Abstract
Intervertebral disc degeneration remains a pervasive and intractable disease arising from a combination of aging and stress on the back and spine. The growing field of regenerative medicine brings the promise of stem cells in the treatment of disc disease. Scientists and physicians hope to employ stem cells not only to stop, but also reverse degeneration. However, there are many important outstanding issues, including the hostile avascular, apoptotic physiological environment of the intervertebral disc, and the difficulty of obtaining mesenchymal stem cells, and directing them towards chondrocytic differentiation and integration within the nucleus pulposus of the disc. Given the recent advances in minimally invasive spine surgery, and developing body of work on stem cell manipulation and transplantation, stem cells are uniquely poised to bring about large-scale improvements in treatment and outcomes for degenerative disc disease. In this review we will first discuss the cellular and molecular factors influencing degeneration, and then examine the efficacy and difficulties of stem cell transplantation.
Introduction
Two anatomically distinct regions comprise the cartilaginous intervertebral discs (IVD) of the human spine. Concurrently, the nucleus pulposus and annulus fibrosus provide both fluid and viscoelastic support within the IVD. First, the central nucleus pulposus (NP) occupies the internal structure of disc. It is filled with collagen type II extracellular matrix (ECM) and hydrophilic proteoglycans. The unique extracellular matrix of the NP provides “shock absorbing” capacity to the IVD derived from the water content of its components. The NP is enveloped by the second component of the IVD, the annulus fibrosus (AF). The AF is mainly collagen type I, and forms a fibrotic circumferential boundary to the more liquid NP (CitationPaesold et al 2007). Consequently, the AF functions to gird the viscoelastic NP and provide structural integrity and resistance to its extrusion when compressive forces are applied to the NP.
The cellular composition of the IVD forms three separate sections. The concentric lamellae of collagen I fibers of the AF surround the NP. The NP contains two unique types of cells: a population of primitive notochordal cells, and a population of chondrocytic cells. The former are most likely the vestige of an embryonic notochord cell that directed the development of the IVD and spine. They disappear in humans after approximately 10 years of age. This may be due to differentiation into chrondrocytic cells or apoptosis. Both regions of the IVD are bound above and below by endplates of cartilage. Some experts believe that these cells play a role in the IVD niche that provides for successful mesenchymal stem cell (MSC) differentiation into the cells of the NP (CitationHunter et al 2003).
Mechanisms of disc degeneration
To attain the goal of cellular regeneration of the IVD, the nature of cellular degeneration within the IVD must be determined. Most accepted cellular mechanism for IVD degeneration focuses on the NP because of its importance in maintaining a healthy and functioning IVD (). Specifically, the ECM of the NP fails to maintain homeostasis for adequate collagen and proteoglycan synthesis (CitationSive et al 2002). The first step in understanding this phenomenon begins with identifying the molecular phenotype of the NP cells. No marker currently exists to distinguish these cells from common hyaline cartilage cells, since both have similar ECM macromolecules. Furthermore, even if stem cells could be instructed to differentiate into hyaline cartilage, they would still lack the essential fluid properties of the NP, and would fail to recreate normal function of the IVD. Also of tantamount importance is being able to separate the different cells. This could be accomplished first by elucidating the ratio of proteoglycans and collagen within the NP (CitationMwale et al 2004). Eventually, both the repopulation of NP cells and the concomitant reproduction of adequate ECM, with a normal proteoglycan/collagen ratio, must be realized for stem cell regeneration of the IVD to be considered.
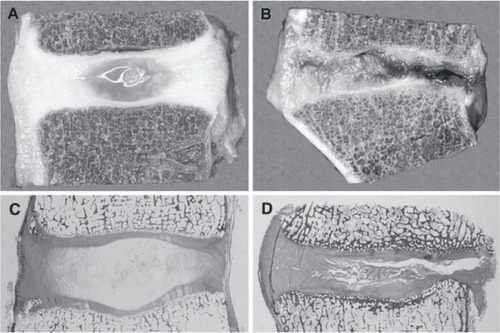
Figure 1 Degenerative changes of the intervertebral disc. Comparison of young and healthy (a, c) and severely degenerated (b, d) discs illustrates that alterations are observed in all anatomical regions of the disc and are obvious on macroscopical (a, b) and histological (c, d) level. Copyright © 2007. Reproduced with permission from CitationPaesold G, Nerlich A, Boos N. 2007. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J, 16:447–68.
