Abstract
Background
Patient-reported outcomes (PROs) are frequently used to evaluate treatment effects and quality of life in clinical trials. The application of PROs in breast cancer clinics is evolving but their use to generate real-time information for use in follow-up care is uncommon. This proactive use might help to shift healthcare delivery toward a more patient-centered approach by acting as a screening tool for unmet needs or a dialogue tool to discuss issues proposed by the patient.
Aims
This review aims to determine the effects and feasibility of using PROs proactively during follow-up care in early breast cancer.
Materials and methods
A systematic search was conducted in January 2019 in PubMed, Cochrane Library, Embase, and CINAHL. Studies that exclusively concerned women treated for early breast cancer where PROs were used as a proactive tool during follow-up were included.
Results
The search revealed a total of 653 records and four eligible studies were identified; three of which concerned the use of PROs both as a screening tool and as a dialogue tool, and one study in which PROs were used solely as a screening tool. The studies explored the feasibility of collecting and integrating PROs in the clinic and their ability to detect otherwise unrecognized problems. All of the included studies were prone to bias, but they point to potential benefits in respect of better symptom management in follow-up care.
Conclusion
Our search identified a small number of low to moderate quality studies of the proactive use of PROs during follow-up after treatment for early stage breast cancer. The limited evidence available suggests that PROs may be useful for providing a more complete picture of the patient’s symptoms and problems, possibly leading to improvements in symptom management.
Introduction
Early stage breast cancer patients experience multiple symptoms following diagnosis and treatment.Citation1,Citation2 In policy-making and healthcare research, patient involvement has been valued as a way to assess whether the healthcare system delivers what matters most to patients.Citation3,Citation4 Accurate assessment of health status and quality of life is essential for improving well-being and rehabilitation in cancer care.Citation4–Citation6
Patient-reported outcomes (PROs) measure quality of life, physical and social functioning, symptoms, side effects, and emotional well-being. According to the US Food and Drug Administration, PRO data are “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”Citation7 These measures are distinct from, but complementary to, disease-focused outcomes such as survival, mortality, and other clinical outcomes.Citation8 Self-reported data are collected using questionnaires that can be repeated over time or used as a single evaluation, depending on the purpose.Citation9 Systematically collected PRO data are being used increasingly in clinical trials of cancer treatments,Citation10,Citation11 mainly as a secondary outcome to support a primary clinical outcome.Citation12,Citation13
Breast cancer is the most frequent cancer among women, with an estimated 2.08 million new cases diagnosed worldwide in 2018, constituting 24.2% of all cancers.Citation14 Advances in breast cancer treatments and improved diagnostics continue to increase the population of breast cancer survivors around the world. Within this population, PRO data have the potential to provide valuable information about long-term side effects and quality of life during survivorship.Citation15,Citation16 PROs are mainly used to evaluate treatment effects. Their use in real time, to inform decisions in follow-up care for individual patients, is relatively rare, but may have the potential to shift healthcare delivery toward a more patient-centered approach.Citation5,Citation17
PROs can potentially be used as a screening tool to customize supportive care for breast cancer survivors. If patients are provided with PROs at several fixed time points during follow-up, the measurements can be used to allocate the patients to the intervention needed. Exposure of new problems may give an early indication of recurrence of breast cancer or of the appearance of unacceptable sequelae to prier treatment.Citation18,Citation19 Reports of fewer problems may be an indicator of effective supportive care, potentially avoiding further interventions and unnecessary clinical appointments, which could be beneficial to both the patient and the healthcare system.Citation20–Citation23 The use of PROs as a dialogue tool in the clinical consultation could help to prompt discussion on the patient’s individual needs, potentially revealing otherwise undetected physical or psychological effects.Citation10,Citation21,Citation24
The potential benefits of using PROs proactively during follow-up care are somewhat different from their use during active treatment. During treatment, symptoms may arise and change rapidly, and patients are usually scheduled for mandatory consultations in order to handle side effects properly and to be prescribed the continued treatment. In this case, PROs are primarily used to illuminate the dialogue about symptom management.Citation6,Citation25,Citation26 In follow-up care, breast cancer patients experience a more varying set of needs, but with fewer opportunities to report problems to the clinician. Here, the potential of a screening tool to detect patients’ individual needs may be helpful to support personalized rehabilitation.Citation1
The present study reviews the literature on the proactive and real-time use of PROs in post-treatment follow-up care for early stage breast cancer patients. We seek to evaluate the feasibility and potential effects of PROs used proactively as screening tools to help clarify the patients’ individual needs and as discussion prompts to enhance the quality, depth, and breadth of dialogue in the clinical consultation.
