Abstract
Epigenetics is a growing field not only in the area of cancer research but recently in stem cells including human embryonic stem cell (hESC) research. The hallmark of profiling epigenetic changes in stem cells lies in maintaining pluripotency or multipotency and in attaining lineage specifications that are relevant for regenerative medicine. Epigenetic modifications including DNA methylation, histone acetylation and methylation, play important roles in regulating gene expressions. Other epigenetic modifications include X chromosome silencing, genomic stability and imprinting and mammalian development. This review attempts to elucidate the mechanism(s) behind epigenetic modifications and review techniques scientists use for identifying each modification. We also discuss some of the trends of epigenetic modifications in the fields of directed differentiation of embryonic stem cells and de-differentiation of somatic cells.
Embryonic stem cells (ESCs)
ESCs derived from the blastocyst are capable of self renewal and can remain in an undifferentiated state for indefinite passages in vitro and also can be coaxed to differentiate to different lineages (CitationMartin 1981). This makes them the favorable candidate for developing cellular therapies against many degenerative diseases such as those outlined in . Following the first successful derivation of 5 hESC lines by Thomson’s group in 1998 (CitationThomson et al 1998), more new hESC lines have been created (CitationGuo et al 2007; CitationZhang et al 2006; CitationCowan et al 2004; CitationSidhu et al 2008). To date it is estimated, that more than 414 new hESC lines have been produced worldwide and out of which ∼78 are listed on the National Institute Health (NIH) Registry (CitationGuhr et al 2006). Only ∼179 of these lines are characterized to some extent and available for research. Many of these hESC lines are not clonal and are derived under different culture conditions and propagated on different feeder layers (MEF, STO, fetal muscle, skin and foreskin, adult fallopian tube epithelial cells and also some feeder free/serum free systems), hence comparison of these lines are very difficult (CitationAmit et al 2000; CitationCowan et al 2004; CitationAmit et al 2003).
Table 1 A concise list of some degenerative diseases that can be treated with cellular transplantation
Epigenetic modifications play a significant role in maintaining pluripotency in ESCs and at the same time, very relevant in determining the somatic status of terminally differentiated cells. Accordingly, epigenetic profiles of pluripotent genes such as Nanog and OCT4, in ESCs are maintained.
The study of epigenetics – a cell’s epigenome
Gene and protein expression profiling has long been the benchmark in characterizing cell specialization during development. However, recent emphasis is shifted in favor of epigenetic profiling (also referred to as the ‘epigenome’) than genomic profiling as the former is considered to play a significant role in lineage specifications. The study of epigenetics involves covalent modifications to the architectural structure of both chromatin and DNA, but not to the sequence itself. However, there are other players which regulate gene expression such as binding of DNA proteins. These modifications are heritable (CitationEl Kharroubi et al 2001) and often regulate gene expression to a certain extent (CitationLi 2002).
In contrast to gene expression profiles observed in somatic and/or differentiated cells, the ESCs have the potential of activating all gene expression profiles of all cell types from one genome. During mammalian development, almost all cells differentiate without changes to the DNA sequence, however their phenotypes are associated with certain activation (or inactivation) of genes. The differential activation/deactivation of genes depends on the presence and arrangement of functional moieties such as methyl (–CH3) and acetyl (–COCH3) groups, which forms the basis of an ‘epigenetic’ environment around the genomic DNA.
Understanding these complex structures of epigenetic modifications can lead to better understandings of how and when genes are activated or repressed. These patterns are established in early embryonic development and are subject to change throughout development (CitationLi 2002; CitationReik et al 2001).
The mechanism of DNA methylation
Protein expressions determine cell phenotypes that are translated from mRNA transcripts of genomic DNA. Despite gene expression being influenced by changes in DNA sequence (single nucleotide polymorphisms – SNPs, within protein encoding sequences), epigenetic modifications such as DNA methylation can also affect gene expression (CitationHattori et al 2004).
DNA methylation forms an important means of epigenetic modifications and was first detected nearly 60 years ago, using chromatography techniques (CitationHotchkiss 1948). Over the past decade, transcriptional silencing of tumor suppressor genes through abnormal DNA methylation patterns has been established (CitationJones and Laird 1999). DNA methylation is also involved in other cellular processes such as, genomic stability (CitationPeters et al 2001), X chromosome inactivation (CitationMohandas et al 1981), genomic imprinting (CitationEl Kharroubi et al 2001), chromatin structure (CitationJones et al 1998) and mammalian development (CitationReik et al 2001).
In mammalian genomes, DNA methylation occurs exclusively at the 5′ position on a cytosine nucleotide in the context of CG sequences (CitationBird, 2002). CpG islands are regions of DNA where CG nucleotides are present at significantly higher levels than the rest of the genome, these islands often reside at 5′ ends of all housekeeping and many tissue-specific genes – the promoter region (CitationGardiner-Garden and Frommer 1987). This methylated 5′ cytosine can act as a 5th DNA base, as different cytosine methylation status affects gene transcription.