Among the culprits to be targeted when considering IVD degeneration are the issues of diffusion of nutrients, cell viability, proteoglycan synthesis, and disruptions in collagen production leading to decreased proteoglycan and collagen II in the IVD disc matrix. Molecular mediators such as degrading enzymes, inflammatory mediators, and growth factors are involved in all the above processes.
Matrix metalloproteinases (MMPs) are the best characterized matrix degrading enzyme, and are major players in IVD degeneration. MMPs degrade various collagens, but their activity is post-transitionally inhibited by the binding of inhibitor of matrix metalloproteinases (TIMPs). The contribution of these enzymes to IVD degeneration most likely results from an imbalance of normal ECM collagen degradation by MMPs and MMP inhibition by TIMPs (CitationLe Maitre et al 2004). Proteoglycan and cathepsins degrading enzymes, such as aggrecanase-1 which targets aggrecan, have also been suspects in IVD degeneration.
Pain manifesting from IVD degeneration may be linked to participating molecular inflammatory mediators. Though heterogeneous models have generally been used to investigate cytokines, they clearly play a role. Some interleukins identified may even recruit MMPs. In the same way, growth factors involved in IVD degeneration, such as transforming growth factor beta (TGF-B), insulin growth factor-1 (IGF-1), and fibroblast growth factor (FGF), have also been examined. These factors function as mitogens that increase rates of mitosis and proliferation of essential IVD components. For instance, TGF-B induces increased proteoglycan production and inhibits MMPs (leading to less ECM degradation) in disc cells (CitationPattison et al 2001). Bone morphogenic proteins (BMPs) produce similar effects as growth factors but are considered morphogenic since they are highly chondrogenic. However, though their function has been delineated, no clear correlation has been seen between factor levels and IVD degeneration. Effects on degeneration varied widely in the presence of different factor levels, although some of this confusion is probably attributable towards the heterogenous assortment of investigations, some of which included extruded discs. Definitive studies remain outstanding, but many hypothesize that increased growth factor expression is a likely physiological response to disc herniation that encourages disc repair.
Transcription factors have also been implicated in degeneration since ECM turnover is also under the direction of genetic regulation. SMADs and latent membrane protein-1 (LMP-1) are intracellular regulators that stimulate proteoglycan and collagen II synthesis by upregulating BMP-2 and BMP-7 (CitationYoon et al 2004). Sox9 is another factor that promotes collagen II expression within the disc by increasing collagen II mRNA transcription (CitationLi et al 2004).
The molecular pathways involved in IVD degeneration are not all the natural consequences of stress and aging, but can also be influenced by genetics. Gene polymorphisms for ECM proteins, like aggrecan, have been associated with disc and early multilevel degeneration (CitationDoege et al 1997). A polymorphism in cartilage intermediate layer protein (CLIP) has also been recently correlated with susceptibility to disc degeneration (CitationSeki et al 2005). Because of the limited innervations and blood supply of the intervertebral discs, the pathology of their degeneration hinges upon the interaction between a wide array of environmental, genetic, and molecular factors. Therefore, the therapeutic strategy will have to be a concerted approach that addresses all these issues.
The aforementioned mechanisms for molecular and cellular degeneration are important components of the clinical presentation of disc degeneration. However, it should be noted that not every patient that has degenerative disc disease has back or leg pain (CitationJensen et al 1994). This underscores the fact that the mechanisms of pain are not well understood. The degenerated IVD is associated with progressive dessication, loss of biomechanical properties, loss of disc height, and in some cases disc herniations (CitationHaefeli et al 2006). These entities must be clinically appreciated because stem cell mediated IVD repair would not obviated the need to remove a herniated disc compressive a spinal root. Current experience and knowledge focus on stem cell mediated IVD repair with the objective of returning the disc ECM to its premorbid state and allowing for the imbibition of water and subsequent return of disc height. This may or may not be associated with pain relief, but currently offers the best cellular strategy for disc repair. In fact, a cellular strategy could be used for multiple aims, not just disc repair, but also the delivery of anti-inflammatory agents to help with the ultimate clinical objective- reduction of pain.