Materials and methods
Scoping reviews are systematic literature reviews in a broad topic area that provide relevant and quantified results about the knowledge available on a particular topic and aim to rapidly map and synthesize the evidence to emphasize what is known. Scoping reviews are used to identify knowledge gaps, set research agendas, and identify implications for decision-making.Citation27,Citation28
Search strategy
A systematic search was conducted in November 2017 and updated through January 9, 2019 in PubMed, using the key words “breast cancer” and the MeSH term “breast neoplasms” combined with the key words “patient reported outcome*” and the MeSH terms “patient outcome assessment” and “patient reported outcome measures.” No restrictions on language, year, or type of study design were applied and relevant references were examined for additional studies. A similar search strategy was applied to the Cochrane Library, Embase, and CINAHL databases. Please refer to Supplementary materials for the entire search string.
Selection criteria
Studies that met the following criteria were included: 1) the study population exclusively concerned women treated for early stage breast cancer; and 2) PROs were used proactively as a screening or a dialogue tool during follow-up care. In the first selection stage, all relevant citations were screened based on the title and abstract for the use of PROs in a breast cancer population during follow-up. Secondly, full-text articles of potentially eligible studies were obtained and assessed for eligibility. Both procedures were performed by one reviewer (CLR), but any doubt of eligibility was resolved by achieving consensus among three reviewers (CLR, TB, KDS). We excluded studies if the population consisted of mixed cancer types or if some patients had metastatic disease, and we excluded studies if PROs were collected as an outcome measure but not used proactively. Hence, only studies concerned with the proactive use of PROs were included, by which we mean data reported by a patient and used to inform care for the same patient during follow-up.
Information was extracted in a standardized format according to a prespecified Data Extraction SheetCitation29 to sum-marize the studies under the following headings: patients, study methods, aims and outcome, assessments, description of the PROs, how they were used, principal findings, and comments. Extracted data are presented in .
Table 1 Summary of studies reviewed
A PRO used as a screening tool was defined as a tool for allocating breast cancer patients to the most optimal supportive care based on symptomatology. A PRO used as a dialogue tool was defined as a tool, which revealed the patient’s physical and psychological symptoms or concerns ahead of a clinical visit and contributed to the discussion of those at the clinical visit. Follow-up care was defined as the post-treatment rehabilitation assignment, which follows and complements the primary surgery and adjuvant treatment with chemotherapy and/or radiotherapy. Defining the initiation of follow-up care may be difficult, since some patients receive only surgery while others have several kinds of adjuvant treatments, including up to 10 years of endocrine therapy. In the current review, the management of acute side effects from chemotherapy and radiotherapy is not considered to be part of follow-up care. Consequently, follow-up initiates at the end of these treatments. Adjuvant endocrine treatment is recommended for 5–10 years and is thereby included as one of the challenges follow-up care needs to provide for.
Assessment of risk of bias
Risk of bias at the individual study level was assessed using the Risk of Bias in Non-randomized Studies – of Interventions (ROBINS-I) tool.Citation30 The ROBINS-I tool is based on the Cochrane Risk of Bias tool for randomized controlled trials (RCTs) and was developed for intervention studies that did not use randomization, so in a review with heterogeneous study designs this tool was found to provide the best comparison. Risk of bias is assessed within specified bias domains, and review authors are asked to document the information on which judgements are based. Seven domains were assessed: confounding, selection, classification, departures from intended interventions, missing data, outcome measurement, and selective reporting. An assessment of bias was reported for each of these domains and summed to an overall judgement presented in . Two review authors (CLR, TB) used the ROBINS-I tool and independently assessed the risk of bias across the seven domains for each included study. Any disagreements were discussed and resolved by consensus.