Methylated DNA is often correlated to gene repression (CitationFuks et al 2000), its precise mechanism remains to be elucidated. One such explanation is that transcriptional binding sites become occupied by a group of methyl-CpG binding proteins (MeCP1, MeCP2, MBD1, MBD2) that specifically bind to methylated DNA. This explanation is supported via the findings that the binding of MeCP2, recruits histone deacetylases to repress transcription (CitationJones et al 1998; CitationNan et al 1998; CitationWade 2001; CitationFuks et al 2003). This is also evident that these 2 epigenetic modifications; DNA methylation and histone deacetylations are inter-related. MBD2 is also known to bind with NuRD, forming a complex; MeCP1 which has gene repressive capabilities (CitationFeng and Zhang 2001).
DNA methyl transferases (DNMT) are enzymes that add methyl groups to DNA. In mammals there are 3 common transferases, DNMT1, DNMT3a, DNMT3b. DNMT1 is a methylation maintenance enzyme and targets newly synthesized DNA via its N-terminal regulatory domain which aims for the replicating foci (CitationLeonhardt et al 1992). Somatic cells are believed to preserve their methylation patterns via this manner. DNMT3a/3b are de novo methylation enzymes and are most active during 2 stages of embryonic development following the 2 rounds of global genomic demethylation (CitationMorgan et al 2005).
Methylation patterns are dynamic, as commonly demonstrated in reprogramming studies and they also change as cells develop physiologically (CitationFreberg et al 2007; CitationTakahashi et al 2007). Demethylation occurs spontaneously as previously demonstrated (CitationCervoni and Szyf 2001). Ectopically methylated genes transfected into somatic cells showed demethylating activity. Another study also showed demethylase activity via a mammalian protein which has DNA encoding for a methyl-CpG binding domain is also shown (CitationBhattacharya et al 1999). However demethylation alone is not sufficient to remove the repressive trait of methylation. This encourages scientists to look at epigenetic modifications as a whole, instead of one entity, specifically at histone modifications, as will be discussed later. Mechanisms that prevent de novo CpG methylation are unclear. Several proposed hypotheses include; DNMT3a/3b requires additional CpG binding proteins (CGBP) or other protein interaction. There may also be a constant demethylase activity, as demonstrated in the above mentioned study (CitationCervoni and Szyf 2001).
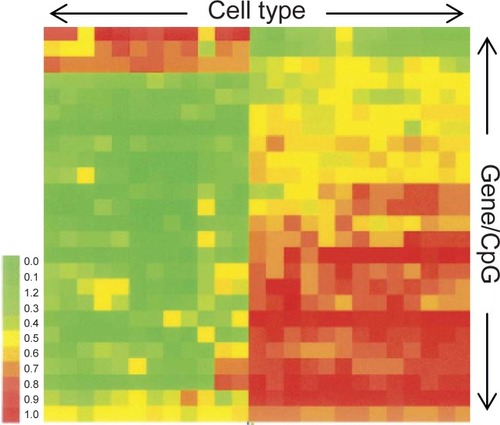
All types of cells would have their own epigenomic signature. This is best depicted using DNA microarrays (for example Illumina’s bead arrays), where an epigenome is expressed in a cluster analysis methylation chart, displaying cell type and CpG methylation levels. A recent study, mapped the methylation status of 23 genes (25 CpG sites) over a range of cell types (CitationBibikova et al 2006) (). From this figure, it is very clear that different cell types are epigenetically (and transcriptionally) divergent from each other, otherwise referred to as being ‘epigenetically unique.’
Figure 1 Methylation analysis chart. Each column represents a different cell type, while each row is a different genes/CpG site. Each cytosine is graded from being heavily methylated (1) to largely unmethylated (0).
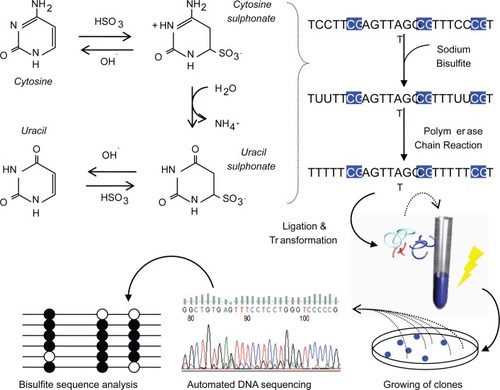
There are many techniques used to study DNA methylation patterns, but the most specific and commonly used, being bisulfite DNA sequencing (CitationFrommer et al 1992). Bisulfite deals structural changes to unmethylated cytosines, while methylated cytosines remain intact. With this change, one can measure the amount of DNA methylation using conventional DNA sequencing procedures (). This is a quantitative measure and is generally correlated to gene expression profiles. A most recent study, mapped the entire genome of Arabidopsis thaliana using a shotgun bisulfite approach using a Next-generation sequencer, which provided a map at single base pair resolution of methylated cytosines (CitationCokus et al 2008).
Figure 2 Process of bisulfite DNA sequencing. Genomic DNA is treated with sodium bisulfite which deals structural and irreversible changes to a cytosine through denaturation, deamination and desulphonation processes. Taking advantage of these changes, the DNA is PCR-amplified and ligated to plasmid vectors for transformation into Escherichia coli. White colonies are hand picked and plasmid DNA extracted for automated DNA sequencing. Closed circles represent methylated CpGs and open represent unmethylated CpGs.