MSCs and the IVD
Originally discovered 30 years ago, mesenchymal stem cells (MSCs) are the elusive vehicle for cell therapy in the IVD (CitationFriedenstein et al 1976). They are valued for their multipotency, or ability to differentiate into cell types of mesenchymal origin (fat, bone, cartilage, etc.), and down the necessary cell lineage for regeneration or replacement of degenerated disc cells (CitationProckop et al 2003). There are two main strategies for acquisition of these desired somatic stem cells: through manipulation of embryonic stem cells (ESCs), or through extraction of MSCs from the fat or bone marrow of the patient.
ESCs are extracted from the inner cell mass of blastocyst stage embryos. The promise of ESCs lies in their plasticity and immortality. These cells are pluripotent, that is, through specialized culture techniques, ESCs can be induced to differentiate into cells of all three germ layers (endoderm, mesoderm, and ectoderm). Indeed, the plasticity of these cells is much greater than any one group of somatic stem cells such as bone marrow derived MSCs. Furthermore, once in culture, ESC lines can be maintained indefinitely, providing an everlasting source of cells for implantation. There is still much to uncover about how to derive chondrocytes or even MSCs from human ESC lines, and the danger of teratoma formation is always present with ESCs (CitationTrounson 2002). Instead of direct MSCs differentiation, recent investigations in the field point towards neural crest stem cells (generated from ESCs) as a source of MSCs, presenting an alternate possibility of a reliable and efficient method of MSCs derivation from human ESCs (CitationLee et al 2007).
Even though ESCs are uniquely versatile, adult MSCs remain the ideal candidate for IVD repair. Readily extracted from adipose tissue or bone marrow, they offer an autologous source of cells with low risk of infection and immunogeniticity. In addition, there is a long history of in vitro manipulation and clinical in vivo investigations for orthopedic trials with these cells (CitationHorwitz et al 2002). Despite the fact that true MSC yield from bone marrow aspirate is less than 0.01%, their proliferative capacity of makes this a sufficient amount for MSC or MSC derived chondrocyte mediated IVD regeneration (CitationPittenger et al 1999). Adipose tissue is also an attractive substitute source of MSCs because of the relative ease in procuring fat over bone marrow. However, some studies have found that the gene expression profiles of bone marrow derived MSCs match that of native cartilage more closely when differentiating into chondrocytes (CitationWinter et al 2003). Bone marrow and adipose derived MSCs also have differing properties when evaluated for cell surface markers (CitationDe Ugarte et al 2003; CitationHuang et al 2005). Thus, until adipose MSCs are more thoroughly investigated and understood, bone marrow MSCs remain the most efficacious option for stem cell IVD therapy.
Preclinical studies of MSC transplantation exist with disc degeneration typically induced with a needle puncture method, and subsequent evaluation of degeneration before and after MSC transplantation. Though this does not accurately represent the complex disease and degeneration process that normally occurs in humans, it is successful in demonstrating the regenerative capabilities of MSCs in the presence of IVD dessication (CitationLeung et al 2006). CitationSakai and colleagues (2006) used a rabbit model of nucleus aspiration to induce degeneration. They injected MSCs embedded in an atelocollagen matrix where they persisted over a month, amplifying the proteoglycan content of targeted discs. Implantation of autogenic MSCs was shown to preserve annular structure, restablishe disc nuclei positive for glycosaminoglycan and keratin sulfate proteoglycans, and partially restore disc height and hydration in similar studies (CitationLeung et al 2006).
MSC derivation
The limiting factor for exploiting stem cells for therapeutic use is obtaining well characterized cells for transplantation. Directing the appropriate differentiation of MSCs (and ESCs) is a complex molecular and cellular puzzle that is contingent upon not only the inherent properties of cells, but also the environment in which they are cultured.