Table 2 Assessment of risk of bias (ROBINS-I)
Results
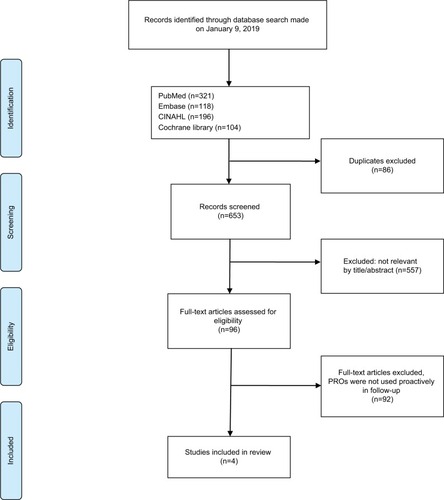
As shown in the PRISMA (),Citation29,Citation31 a total of 653 records were identified and screened for eligibility. Ninety-six articles concerned women treated for early stage breast cancer with PROs used as a primary or secondary outcome measure in a follow-up setting. These were further scrutinized and those articles not concerned with the proactive use of PROs in follow-up care were discarded, leaving four studies that met the eligibility criteria.
The extracted four articles were of recent date, published from 2012 until 2017. A summary of the abstracted information is presented in . Sample sizes ranged from 102 to 172 patients. Three out of four articles used electronic surveys and multiple assessments over time to achieve a longitudinal perception of the patient’s condition during follow-up.Citation32–Citation34 In the fourth study, printed PRO questionnaires were provided to patients with a prepaid envelope for completion at a single time-point as a cross-sectional survey analysis.Citation35
Characteristics of PRO tools
Three studies used generic, validated PRO tools to capture the patient’s perspective of health, quality of life, anxiety, depression, or other related issues.Citation32,Citation33,Citation35 In one study,Citation33 the content of the questionnaires included both validated surveys such as the 36-Item Short-Form Health SurveyCitation36 and the 8-item Patient Health Questionnaire depression scale (PHQ-8),Citation37 as well as a non-validated symptom questionnaire modified from the Memorial Symptom Assessment Scale. The questionnaire also included a free text area for patients to pose questions or report concerns to their provider. The PHQ-8 is validated as a diagnostic and severity measure for depressive disorders in large clinical trials. The Hospital Anxiety and Depression Scale (HADS) is another widely used and validated tool to measure anxiety and depression.Citation38 The HADS was used together with the European Organization for Research and Treatment of Cancer quality of life questionnaireCitation39 in a pilot study.Citation32 This study also used the validated distress thermometer, a numerical scale from 0 (no distress) to 10 (extreme distress), to allocate patients into the study by a cutoff point of >7. This score has been shown to identify breast cancer patients suffering from moderate to severe distress.Citation40 The fourth study used a non-specified web-based health questionnaire with no further documentation about the origin or validation.Citation34
Design, feasibility, and principal findings
The use of PROs both as a screening tool and as a dialogue tool was investigated in three studies.Citation32–Citation34 In a Danish RCT study,Citation32 PROs were used as a screening tool to detect those patients who were in need of a nurse navigator intervention to relieve distress, anxiety, and depression. From a cohort of 116 newly diagnosed breast cancer patients, 50 patients reported scores that indicated a high level of distress. These 50 patients were randomized 1:1 to the intervention group or the control group. The remaining 66 patients were observed and completed PROs at baseline, 6 months, and 12 months. The nurse navigator aimed to improve rehabilitation and supportive care by empathetic listening and actively engaging in dialogue. Using PROs for assessment of the patients’ needs provided topics for psychoeducation, goal-setting, and debriefing. The dialogue was conducted face-to-face or by telephone and could be assisted by referrals to existing rehabilitation offers. At 12 months, the intervention group reported lower levels of distress, anxiety, and depression compared to the control group. No significant effects were seen on health-related quality of life. The intervention was found to be feasible and useful for patients with high distress scores at the time of diagnosis.Citation32
In an RCT from the USA, a web-based system for symptom management after treatment of breast cancer was evaluated against standard care.Citation33 The online questionnaire included a free text area for patients to pose questions or report concerns to their provider. The information was used in the triage of patients for additional follow-up appointments and aimed at facilitating patient–clinician dialogue, when present. The authors hypothesized that PROs would reduce time to symptom management and decrease the number of breast cancer-related hospital appointments, but no reduction in the use of healthcare resources was demonstrated.Citation33 Participants in the intervention group reported a significant higher mean of 7.36 new or changed symptoms during the 18-month study period compared to the standard care arm with a mean of 3.2 new or changed symptoms. The authors concluded that the online questionnaires facilitated better reporting and more timely assessment of symptoms, particularly for those symptoms not deemed by the patient to be urgent and in need of immediate attention. There was a comparatively low questionnaire completion rate of 50%, which the authors suggested could be caused by the electronic interface, which might have inhibited its use by some patients.Citation33