5′aza-2-deoxycytidine (5azadC) is a global DNA demethylation reagent that clips off methyl groups bound on 5′methyl-cytosines. 5azadC is a deoxy- form of 5-azacytidine and is more readily incorporated into DNA. This causes a more efficient inhibition of methylation than the latter (CitationMomparler et al 1984). Its activity is characterized by the covalent trapping of DNMT which depletes the cell of its enzymatic activity (CitationJuttermann et al 1994). 5azadC is commonly used as a DNA methylation inhibitor and its activity has been correlated with gene expression (CitationJones and Taylor 1980; CitationTaylor 1993; CitationJuttermann et al 1994; CitationGrassi et al 2003), cellular differentiation (CitationPinto et al 1989; CitationChoi et al 2004a) and specifically in enhancing hESC differentiation (CitationXu et al 2002).
Maintaining methylation but not de novo methylation is required for in vitro differentiation, as demonstrated previously (CitationJackson et al 2004). Briefly, hypomethylated cell lines with Dnmt3a/3b gene knockouts restored their methylation after stable integration of DNMT1 cDNA transgene.
Although DNA methylation has been intensively studied, many questions remain to be answered, including what mechanisms prevent the de novo methylation of normal somatic cells? And the proteomic network of DNMT remains to be elucidated.
Chromatin remodeling and histone modifications
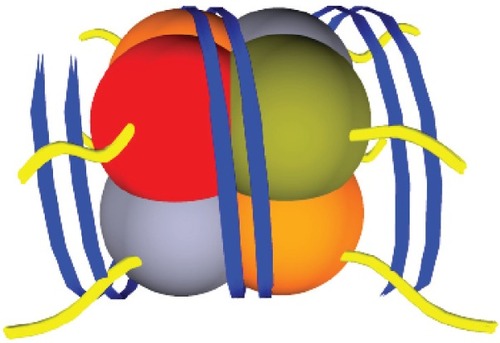
The fundamental unit of chromatin is a nucleosome, which consists of a core of 8 histones; H2A, H2B, H3 and H4 (2 of each). Each core is surrounded by ∼147bp of DNA and is tightly wound around 1.75 turns (). There is increasing evidence that transcriptional factors recognize signals given off by histone tail modifications. As there is an association between DNA and histones, it is not surprising that histone tail modifications (acetylation methylation, ubiquitylation and phosphorylation) also affect gene transcription.
Figure 3 A diagrammatic representation of one chromatin unit. A nucleosome, consisting of 4 histones types; H2A, H2B, H3 and H4 with DNA (blue) tightly wound around the core unit. Histone tails (yellow) protrude from the centre of the histones through the DNA strands (blue).
At the molecular level, the revealing (or hiding) of binding sites that influence gene transcription are outcomes of histone tail modifications. This hiding and revealing of binding sites is determined by overall chromatin structure whether it is relaxed or compact. Acetylation of histone tails removes the positive charge, thus decreasing the affinity between the DNA and histones. This results in a structure called euchromatin and allows easier access of transcriptional factors. In contrast, the result of deacetylation, caused by histone deacetylases (HDACs) is heterochromatin, which results in tightly compacted chromatin and conceals transcriptional DNA binding sites.
Histone tails of H3 holds several amino acids that are notably studied for their correlation with gene expression; these are lysine, arginine, serine and threonine residues. Transcriptionally active genes generally harbors histone H3 lysine 9 acetylation (H3K9ac), H3K4 di-methylation (H3K4me2), tri-methylation (H3K4me3), H3K36me3 and H3K79me3. Transcriptionally repressed genes tend to harbor H3K9me2, H3K9me3, H3K27me3 and histones H4 lysine 20 tri-methylation (H4K20me3) (CitationDahl and Collas 2007; CitationFreberg et al 2007; CitationMaherali et al 2007). Cell populations expressing high levels of gene(s) are generally enriched with euchromatic markers in their promoter regions as demonstrated in pluripotent genes OCT4, NANOG and heterochromatic markers of somatic gene PAX6 of pluripotent undifferentiated carcinoma cells (CitationDahl and Collas 2007). A recent study mapped the histone methylation marks in mouse- ESCs, neural progenitor cells and embryonic fibroblasts and highlighted the impact of H3K4me3 and H3K27me3, on transcriptionally active and inactive genes respectively (CitationMikkelsen et al 2007). Gene promoters which contained both the euchromatic and heterochromatic markers above determine switching cell developmental fates (CitationBernstein et al 2006).
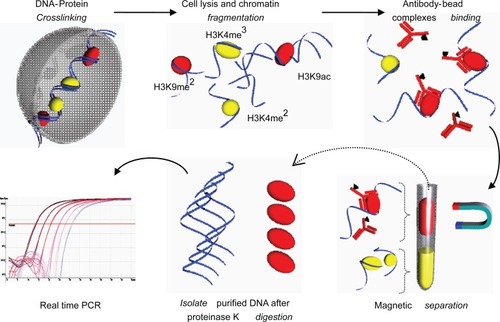
Chromatin immunoprecipitation (ChIP) is a technique used to study chromatin remodeling including histone de/acetylation and de/methylation. Protein-DNA interaction is the basis of this technique and has been used for the past 20 years. Conventional ChIP analysis requires large numbers of starting material; cells and hence, a simplified recipe, Q2ChIP Assay was invented (CitationDahl and Collas 2007). Briefly, cells are cross linked using sodium butyrate prior to lysis and sonication. Cell lysate is immunoprecipitated and reversed cross linked; unbinding of DNA-histone complexes, DNA is then isolated and used for polymerase chain reaction (PCR) assays ().