The soluble factors TGF-B and BMP are necessary components of culture media used to induce in vitro chondrogenic differentiation of MSCs. In fact, careful use of soluble factors in media can lead to chondrogenesis with a genetic profile more analogous to IVD tissue than articular cartilage () (CitationSteck et al 2005). Another method of alter MSC microenvironment to trigger chondrogenic differentiation involves co-culturing with different cell populations to take advantage of cell-cell contact and molecular signal activation. Utilizing the autocrine and paracrine factors secreted by one cell type leads to the activation of cell surface receptors on MSCs. Experiments culturing human NP cells and MSCs found that differentiation was reliant on cell-cell contact by looking at gene expression of Sox9, type II collagen, and aggrecan () (CitationRichardson et al 2006).
Figure 2 Quantitative analysis of gene expression levels of selected genes (signal intensity above 15% in IVD tissue, except collagen type X, which was negative). Spheroid cultures of MSCs 2 weeks after TGF-mediated induction (n = 7) were compared with IVD tissue (n = 6) A) and articular cartilage tissue (n = 5) B). The signal intensities were normalized to the gene expression levels of the housekeeping genes on each filter. The medians of independent experiments are shown and expressed as relative values in percent of the housekeeping genes. Copyright © 2005. Reproduced with permission from CitationSteck E, Bertram H, Abel R, et al 2005. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells, 23:403–11.
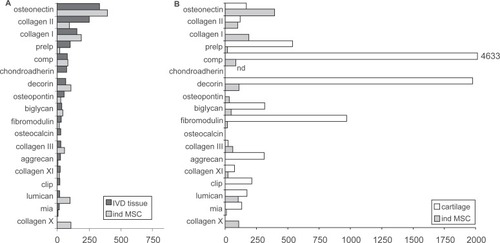
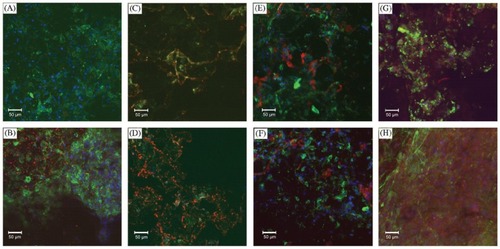
Figure 3 Confocal microscopy images showing deposition of matrix proteins on PLLA scaffolds. Blue is Hoechst of nuclei, green in F-actin and red in specific stain: A) MSC+SM 1 week type II collagen; B) MSC+SOX−9+CM 1 week type II collagen; C) MSC+SM 4 weeks type II collagen; D) MSC+SOX−9+CM 4 weeks type II collagen; E) MSC+SM 1 week type I collagen; F) MSC+SOX−9+CM 1 week type I collagen; G) MSC+SM 1 week aggrecan; and H) MSC+SOX−9+CM 1 week aggrecan. Copyright © 2006. Reproduced with permission from CitationRichardson S, Curran J, Chen R, et al 2006. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-l-lactic acid (PLLA) scaffolds. Biomaterials, 27:4069–78.
The three dimensional properties of the culture system have also shown to exercise substantial influence on the process of cell fate determination. MSCs are pelleted down into a dense micromass before addition of soluble factors to recreate the in vivo state that leads to cartilage formation. This structure helps direct the chrondrogenic cascade of MSC differentiation from micromass into cartilage. Mesenchymal condensation allows for extracellular signaling molecules such as Wnt glycoproteins and N-cadherin to form cadherin and connexin adhesion complexes for the beginning stages of ECM formation. Cartilage then begins to form on this three dimensional scaffold. Plating density of MSCs prior to soluble factor addition also influences the efficiency of differentiation (). This is because plating density can change the cell morphology; specifically, wider spindle shaped cells corresponding with denser MSCs plating. (). Wider cells also have an increased propensity to differentiate after exposure to soluble factors in vitro (CitationSekiya et al 2002). In this way, employing density dependent culturing techniques can produce cartilage formation from MSCs in vitro, and increase the efficiency of MSC differentiation. Another technique described to direct MSCs toward the desired cell fate has been with co-culture systems, where the differentiated cell types provide the autocrine factors to increase the amount of nucleus pulposus cells in vitro (CitationYamamoto et al 2004). Clearly, there are many microenvironmental considerations to be made when designing an optimal in vitro MSC culturing system (CitationLe Visage et al 2006). Exposure to a particular microenvironment may result in physiological variations that can be genetically perpetuated to daughter cells, epigenetically conditioning them to a particular cell fate (CitationGregory et al 2005).
