change is unclear but likely multifactorial. Similar findings have been observed in HIV mono-infected populations.Citation1 The proportion of men and women on the same HAART regimen after one year was low and did not differ by sex. Women more typically interrupted HAART with a break prior to resumption of therapy whereas men where more likely to immediately switch to an alternate regimen. The reason for this is unclear. However, post-partum discontinuation of therapy did not explain this observation.
It is plausible that pregnancy may influence the timing of HAART initiation and therapeutic outcomes in HIV. Pregnancy may affect pharmacokinetics that could influence side effects.Citation18–Citation20 Pregnancy may contribute to greater drug intolerance in female recipients and higher rates of treatment discontinuation as following delivery HAART may be stopped depending on patient and physician preferences towards remaining on treatment (dependent on disease stage). Consistent with other studies, we did not identify a pregnancy effect on HIV disease progression or outcome.Citation21,Citation22 However, the number of pregnancies in our cohort was low.
In our study, virological and immunological outcomes did not differ by sex in those remaining on therapy. It is noteworthy that the baseline CD4 count at treatment initiation was higher in women. This difference was not explained by pregnant females with high CD4 counts initiating HAART to prevent maternal to fetal HIV transmission. It is plausible that more women initiated HAART earlier in the HAART era when higher CD4 counts triggered the introduction of therapy. This was not the case in our cohort as the average date of treatment initiation was similar by sex. We suspect that better compliance with scheduled clinic visits in women may have contributed to HAART-initiation at higher CD4 counts. There are discrepancies between other studies with some reporting higher,Citation23 lowerCitation2 or similarCitation1,Citation7,Citation24,Citation25 baseline CD4 counts by sex. This may reflect the influence of the above mentioned confounders.
Consistent with other work, the primary reason for therapy interruption differed by sex in our analysis.Citation26 Treatment adherence in our female patients was not a significant factor leading to treatment interruption or change. This finding is consistent with some, but not all analyses.Citation24,Citation27 It is unclear why so many men where lost to follow-up and to what effect this had on the outcomes in the study.Citation3 Previous research by our group indicated that our HCV–HIV coinfected population faces multiple barriers to treatment including substance abuse, psychiatric illness, anxiety related to workup or treatment, and poor social circumstances which contribute to suboptimal follow-up.Citation4
Neuropsychiatric issues were identified as an important reason for therapy interruption in females. Depression and other mental health issues are common in HIV–HCV coinfection.Citation28–Citation30 In HCV populations, current levels of psychiatric disorders range from 31% to 58% with lifetime rates as high as 95%.Citation5 This may be higher in women compared to men.Citation10 In HIV populations, psychiatric disorders are also common with up to 36% of individuals suffering from major depression, a rate that is three times higher than non-HIV infected individuals.Citation31,Citation32 Several studies suggest mental illness is more prevalent and more severe in the HIV–HCV coinfection.Citation31–Citation34 Studies evaluating HIV mono-infected patients suggest that much of the variation in HIV progression is related to depression, stressful life events, and trauma.Citation32 In our analysis women were more likely to have a psychiatric illness and for this to affect their treatment. Mental health issues may also explain why women in our study had a decreased cumulative survival. Despite the well-established influence on mental health, the use of efavirenz did not appear to influence the likelihood of interrupting HAART in our cohort.Citation35 This data highlight the importance of thoroughly evaluating and treating mental health issues, especially in women, as such interventions may significantly improve outcomes in HIV–HCV coinfection.