Figure 4 Quick and Quantitative Chromatin Immuno-precipitation (Q2ChIP). Cells were harvested and treated with sodium butyrate to allow DNA-protein crosslinking. Cells were lysed and sonicated to produce fragments (∼500 bp). Chromatin fragments were allowed to conjugate to ‘antibody-paramagnetic bead complexes’ (specific for H3K9ac). The solution is magnetically separated and purified fragments are reversed crosslinked and subjected to proteinase K digestion. Isolated DNA is now ready for downstream PCR processes.
Recruitment of histone acetyl transferases (HATs) or presence of histone deactylases (HDAC) inhibitor(s) results in histone acetylation (CitationCervoni and Szyf 2001). Hyper-acetylated promoter regions correspond to gene activity (CitationHattori et al 2004). Trichostatin A (TSA) is a commonly used deacetylase inhibitor, and allows re-expression of genes regulated by histone acetylations (CitationCameron et al 1999), methylation (CitationOu et al 2007) and also inducing cellular differentiation (CitationHosseinkhani et al 2007). Other studies have determined common enzymes that demethylate specific lysine residues which generally lead to gene activation/repression (depending on the traits of specific lysine residues): Lysine specific demethylase (LSD1) specifically demethylates H3K4 (CitationShi et al 2004), Ubiquitously transcribed tetratricopeptide repeat, X chromosome (UTX) and Jumonji domain containing 3 (JMJD3) specifically demethylates H3K27 (CitationHong et al 2007).
The increase of histone acetylation is associated with a decrease in global methylation (CitationJackson et al 2004; CitationOu et al 2007) and a gene’s methylated state is determined by the balance between demethylase activity and state of histone acetylation (CitationCervoni and Szyf 2001). Treatment of cells with TSA showed re-expression of some methylation-silenced tumor suppressor genes indicating that methylation is the dominant epigenetic suppressor of densely methylated genes (CitationCameron et al 1999). Another study displays similar results while providing evidence that TSA inhibits DNMT1 (CitationJanuchowski et al 2007), which plays the role of 5azadC (as mentioned earlier); demethylation of tumor suppressor genes (CitationMerlo et al 1995).
Lineage specifications
Recently, the study of epigenetic changes during differentiation of ESCs and de-differentiation of somatic cells has revealed some interesting results. More differentiated cells generally harbor more epigenetic modifications as demonstrated in mouse fibroblast cells (fully differentiated cells), where a combination of Trichostatin A (TSA) and 5′aza-2-deoxycytidine (5azadC), was required for OCT4 re-expression, whereas in trophoblast stem cells (less differentiated cells), either 5′azadC or TSA alone can re-activate OCT4 expression (CitationHattori et al 2004). However, certain cells do retain their ability to switch cell developmental fates through balancing of euchromatic and heterochromatic markers (CitationMikkelsen et al 2007). One exception to the above rule, applies to germ line cells (non-somatic cells), namely – primordial germ cells (PGCs). PGCs undergoes dynamic methylation changes (CitationTrent 2005) which forms the basis of regulating germ cell-specific gene expression (CitationMaatouk et al 2006). Derivatives of PGCs, such as; arrested metaphase II eggs and mature sperm exhibit hypomethylated major and minor satellite sequences whereas in comparison to somatic cells, are hypermethylated (CitationYamagata et al 2007). This is one of the best examples using epigenetic modifications in lineage specification. Somatic cells maintain their gene expressions after mitosis through epigenetic mechanisms, a process often referred to as ‘cellular memory.’ However, the cellular memory present in PGCs remains in a ‘reprogrammable’ state with the potential event of totipotency.
Another example of epigenetic lineage specification include specific CpG methylation of promoter regions as previously demonstrated (CitationTakizawa et al 2001). Briefly, a certain CG found in the STAT3 binding element within the GFAP promoter region of neuroepitheilial cells/post-mitotic neurons is highly methylated and is a sign of GFAP suppression. As these cells differentiate into astrocytes, this level of methylation is decreased resulting in the expression of GFAP (CitationTakizawa et al 2001). Therefore methylation of the STAT3 recognition sequence results in GFAP suppression.
Epigenetic modifications of stem cells
Cellular differentiation
There are 3 major stages of epigenetic modifications during gametogenesis and embryonic development. The first occurs in primordial germ cells, where the imprints of genes are erased, shown by the lack of methylation (CitationSeki et al 2007; CitationMaatouk et al 2006). The second stage involves epigenetic acquisition in maturing gametes, namely oocytes (CitationLucifero et al 2002) and spermatozoan (CitationOakes et al 2007). The third stage occurs during fertilization and usually involves maintenance of methylation in imprinted genes, while other genes gradually lose their methylation (CitationMayer et al 2000; CitationOkamoto et al 2004).