In a retrospective analysis of 106 breast cancer patients, the impact of using a web-based health questionnaire on symptom reporting, physician documentation of symptoms, and symptom management was investigated for its potential of screening for otherwise undetected symptoms and as a dialogue tool in the management of those.Citation34 The study sample was randomly selected from a population of patients who had filled in a follow-up questionnaire before their appointment and had given consent for data to be used in clinical research. The sample was argued to be representative for the whole breast cancer population. A summary of the information reported by the patients was available for clinician review before the patient’s visit. The questionnaire completion rate was nearly 80% for first appointments and about 40% for follow-up visits in the population from which the sample was extracted. The primary finding was a significantly higher incidence of symptoms reported by the patient than documented by the clinician.Citation34
In the fourth study, a prospective study of 172 participants, PRO measures were used solely as a screening tool to identify levels of distress in breast cancer survivors approaching discharge from hospital-based follow-up care to community-based care at least 2 years past diagnosis.Citation35 This study met the inclusion criterion of proactive use by the fact that patients who reported scores that indicated significant distress were contacted and referred to supportive services. Only a minority of patients reported high anxiety and depression scores on the generic PRO instruments used in this study, but the authors concluded that screening with PROs for psychological/emotional distress should be a vital part of follow-up care. The response rate was 75%.Citation35
Biases in the studies
The assessment of risk of bias by the ROBINS-I tool is presented in . Only the pilot studyCitation32 achieved moderate bias assessment as a consequence of the RCT design and high response rates. The remaining three studies were assessed as having a high risk of selection bias, confounding, and missing data. The scores according to the ROBINS-I tool across seven domains are reported in .
Discussion
The majority of the publications on the use of PROs within breast cancer patients deal with the patient’s evaluation of a treatment.Citation10,Citation41 Using PRO data in clinical trials examining side effects of adjuvant endocrine therapy has revealed a higher frequency of both physical and psychosocial symptom burden than based on clinicians’ assessment.Citation42–Citation46 Thus, one of the potential benefits with the application of systemically obtained PRO data, used proactively in survivorship, may be to provide a more comprehensive evaluation that includes the patient’s perception of problems experienced during follow-up care.Citation11,Citation42–Citation46
PROs collected ahead of a clinical visit and actively used as part of informed care for individual patients seem far more challenging than including PROs as an outcome measure in a clinical trial to assess treatment effects.Citation47,Citation48 Implementation of the proactive use of PROs in everyday care involves changing the clinical culture and workflow, requiring extra training and support for those unfamiliar with these tools. Introducing changes in clinical procedures in the face of busy work schedules can lead to a disturbed work pattern, delays, and resistance from clinical staff. Clarification of the appropriate use and interpretation of PROs and their impact on the healthcare system still needs further investigation.Citation49–Citation52
The present review identified four studies exploring the potential of using PROs as a dialogue tool and a screening tool during follow-up in breast cancer patients. The studies differ concerning design, the selected PRO tools, and how they evaluated the potential of applying PROs proactively during follow-up care. The range of study designs, from a randomized clinical trial to an observational study, complicates comparison of the principal findings but still gives a perspective on the potential for using PRO data proactively.