Figure 4 Relation between initial plating density and expansion of MSCs. Passage 3 MSCs (donor 89L) were plated on 60 cm2 dishes at 10, 50, 100, and 1,000 cells/cm2. The cells were harvested and counted at 1 to 12 days. Fold increase A) and total cell numbers per 60 cm2 dish B) are shown. Data are expressed as mean ± standard deviation (n = 3). Copyright © 2002. Reproduced with permission from CitationSekiya I, Larson B, Smith J, et al 2002. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 20:530–41.
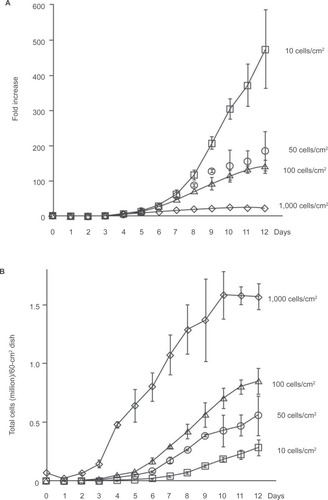
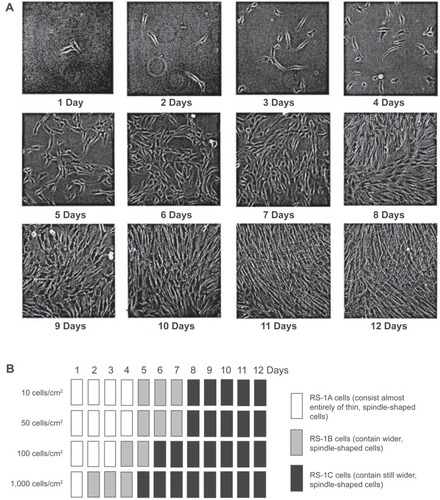
Figure 5 Initial cell density and time in culture affect cell morphology. Passage 3 MSCs were plated at 10, 50, 100, and 1,000 cells/cm2. A) Representative pictures of MSCs plated at an initial cell density of 50 cells/cm2 at 1 to 12 days. B) Schematic diagram of MSC culture morphologies at four different initial cell densities from 1 to 12 days. Copyright © 2002. Reproduced with permission from CitationSekiya I, Larson B, Smith J, et al 2002. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 20:530–41.
MSC implantation to IVD and its options
Stem cell therapy can easily be adapted in the clinical setting (with or without MHC typing) once an appropriate delivery “package” of cells and molecular adjuncts is devised. The objective, with our present understanding, would be to restore disc height by decreasing the dessication of degenerated discs. Although, disc degeneration is associated with back pain, it is clearly not the only contributor. As such, stem cell mediated disc repair would aim to remedy on component of a complex disease entity. Knowledge and experience gained, along with increasing understanding of multifactorial mechaisms of back pain would lay the foundation for further advances. With that noted, the delivery “package” would not only consist of growth factors for effective MSC engraftment, but could also include various biodegradable gels or grafts to provide initial biomechanical support and a structure for biological reconstruction of more severely degenerated discs. Allogenic stem cells could be implanted in minimally degenerated discs via percutaneous techniques. If disc degeneration was more sever, stem cells could be transplanted with minimally invasive surgical and radiological techniques through a true lateral retroperitoneal approached guided by discogram. Employing this minimally invasive approach would also allow for co-implantation of a biodegradable interbody graft, that would be replaced by the engrafted stem cells. Ultimately, the best clinical intervention would incorporate a personalized strategy for each patient and the degree and type of disc degeneration identified. The tools for this strategy would include, but not limited to, cell transplants, biodegradable implants, and internal fixation as needed.