As described above, epigenetic modifications are dynamic throughout cellular differentiation. Many recent studies on directed differentiation of ESCs used epigenetic modifying agents 5azadC and TSA to enhance the efficiency of differentiation. Differentiation of ESCs to cardiomyocytes was enhanced with 5azadC and TSA (CitationXu et al 2002; CitationHosseinkhani et al 2007). A recent report attempts to describe the dynamics of DNA methylation of differentially methylated regions (DMRs) in 5azadC-induced adipocyte differentiation (CitationSakamoto et al 2008). Although a majority of associated genes exhibited no change in methylation profiles, a series of 8 out of 65 tissue-dependent DMRs underwent either methylation, demethylation or were transient, from stem cell to mature adipocytes.
In a recent study it is demonstrated that although TSA does not improve astrocyte differentiation, it assisted in the derivation of neurons with normal electrophysiological membrane properties and elongation of dendrites (CitationBalasubramaniyana et al 2006).
Cellular reprogramming and dedifferentiation
Somatic cell nuclear transfer (SCNT) is a process whereby the derivation of cloned animals (CitationWilmut et al 1997) and donor-matched cell lines (CitationWakayama et al 2005) is made possible. SCNT is an emerging area in stem cell research and involves transferring nuclei of somatic cells into enucleated oocytes with the aim to derive embryonic stem cells with the same genetic makeup as the ‘donor’ somatic cell. From a clinical perspective, such cells, when transplanted, should not be rejected due to incompatible immune response.
SCNT is also useful for studying drug toxicologies in embryonic cell lines derived from a somatic cell’s genome carrying a certain disease(s) such as those listed in . This technique has also been used to generate live cloned offspring in various animal species but not in humans (CitationCibelli et al 1998b; CitationMeng et al 1997; CitationRideout III et al 2000). However, it remains as an inefficient technique. Many factors influencing SCNT efficiency include; donor cell phenotype (), developmental stage of recipient oocytes during nuclear transfer (CitationHall et al 2007), cell cycle stage of donor somatic cell (CitationKasinathan et al 2001), type of zygote/embryo activation method (CitationChoi et al 2004b) and recently, epigenetic modifications including DNA methylation and histone acetylation (CitationKishigami et al 2006).
Table 2 Efficiencies of obtaining live offspring or establishing nt-ESC lines between species across a range of donor cells
Epigenetic studies play a significant role in determining reprogramming during SCNT (CitationByrne et al 2007) and also in a recently developed technology; induced pluripotent stem (iPS) cells, that is, reprogramming somatic cells to pluripotent state by transducing specific pluripotency-associated genes into the somatic cells (CitationTakahashi and Yamanaka 2006; CitationTakahashi et al 2007; CitationWernig et al 2007; CitationYu et al 2007). These studies have analysed DNA methylation and histone modifications to confirm their reprogrammed somatic cells have reverted back into a pluripotent state (and even to totipotent status in mice). These epigenetic analyses along with gene expression patterns are essential to specify and confirm the identity of the pluripotent lineage.
As indicated in , SCNT remains as an inefficient process and many studies are being carried out to improve SCNT efficiency. In a recent study led by Jaenisch (CitationBlelloch et al 2006), it has been demonstrated that less differentiated somatic cells increases SCNT efficiency. It has been shown, by using cells which carry a hypomorphic allele of Dnmt1 (which results in ‘global demethylation’ of the donor genome), higher efficiency could be achieved (65%). This paper may well provide a spark in the future for efficient establishment of nuclear transfer-ESC lines. However higher efficiency of cloning was also achieved by using differentiated cells (namely, granulocytes) compared to that with less differentiated hematopoietic stem cells (CitationSung et al 2006). Conclusions from both these studies, although correct, encourage us to look at the epigenetic nature of donor cells and how certain somatic epigenomes influences SCNT efficiency.
Bisulfite sequencing and Q2ChIP analyses are useful for studying a limited number of genes at one time. However, it is more convenient to use immunocytochemistry to visually observe global epigenetic patterns and/or dynamics within cell populations. Antibodies specific to epigenetic markers (for eg, 5′methyl-cytosines and H3K9 methylation) is an invaluable tool to study the epigenetic dynamics by observing intensities of fluorescence (CitationBeaujean et al 2004; CitationYang et al 2006; CitationSeki et al 2007).
SCNT embryos in animals tend to exhibit aberrant epigenetic makers as compared to those produced with traditional IVF technology These embryos tend to exhibit abnormally higher methylation levels during pre-implantation embryogenesis (CitationDean et al 2001; CitationBeaujean et al 2004). H3K9 acetylation levels in SCNT embryos, on the other hand, were at a lower level than IVF embryos and suggest active histone deacetylase activity. Inhibition of histone deacetylases (HDACs) via trichostatin A, may bring up the levels of acetylation consistent with IVF embryos. These strategies have previously been demonstrated in mice (CitationEnright et al 2003; CitationKishigami et al 2006) and should be adapted in future studies, for efficient reprogramming of donor nuclei, which is believed to be the major cause for SCNT low efficiency.
Conclusions
Epigenetics is an exponentially growing field in ESC research, especially in cellular reprogramming studies. The aim to establish patient-matched ESC lines is currently hindered by the fact that there are aberrant epigenetic modifications during the reprogramming process and needs to be addressed. Epigenetic modifications are dynamic and directed differentiation studies should aim to address these issues, since growth factors and supplements is not sufficient for the directed differentiation of ESCs. Both DNA methylation and histone modifications are inseparable entities when it comes to cellular differentiation and de-differentiation. Epigenetic changes induced by using specific reagents have the prospects of studying both the cellular differentiation and de-differentiation processes as demonstrated in some previous studies.