Patients with HIV–HCV coinfection have higher mortality rates.Citation11 Little is known about gender differences among coinfected populations. Our results suggest that in the HIV–HCV coinfected population, mortality in women may be higher despite similar viral suppression and CD4 counts, and when controlling for IDU history, race, and baseline CD4 levels. Recent data in HIV mono-infected individuals reveals that women generally live longer with HIV, which contradicts our findings and may indicate that the negative interaction between these two viruses is particularly harmful in women.Citation6 The primary cause for death was related to end-stage liver disease. HCV-related liver disease does progress more rapidly in women, which partially explains our results.Citation36 This suggests that HIV–HCV coinfected women should be aggressively evaluated and treated for HCV. Mental health issues in women may have contributed to our findings. Others have reported that HIV-positive women with chronic depressive symptoms are two times more likely to die and show greater declines in CD4 counts.Citation10 We do not believe that the differential mortality rates observed in our analysis are a consequence of an era effect as the average date of initiation of HAART and the median duration of follow-up was similar by sex. We acknowledge that the high number of loss to follow-up in males may have skewed the data as the true outcomes of these patients, including the possibility of death, is unknown. Furthermore, it is difficult to separate biological differences from confounding socioeconomic and mental health factors which are well-established to influence adherence, morbidity, and survival.Citation12,Citation13
There are several limitations to the current study. Most patients were White. It is unclear whether race directly influences the natural history of HIV–HCV coinfected individuals.Citation22 The patients evaluated in this study were managed at a tertiary center offering specialized HIV care. Due to referral bias, a broader population of HIV–HCV coinfected individuals that are not referred or who have yet to seek care may have been missed. As a retrospective evaluation, reporting bias and missing data may have influenced our findings. Nonetheless, our analysis convincingly indicates that biological sex is not associated with differences in virological and immunological outcome in HIV–HCV coinfection initiating HAART. However, reasons for treatment interruption and mortality itself may differ by sex. These findings are of value in guiding health care providers as they plan and manage gender-specific treatment in HIV–HCV coinfection.
Disclosures
The authors report no conflict of interest in this work.
References
- CollazosJAsensiVCartonJASex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAARTAIDS200721783584317415038
- CunninghamWEMarksonLEAndersenRMPrevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. HCSUS Consortium. HIV Cost and Services UtilizationJ Acquir Immune Defic Syndr200025211512311103041
- FurlerMDEinarsonTRWalmsleySMillsonMBendayanRLongitudinal trends in antiretroviral use in a cohort of men and women in Ontario, CanadaAIDS Patient Care STDS200620424525716623623
- GeboKAFleishmanJAConviserRRacial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001J Acquir Immune Defic Syndr20053819610315608532
- GandhiMBacchettiPMiottiPQuinnTCVeroneseFGreenblattRMDoes patient sex affect human immunodeficiency virus levels?Clin Infect Dis200235331332212115098
- FarzadeganHHooverDRAstemborskiJSex differences in HIV-1 viral load and progression to AIDSLancet19983529139151015149820299
- BoulasselMRMoralesRMurphyTLalondeRGKleinMBGender and long-term metabolic toxicities from antiretroviral therapy in HIV-1 infected personsJ Med Virol20067891158116316847953
- HewittRGParsaNGuginoLWomen’s health. The role of gender in HIV progressionAIDS Read2001111293311215085
- ZierlerSKriegerNReframing women’s risk: social inequalities and HIV infectionAnnu Rev Public Health1997184014369143725
- IckovicsJRHamburgerMEVlahovDHIV Epidemiology Research Study GroupMortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research StudyJAMA2001285111466147411255423
- GreubGLedergerberBBattegayMClinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort StudyLancet200035692441800180511117912
- FalusiOMPulvirentiJSarazineJShastriPGailCGlowackiRHIV-infected inpatients in the HAART era: how do hepatitis C virus coinfected patients differ?AIDS Patient Care STDS2003171131612614516
- WoodEKerrTTyndallMWMontanerJSA review of barriers and facilitators of HIV treatment among injection drug usersAIDS200822111247125618580603
- Antiretroviral Therapy Cohort CollaborationLife expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studiesLancet2008372963529329918657708
- GrebelyJRaffaJDLaiCKrajdenMConwayBTyndallMWFactors associated with spontaneous clearance of hepatitis C virus among illicit drug usersCan J Gastroenterol200721744745117637948
- BurruanoLKruglovYHIV/AIDS epidemic in Eastern Europe: recent developments in the Russian Federation and Ukraine among womenGend Med20096127728919467524
- FarrSLKraftJMWarnerLAndersonJEJamiesonDJThe integration of STD/HIV services with contraceptive services for young women in the United StatesAm J Obstet Gynecol20092012142e1e8
- BardeguezADLindseyJCShannonMPACTG 1025 Protocol TeamAdherence to antiretrovirals among US women during and after pregnancyJ Acquir Immune Defic Syndr200848440841718614923
- ClarkRConsiderations for the antiretroviral management of women in 2008Womens Health (Lond Engl)20084546547719072486
- StekAMAntiretroviral medications during pregnancy for therapy or prophylaxisCurr HIV/AIDS Rep200962687619358777
- AhdiehLPregnancy and infection with human immunodeficiency virusClin Obstet Gynecol200144215416611344985
- HershowRCO’DriscollPTHandelsmanEHepatitis C virus coinfection and HIV load, CD4+ cell percentage, and clinical progression to AIDS or death among HIV-infected women: Women and Infants Transmission StudyClin Infect Dis200540685986715736020
- Perez-HoyosSRodríguez-ArenasMAGarcía de la HeraMProgression to AIDS and death and response to HAART in men and women from a multicenter hospital-based cohortJ Womens Health (Larchmt)20071671052106117903082
- NicastriELeoneSAngelettiCSex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic reviewJ Antimicrob Chemother200760472473217715125
- SterlingTRVlahovDAstemborskiJHooverDRMargolickJBQuinnTCInitial plasma HIV-1 RNA levels and progression to AIDS in women and menN Engl J Med20013441072072511236775
- KumarasamyNVenkateshKKCeceliaAJGender-based differences in treatment and outcome among HIV patients in South IndiaJ Womens Health (Larchmt)20081791471147518954236
- KuyperLMWoodEMontanerJSYipBO’ConnellJMHoggRSGender differences in HIV-1 RNA rebound attributed to incomplete antiretroviral adherence among HIV-Infected patients in a population-based cohortJ Acquir Immune Defic Syndr20043741470147615602125
- McLarenMGarberGCooperCBarriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinicCan J Gastroenterol200822213313718299730
- RestrepoAJohnsonTCWidjajaDThe rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centreJ Viral Hepat2005121869015655053
- RighiEBeltrameABassettiMTherapeutical aspects and outcome of HIV/HCV coinfected patients treated with pegylated interferon plus ribavirin in an Italian cohortInfection200836435836118642111
- DouaihyAHilsabeckRCAzzamPJainADaleyDCNeuropsychiatric aspects of coinfection with HIV and hepatitis C virusAIDS Read200818842543218770900
- LesermanJRole of depression, stress, and trauma in HIV disease progressionPsychosom Med200870553954518519880
- ButtAAKhanUASkandersonMComorbidities and their impact on mortality in HCV and HCV-HIV-coinfected persons on dialysisJ Clin Gastroenterol20084291054105919013829
- SiikaAMAyuoPOSidleMJWools-KaloustianKKimaiyoSNTierneyWMAdmission characteristics, diagnoses and outcomes of HIV-infected patients registered in an ambulatory HIV-care programme in western KenyaEast Afr Med J2008851152352819413204
- QueredaCCorralIMorenoAEffect of treatment with efavirenz on neuropsychiatric adverse events of interferon in HIV/HCV-coinfected patientsJ Acquir Immune Defic Syndr2008491616318667933
- Rodríguez-TorresMRíos-BedoyaCFRodríguez-OrengoJProgression to cirrhosis in Latinos with chronic hepatitis C: differences in Puerto Ricans with and without human immunodeficiency virus coinfection and along genderJ Clin Gastroenterol200640435836616633110