Three out of four studies used electronic surveys. Apps for phones and tablets focusing on easily accessible electronic surveys are of great importance for the rapid progress of PRO data research.Citation53,Citation54 It is crucial for the collection of real-time data that patients are able to assess their PROs where ever they are.Citation22,Citation55 Electronic collections of PROs also provide the possibility of immediate feedback to the patient and could be combined with some kind of web-based self-management application to support patient education, activation, and empowerment.Citation56 However, electronic questionnaires may not be feasible for all patients due to language or skill barriers, particularly older people who lack computer experience or those with multiple morbidities.Citation57 The technical feasibility of integrating PRO data into the electronic medical record has been provided in some settings and makes the collection of PRO measurements more cost-effective.Citation49,Citation58 Success with the task elsewhere is evolving due to technological improvements and the awareness of the potential benefits of using PROs proactively.Citation58–Citation60
In the study by Thompson et al,Citation35 patients completed paper questionnaires. The researchers obtained a high compliance in completed items for the returned PROs, which may be attributable to the simple design of the study with a single evaluation. Of the 227 patients who voluntarily agreed to participate and who were provided with questionnaires, from a cohort of 323 eligible candidates, only 172 actually completed and returned them. The sample was thus highly selected, with only 53.2% of the cohort represented, calling into question its representativeness, and there was no discussion in the paper to inform judgment about the extent of bias this might have introduced. In research it is acceptable to allocate resources to the management of paper questionnaires, but in routine care it is necessary to minimize workloads and resource use. Infrastructure for data collection and safe storage, data analysis, and data presentation that is readily understood by patients and clinicians are further challenging factors for the implementation of PROs.Citation16,Citation52–Citation54,Citation57,Citation59 In the RCT by Wheelock et al,Citation33 low response rates were suggested to be caused by lack of skills toward the management of an electronic survey. The development of user-friendly, easy-access electronic PRO instruments, and timing – what to measure, when, and why – is crucial for standardized implementation, integration, and feasibility.Citation61–Citation66
Collection of PRO data has been recommended in the evaluation of lifestyle interventions in breast cancer survivorship and to provide complementary information to more traditional clinical indicators.Citation28 Multiple studies have demonstrated that PROs more accurately capture patients’ experience of symptoms and other problems than physicians’ assessments.Citation46,Citation67–Citation69 The studies we reviewed on use of PROs during follow-up care are suggestive of more complete symptom reporting, but they were too biased to be conclusive.Citation33,Citation34
Conclusion
The potential use of PROs in early stage breast cancer as a symptom screening and dialogue tool during follow-up is promising. However, our review reveals limited knowledge of its potential. Of the four studies we found, three were prone to bias and the fourth has limitations general for pilot studies, with low reproducibility and generalizability, since there was only one nurse navigator and a small sample size. We believe PROs could be useful to provide more complete and accurate information in patient–clinician communication, enhancing the quality of dialogue and revealing otherwise undetected symptoms or needs, but this assumption requires testing in more robust studies. If proven, this could lead to better follow-up care and improvements in health-related quality of life. The challenges, which must be overcome to provide more reliable evidence on this matter, include resistance from clinicians and the need to develop user-friendly, easily accessible technology, available for all involved parties. Further investigations in larger-scale studies are needed to understand every aspect of using PROs proactively in followup after treatment for breast cancer.
Supplementary material
Supplementary S1 search string: ((((“Patient Reported Outcome Measures”[Mesh]) OR “Patient Outcome Assessment”[Mesh]) OR “patient reported outcome*”[Title/Abstract])) AND ((“breast cancer”[Title/Abstract]) OR “Breast Neoplasms”[Mesh]).
Disclosure
The authors report no conflicts of interest in this work.
References
- HansenDGLarsenPVHolmLVAssociation between unmet needs and quality of life of cancer patients: a population-based studyActa Oncol201352239139923244672
- EllegaardM-BBGrauCZachariaeRBonde JensenAFear of cancer recurrence and unmet needs among breast cancer survivors in the first five years. A cross-sectional studyActa Oncologica201756231432028093034
- Organisation for Economic Co-operation and DevelopmentThe next generation of health reforms. Ministerial StatementParisOECD2017 Available from: https://www.oecd.org/health/ministerial-statement-2017.pdfAccessed March 21, 2019