Disclosures
The authors have no conflicts of interest to declare.
References
- AmitMCarpenterMKInokumaMS2000Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of cultureDev Biol227271811071754
- AmitMMarguletsVSegevH2003Human feeder layers for human embryonic stem cellsBiol Reprod682150612606388
- BalasubramaniyanaVBoddekeaEBakelsaR2006Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cellsNeuroscience1439395117084985
- BeaujeanNTaylorJGardnerJ2004Effect of limited DNA methylation reprogramming in the normal sheep embryo on somatic cell nuclear transferBiol Reprod711859314998909
- BernsteinBEMikkelsenTSXieX2006A bivalent chromatin structure marks key developmental genes in embryonic stem cellsCell1253152616630819
- BetthauserJForsbergEAugensteinM2000Production of cloned pigs from in vitro systemsNat Biotech18105559
- BhattacharyaSKRamchandaniSCervoniN1999A mammalian protein with specific demethylase activity form CpG DNANature3975798310050851
- BibikovaMChudinEWuB2006Human embryonic stem cells have a unique epigenetic signatureGenome Res1610758316899657
- BirdA2002DNA methylation patterns and epigenetic memoryGenes Dev1662111782440
- BlellochRWangZDMeissnerA2006Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleusStem Cells2420071316709876
- BlellochRHHochedlingerKYamadaY2004Nuclear cloning of embryonal carcinoma cellsProc Natl Acad Sci U S A101139859015306687
- ByrneJAPedersenDAClepperLL2007Producing primate embryonic stem cells by somatic cell nuclear transferNature45049750218004281
- CameronEEBachmanKEMyöhänenS1999Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancerNat Genet2110379916800
- CervoniNSzyfM2001Demethylase activity is directed by histone acetylationJ Biol Chem276407788711524416
- ChenHFKuoHCChienCL2007Derivation, characterization and differentiation of human embryonic stem cells: Comparing serum-containing versus serum-free media and evidence of germ cell differentiationHum Reprod225677717071820
- ChoS-KKimJ-HParkJ-Y2007Serial cloning of pigs by somatic cell nuclear transfer:Restoration of phenotypic normality during serial cloningDev Dyn23633698217849457
- ChoiSCYoonJYShimWJ2004a5-azacytidine induces cardiac differentiation of P19 embryonic stem cellsExp Mol Med365152315665584
- ChoiYHLoveLBWesthusinME2004bActivation of equine nuclear transfer oocytes:methods and timing of treatment in relation to nuclear remodelingBiol of Reprod70465312954733
- CibelliJBSticeSLGoluekePJ1998aTransgenic bovine chimeric offspring produced from somatic cell-derived stem-like cellsNat Biotech166426
- CibelliJBSticeSLGoluekePJ1998bCloned transgenic calves produced from nonquiescent fetal fibroblastsScience280125689596577
- CokusSJFengSZhangX2008Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterningNature4522151918278030
- CowanCAKlimanskayaIMcMahonJ2004Derivation of embryonic stem-cell lines from human blastocystsNew Engl J Med3501353614999088
- DahlJACollasP2007Q2ChIP, a quick and quantitative chromatin immunoprecipitation assay, unravels epigenetic dynamics of developmentally regulated genes in human carcinoma cellsStem Cells2510374617272500
- DeanWSantosFStojkovicM2001Conservation of methylation reprogramming in mammalian development:Aberrant reprogramming in cloned embryosProc Natl Acad Sci U S A9813734811717434
- El KharroubiAPirasGStewartCL2001DNA demethylation reactivates a subset of imprinted genes in uniparental mouse embryonic fibroblastsJ Biol Chem27686748011124954
- EnrightBPKubotaCYangX2003Epigenetic characteristics and development of embryos cloned from donor cells treated by trichostatin A or 5-aza-2’-deoxycytidineBiol Reprod6989690112748129
- FengQZhangY2001The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomesGenes Dev158273211297506
- FrebergCTDahlJATimoskainenS2007Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extractMol Biol Cell1815435317314394
- FrenchAJAdamsCAAndersonLS2008Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblastsStem Cells264859318202077
- FrommerMMcDonaldLEMillarDS1992A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strandsProc Natl Acad Sci U S A891827311542678
- FuksFBurgersWABrehmA2000DNA methyltransferase Dnmt1 associates with histone deacetylase activityNat Genet24889110615135
- FuksFHurdPJWolfD2003The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylationJ Biol Chem27840354012427740
- Gardiner-GardenMFrommerM1987CpG Islands in vertebrate genomesJ Mol Biol196261823656447
- GibbonsaJAratSRzucidloJ2002Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cellsBiol Reprod6689590011906906
- GrassiGMaccaroniPMeyerR2003Inhibitors of DNA methylation and histone deacetylation activate cytomegalovirus promoter-controlled reporter gene expression in human glioblastoma cell line U87Carcinogenesis2416253512869421
- GuhrAKurtzAFriedgenK2006Current state of human embryonic stem cell research: an overview of cell lines and their usage in experimental workStem Cells117
- GuoHWei-qiangLRuiC2007Establishment and characterization of two new human embryonic stem cell lines, SYSU-1 and SYSU-2Chinese Med J12058994