A large number of NP like cells will need to be generated for proficient IVD repair. The NP cell type is not well characterized, but NP cells identified by their production of the requisite ECM components can suffice. Though autologous bone marrow derived MSCs are the ideal candidates for therapy, allogenic MSCs could serve as a more economical substitute. MSCs lack HLA class II receptors and sibling donors should not generate an immune response in recipients (CitationYang 2007). A cocktail of MSCs or differentiated NP cells would have to include molecules that promote the survival and engraftment of transplanted cells, and be rigorously tested in large animal models before clinical trials are undertaken. It will also be imperative to develop approved protocols of MSC culture and differentiation with strict quality controls to ensure safety before application in humans. Once the science and methods of MSC manipulation are better elucidated, IVD regeneration with stem cell therapy could be a viable treatment option for patients. In fact, osteogenesis imperfecta is already being treated effectively with MSCs and presents a model of the potential of MSCs for regenerative medicine. Developing this therapy to the unique cartilaginous tissue of the IVD could be the safest, most efficient application of stem cell biology to disease processes treated by surgeons.
Disclosure
The authors report no conflicts of interest in this work.
References
- De UgarteDAlfonsoZZukP2003Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrowImmunol Lett892677014556988
- DoegeKCoulterSMeekL1997A human-specific polymorphism in the coding region of the aggrecan gene. Variable number of tandem repeats produce a range of core protein sizes in the general populationJ Biol Chem2721397499153261
- FriedensteinAGorskajaJKulaginaN1976Fibroblast precursors in normal and irradiated mouse hematopoietic organsExp Hematol426774976387
- GregoryCYlostaloJProckopD2005Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental niches in culture: a two-stage hypothesis for regulation of MSC fateSci STKE2005pe3716046665
- HaefeliMKalbererFSaegesserD2006The course of macroscopic degeneration in the human lumbar intervertebral discSpine3115223116778683
- HorwitzEGordonPKooW2002Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of boneProc Natl Acad Sci U S A998932712084934
- HuangJKazmiNDurbhakulaM2005Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparisonJ Orthop Res231383915936917
- HunterCMatyasJDuncanN2003The notochordal cell in the nucleus pulposus: a review in the context of tissue engineeringTissue Eng96677713678445
- JensenMBrant-ZawadzkiMObuchowskiN1994Magnetic resonance imaging of the lumbar spine in people without back painN Engl J Med33169738208267
- Le MaitreCFreemontAHoylandJ2004Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral discJ Pathol204475415307137
- Le VisageCKimSTatenoK2006Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesisSpine3120364216915085
- LeeGKimHElkabetzY2007Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cellsNat Biotechnol2514687518037878
- LeungVChanDCheungK2006Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future directionEur Spine J15Suppl 3S4061316845553
- LiYTewSRussellA2004Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9Tissue Eng105758415165474
- MwaleFRoughleyPAntoniouJ2004Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral discEur Cell Mater85863 Discussion, 63–4.15602703
- PaesoldGNerlichABoosN2007Biological treatment strategies for disc degeneration: potentials and shortcomingsEur Spine J164476816983559
- PattisonSMelroseJGhoshP2001Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by transforming growth factor-beta(1)and insulin like growth factor-ICell Biol Int256798911448107
- PittengerMMacKayABeckS1999Multilineage potential of adult human mesenchymal stem cellsScience284143710102814
- ProckopDGregoryCSpeesJ2003One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissuesProc Natl Acad Sci U S A100Suppl 1119172313679583
- RichardsonSCurranJChenR2006The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffoldsBiomaterials2740697816569429
- SakaiDMochidaJIwashinaT2006Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral discBiomaterials273354516112726
- SekiSKawaguchiYChibaK2005A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc diseaseNat Genet376071215864306
- SekiyaILarsonBSmithJ2002Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their qualityStem Cells205304112456961
- SiveJBairdPJeziorskM2002Expression of chondrocyte markers by cells of normal and degenerate intervertebral discsMol Pathol5591711950957
- SteckEBertramHAbelR2005Induction of intervertebral disc-like cells from adult mesenchymal stem cellsStem Cells234031115749935
- TrounsonA2002Human embryonic stem cells: mother of all cell and tissue typesReprod Biomed Online4Suppl 1586312470337
- WinterABreitSParschD2003Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cellsArthritis Rheum484182912571852
- YamamotoYMochidaJSakaiD2004Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: significance of direct cell-to-cell contact in coculture systemSpine2915081415247571
- YangX2007Immunology of stem cells and cancer stem cellsCell Mol Immunol41617117601370
- YoonSParkJKimK2004ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivoSpine2926031115564908