- KoolMvan der SijpJRMKroepJRImportance of patient reported outcome measures versus clinical outcomes for breast cancer patients evaluation on quality of careThe Breast201627626827026219
- BaschEAbernethyAPSupporting clinical practice decisions with real-time patient-reported outcomesJ Clin Oncol201129895495621282536
- BaschEDealAMKrisMGSymptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trialJ Clin Oncol201634655756526644527
- Center for Drug Evaluation and ResearchGuidance for Industry, Patient-reported outcome measures: use in medical product development to support labeling claimsSilver Spring, MDCDER2009 Available from: https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdfAccessed March 21, 2019
- HowellDFitchMBakkerDCore domains for a person-focused outcome measurement system in cancer (PROMS-Cancer core) for routine care: a scoping review and Canadian Delphi consensusValue Health2013161768723337218
- CoulterAPotterCMPetersMFitzpatrickRCancer PROMs: a scoping studyMacmillan Cancer Support2015
- OhsumiSShimozumaKCurrent status and future perspectives of patient-reported outcome research in clinical trials for patients with breast cancer in JapanBreast Cancer201320429630122569680
- TevaarwerkAJWangMZhaoFPhase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in pre-menopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology GroupJ Clin Oncol201432353948395825349302
- RibiKLuoWBernhardJAdjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: patient-reported outcomes in the suppression of ovarian function trialJ Clin Oncol201634141601161027022111
- BernhardJLuoWRibiKPatient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (text and soft): a combined analysis of two phase 3 randomised trialsLancet Oncol201516784885826092816
- FerlayJColombetMSoerjomataramIEstimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methodsInt J Cancer201839113
- FallowfieldLJenkinsVPsychosocial/survivorship issues in breast cancer: are we doing better?J Natl Cancer Inst2015107133525432407
- PalmerSCStrickerCTDemicheleAMThe use of a patient-reported outcome questionnaire to assess cancer survivorship concerns and psychosocial outcomes among recent survivorsSupport Care Cancer20172582405241228233121
- GilbertASebag-MontefioreDDavidsonSVelikovaGUse of patient-reported outcomes to measure symptoms and health related quality of life in the clinicGynecol Oncol2015136342943925448486
- FuMRAxelrodDGuthAA Web- and Mobile-Based intervention for women treated for breast cancer to manage chronic pain and symptoms related to lymphedema: randomized clinical trial rationale and protocolJMIR Res Protoc201651e7p26795447
- RushCLDarlingMElliottMGEngaging Latina cancer survivors, their caregivers, and community partners in a randomized controlled trial: Nueva Vida interventionQual Life Res20152451107111825377349
- LinNUThomssenCCardosoFInternational guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)–MBC Task Force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancerBreast201322320321023601761
- ToddBLFeuersteinMGehrkeAHydemanJBeaupinLIdentifying the unmet needs of breast cancer patients post-primary treatment: the Cancer Survivor Profile (CSPro)J Cancer Surviv20159213716025820913
- PatelRAKlasnjaPHartzlerAUnruhKTPrattWProbing the benefits of real-time tracking during cancer careAMIA Annu Symp Proc201220121340134923304413
- PorterIGonçalves-BradleyDRicci-CabelloIFramework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunitiesJ Comp Eff Res20165550751927427277
- StoverAMBaschEMUsing patient-reported outcome measures as quality indicators in routine cancer careCancer2016122335535726619153
- BaschEDealAMDueckACOverall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatmentJAMA2017318219728586821
- BaschEThe rationale for collecting patient-reported symptoms during routine chemotherapyAmerican Society of Clinical Oncology Educational Book201434161165
- TriccoACLillieEZarinWA scoping review on the conduct and reporting of scoping reviewsBMC Medical Research Methodology201616111026728979
- HowellDMolloySWilkinsonKPatient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factorsAnn Oncol20152691846185825888610
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnnals of Internal Medicine2009151426419622511
- SterneJACHernánMaReevesBCSavovićJROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventionsBMJ2016355410
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaborationPLoS Medicine200967e100010019621070
- MertzBGDunn-HenriksenAKKromanNThe effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: a randomized pilot studyActa Oncologica201756121682168928758822
- WheelockAEBockMAMartinELSIS.NET: a randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancerCancer2015121689389925469673
- BockMMooreDHwangJThe impact of an electronic health questionnaire on symptom management and behavior reporting for breast cancer survivorsBreast Cancer Research and Treatment201213431327133522798157
- ThompsonJColemanRColwellBLevels of distress in breast cancer survivors approaching discharge from routine hospital follow-upPsycho-Oncology20132281866187123203833
- WareJESherbournCDThe MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection Author (s): John E. Ware, Jr. and Cathy Donald Sherbourne Published by : Lippincott Williams & Wilkins Stable URL : http://www.jstor.org/stable/3765916 AcMedical Care19923064734831593914