- HallVJComptonDStojkovicP2007Developmental competence of human in vitro aged oocytes as host cells for nuclear transferHum Reprod22526216957049
- HamanoSWatanabeYAzumaS1998Establishment of embryonic stem ES. cell-like cell lines derived from bovine blastocysts obtained by in vitro culture of oocytes matured and fertilized in vitroJ Reprod Dev44297303
- HattoriNNishinoKY-gKo2004Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cellsJ Biol Chem27917063914761969
- HeindryckxBSutterPDGerrisJ2007Embryo development after successful somatic cell nuclear transfer to in vitro matured human germinal vesicle oocytesHum Reprod2219829017513316
- HiiraguiTSolterD2005Reprogramming is essential in nuclear transferMol Reprod Dev704172115685639
- HongSChoY-WYuL-R2007Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylasesProc Natl Acad Sci U S A1041843944418003914
- HosseinkhaniMHasegawaKOnoK2007Trichostatin A induces myocardial differentiation of monkey ES cellsBiochem Biophys Res Commun3653869117996726
- HotchkissRD1948The quantitative separation of purines, pyrimidines and nucleosides by paper chromatographyJ Biol Chem1753153218873306
- JacksonMKrassowskaAGilbertN2004Severe global DNA hypomethylation blocks differentiation and induces histone hyperacetylation in embryonic stem cellsMol Cell Biol2488627115456861
- JanuchowskiRDąbrowskiMOforiH2007Trichostatin A down-regulates DNA methyltransferase 1 in Jurkat T cellsCancer Lett246313716624484
- JonesPALairdPW1999Cancer-epigenetics comes of ageNat Genet2116379988266
- JonesPATaylorSM1980Cellular differentiation, cytidine analogs, and DNA methylationCell2085936156004
- JonesPLVeenstraGJCWadePA1998Methylated DNA and MeCP2 recruit histone deacetylase to repress transcriptionNat Genet19187919620779
- JuttermannRLiEJaenischR1994Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of dna methyltransferase rather than DNA demethylationProc Natl Acad Sci U S A91117978017527544
- KasinathanPKnottJGWangZD2001Production of calves from G1 fibroblastsNat Biotech1911768
- KishigamiSMizutaniEOhtaH2006Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transferBiochem Biophys Res Commun340183916356478
- LeeJ-WWuS-CTianXC2003Production of cloned pigs by whole-cell intracytoplasmic microinjectionBiol Reprod69995100112773418
- LeonhardtHPageAWWeierH-U1992A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nucleiCell71865731423634
- LiE2002Chromatin modification and epigenetic reprogramming in 1 developmentNat Rev Genet36627312209141
- LiMLiY-HHouY2004Isolation and culture of pluripotent cells from in vitro produced porcine embryosZygote1243815214579
- LuciferoDMertineitCClarkeHJ2002Methylation dynamics of imprinted genes in mouse germ cellsGenomics79530811944985
- MaatoukDMKellamLDMannMRW2006DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineagesDevelopment13334111816887828
- MaheraliNSridharanRXieW2007Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contributionCell Stem Cell1
- MaiQYuYLiT2008Derivation of human embryonic stem cell lines from parthenogenetic blastocystsCell Res1710081918071366
- MartinGR1981Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cellsProc Natl Acad Sci U S A78763486950406
- MayerWNiveleauAWalterJ2000Demethylation of the zygotic paternal genomeNature403501210676950
- MengLElyJJStoufferRL1997Rhesus monkeys produced by nuclear transferBiol Reprod5745499241063
- MerloAHermanJGMaoL19955′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancersNature Med1686927585152
- MikkelsenTSKuMJaffeDB2007Genome-wide maps of chromatin state in pluripotent and lineage-committed cellsNature4485536017603471
- MitalipovSYeomanRRNusserKD2002Rhesus monkey embryos produced by nuclear transfer from embryonic blastomeres or somatic cellsBiol Reprod6613677311967199
- MitalipovSMNusserKDWolfDP2001Parthenogenetic activation of rhesus monkey oocytes and reconstructed embryosBiol Reprod65253911420247
- MitalipovaSMZhouQByrneJA2007Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodelingHuman Reproduction2222324217562675
- MohandasTSparkesRSShapiroLJ1981Reactivation of an inactive human X chromosome:evidence for X inactivation by DNA methylationScience21139366164095
- MomparlerRLMomparlerLFSamsonJ1984Comparison of the antileukemic activity of 5-aza-2′-deoxycytidine, 1-beta-d-arabinofuranosylcytosine and 5-azacytidine against l1210 leukemiaLeukemia Res8104396083417
- MorganHDSantosFGreenK2005Epigenetic reprogramming in mammalsHum Mol Genet144758
- NanXNgHHJohnsonCA1998Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complexNature39338699620804
- OakesCCSalleSLSmiragliaDJ2007Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cellsDev Biol3073687917559830
- OkamotoIOtteAPAllisCD2004Epigenetic dynamics of imprinted X inactivation during early mouse developmentScience303644914671313
- OnishiAIwamotoMAkitaT2000Pig cloning by microinjection of fetal fibroblast nucleiScience28911889010947985