- Reyes-GibbyCCAndersonKOMorrowPKSheteSHassanSDepressive symptoms and health-related quality of life in breast cancer survivorsJ Womens Health2012213311318
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- GroenvoldMKleeMCSprangersMAAaronsonNKAaronsonNKSprangersMirjamAGAaronsonValidation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreementJ Clin Epidemiol19975044414509179103
- Ploos van AmstelFKTolJSessinkKHA specific distress cutoff score shortly after breast cancer diagnosisCancer Nurs2017403E35E4027135753
- PeMDormeLCoensCStatistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic reviewLancet Oncol2018199e459e46930191850
- SnyderCFBlackfordALAaronsonNKCan patient-reported outcome measures identify cancer patients’ most bothersome issues?J Clin Oncol20112991216122021343558
- OberguggenbergerAGoebelGBeerBGetting the whole picture: adding patient-reported outcomes to adjuvant endocrine treatment evaluation in premenopausal breast cancer patientsBreast J201420555555725040193
- SnyderCFBlackfordALOkuyamaTUsing the EORTC-QLQ-C30 in clinical practice for patient management: identifying scores requiring a clinician’s attentionQual Life Res201322102685269123532341
- GanzPAPetersenLBowerJECrespiCMImpact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body studyJ Clin Oncol201634881682426786934
- EllegaardM-BBGrauCZachariaeRJensenABWomen with breast cancer report substantially more disease- and treatment-related side or late effects than registered by clinical oncologists: a cross-sectional study of a standard follow-up program in an oncological departmentBreast Cancer Res Treat2017164372773628536950
- NordanLBlanchfieldLNiaziSImplementing electronic patient-reported outcomes measurements: challenges and success factorsBMJ Qual Saf20182710852856
- GreenhalghJMeadowsKThe effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature reviewJ Eval Clin Pract19995440141610579704
- SchougaardLMVLarsenLPJessenAAmbuFlex: tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseasesQual Life Res201625352553426790427
- CalvertMKyteDDuffyHPatient-reported outcome (PRO) assessment in clinical trials: a systematic review of guidance for trial protocol writersPLoS ONE2014910e11021625333995
- SnyderCFHermanJMWhiteSMWhen using patient-reported outcomes in clinical practice, the measure matters: a randomized controlled trialJ Oncol Practice2014105e299e306
- SmithTGCastroKMTroeschelANThe rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving itCancer2016122334435126619031
- KuijpersWGroenWGOldenburgHSeHealth for breast cancer survivors: use, feasibility and impact of an interactive portalJMIR Cancer201621e328410178
- MinYHLeeJWShinY-WDaily collection of self-reporting sleep disturbance data via a smartphone APP in breast cancer patients receiving chemotherapy: a feasibility studyJ Med Internet Res2014165e13524860070
- AbernethyAPLeBlancPatient-reported outcomes in cancer care - hearing the patient voice at greater volumeNat Rev Clin Oncol2017141276377228975931
- MelissantHCVerdonck-de LeeuwIMLissenberg-WitteBIKoningsIRCuijpersPvan Uden-KraanCF“Oncokompas”, a web-based self-management application to support patient activation and optimal supportive care: a feasibility study among breast cancer survivorsActa Oncol201857792493429451059
- GrafJSimoesEWißlicenKWillingness of patients with breast cancer in the adjuvant and metastatic setting to use electronic surveys (ePRO) depends on sociodemographic factors, health-related quality of life, disease status and computer skillsGeburtshilfe und Frauen-heilkunde20167605535541
- BennettAVJensenREBaschEElectronic patient-reported outcome systems in oncology clinical practiceCA: A Cancer Journal for Clinicians2012625336347
- SnyderCFBlackfordALWolffACFeasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practicePsychooncology201322489590122544513
- SnyderCFAaronsonNKChoucairAKImplementing patient-reported outcomes assessment in clinical practice: a review of the options and considerationsQuality of Life Research20122181305131422048932
- WuAWWhiteSMBlackfordALImproving an electronic system for measuring PROs in routine oncology practiceJ Cancer Survivor-ship2016103573582
- LeblancMStinemanMDemicheleAStrickerCMaoJJValidation of QuickDASH outcome measure in breast cancer survivors for upper extremity disabilityArch Phys Med Rehabil201495349349824095658
- FayanjuOMMayoTLSpinksTEValue-based breast cancer care: a multidisciplinary approach for defining patient-centered outcomesAnnals of Surgical Oncology20162382385239026979306
- JensenRESnyderCFAbernethyAPReview of electronic patient-reported outcomes systems used in cancer clinical careJ Oncol Pract2014104e215e22224301843
- ZhangJYaoY-FengZhaX-MingDevelopment and evaluation of a patient-reported outcome (PRO) scale for breast cancer2015161885738578
- BlackNPatient reported outcome measures could help transform healthcareBMJ20133461f16723358487
- GordonB-BEChenRCPatient-reported outcomes in cancer survivor-shipActa Oncol201756216617328084867
- BerryDLBlumensteinBAHalpennyBEnhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trialJ Clin Oncol20112981029103521282548
- di MaioMBaschEBryceJPerroneFPatient-reported outcomes in the evaluation of toxicity of anticancer treatmentsNat Rev Clin Oncol201613531932526787278