- OuJ-NTorrisaniJUnterbergerA2007Histone deacetylase inhibitor trichostatin A induces global and gene-specific DNA demethylation in human cancer cell linesBiochem Pharm73129730717276411
- PetersAHFMO’CarrollDnScherthanH2001Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stabilityCell1073233711701123
- PintoAZagonelVAttadiaV19895-Aza-2-deoxycytidine induces terminal differentiation of leukemic blasts from patients with acute myeloid leukemiasBone Marrow Transplant328322483349
- PolejaevaIAChenS-HVaughtTD2000Cloned pigs produced by nuclear transfer from adult somatic cellsNature407869010993078
- ReikWDeanWWalterJ2001Epigenetic reprogramming in mammalian developmentScience29310899311498579
- RevazovaESTurovetsNAKochetkovaOD2007Patient-specific stem cell lines derived from human parthenogenetic blastocystsCloning Stem Cells9118
- RideoutWMIIIWakayamaTWutzA2000Generation of mice from wild-type and targeted ES cells by nuclear cloningNat Genet241091010655052
- PratherRSSimsMFirstN1989Nuclear transplantation in early pig embryosBiol Reprod4141482590712
- SakamotoHKogoaYOhganeJ2008Sequential changes in genome-wide DNA methylation status during adipocyte differentiationBiochem Biophys Res Comm366360618062916
- SekiYYamajiMYabutaY2007Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in miceDevelopment13426273817567665
- ShaoHWeiZWangL2007Generation and characterization of mouse parthenogenetic embryonic stem cells containing genomes from non-growing and fully grown oocytesCell Biol Int3113364417601752
- ShiYLanFMatsonC2004Histone demethylation mediated by the nuclear amine oxidase homolog LSD1Cell1199415315620353
- SidhuKSRyanJPTuchBE2008Derivation of a new hESC line, Endeavour-1 and its clonal propagationStem Cells Dev17415218271699
- SuemoriHTadaTToriiR2001Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSIDev Dyn222273911668604
- SungLYGaoSShenH2006Differentiated cells are more efficient than adult stem cells for cloning by somatic cell nuclear transferNat Genet381323817013394
- TakahashiKTanabeKOhnukiM2007Induction of pluripotent stem cells from adult human fibroblasts by defined factorsCell1318617218035408
- TakahashiKYamanakaS2006Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell126652516923385
- TakizawaTNakashimaKNamihiraM2001DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brainDev Cell17495811740937
- TaylorSM19935-Aza-2′-deoxycytidine: cell differentiation and DNA methylationLeukemia7387683353
- ThomsonJAItskovitz-EldorJShapiroSS1998Embryonic stem cell lines derived from human blastocystsScience2821145479804556
- TrentRJ2005Molecular MedicineUSAElsevier Academic Press
- WadePA2001Methyl CpG-binding proteins and transcriptional repressionBioessays231131711746232
- WakayamaSOhtaHKishigamiS2005Establishment of male and female nuclear transfer embryonic stem cell lines from different mouse strains and tissuesBiol Reprod72932615601921
- WalkerSCShinTZaunbrecherGM2002A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytesCloning Stem Cells41051212171703
- WangLDuanESungLY2005Generation and characterization of pluripotent stem cells from cloned bovine embryosBiol Reprod731495515744021
- WellsDNLaibleGTuckerFC2002Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattleTheriogenology59455912499017
- WernigMMeissnerAForemanR2007In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like stateNature4483182417554336
- WilmutISchniekeAEMcWhirJ1997Viable offspring derived from fetal and adult mammalian cellsNature38581039039911
- WolfDPThormahlenSRamseyC2004Use of assisted reproductive technologies in the propagation of rhesus macaque offspringBiol Reprod714869315044263
- XuCHPoliceSRaoN2002Characterization and enrichment of cardiomyocytes derived from human embryonic stem cellsCirculation Res91501812242268
- YamagataKYamazakiTMikiH2007Centromeric DNA hypomethylation as an epigenetic signature discriminates between germ and somatic cell lineagesDev Biol3124192617964565
- YamazakiWFerreiraCRMéoSC2005Use of strontium in the activation of bovine oocytes reconstructed by somatic cell nuclear transferZygote1329530216388697
- YangJYangSBeaujeanN2007aEpigenetic marks in cloned rhesus monkey embryos:comparison with counterparts produced in vitroBiol Reprod76364217021347
- YangX-yLiHMaQ-w2006Improved efficiency of bovine cloning by autologous somatic cell nuclear transferReproduction132733917071774
- YangXZSmithSLTianXC2007bNuclear reprogramming of cloned embryos and its implications for therapeutic cloningNat Genet3929530217325680
- YongHYHaoYLaiL2006Production of a transgenic piglet by a sperm injection technique in which no chemical or physical treatments were used for oocytes or spermMol Reprod Dev73595916489622
- YuJVodyanikMASmuga-OttoK2007Induced pluripotent stem cell lines derived from human somatic cellsScience31819172018029452
- ZhangXStojkovicPPrzyborskiS2006Derivation of human embryonic stem cells from developing and arrested embryosStem Cells2426697616990582
- ZhouQYangSHDingCH2006A comparative approach to somatic cell nuclear transfer in the rhesus monkeyHum Reprod2125647116793991