Abstract
Primary hepatocellular carcinoma (HCC) is a common malignancy that has a poor prognosis because it is often diagnosed at an advanced stage. HCC normally develops as a consequence of underlying liver disease and is most often associated with cirrhosis. Surgical resection and liver transplantation are the current best options to treat liver cancer. However, problems associated with liver transplantation, such as shortage of donors, risk of immune rejection, and tissue damage following surgery provided the impetus for development of alternative therapies. The emerging field of stem cell therapy has raised hopes for finding curative options for liver cancer. Stem cells have the ability not only to proliferate after transplantation but also to differentiate into most mammalian cell types in vivo. In this review, progress on stem cell-derived technologies for the treatment of liver cancer is discussed.
Introduction
Primary liver cancer can be classified into one of several histologically different hepatic malignancies, including hepatocellular carcinoma (HCC), cholangiocarcinoma, hepatoblastoma, and hemangiosarcoma.Citation1 Of these, HCC is the most common liver cancer, accounting for 70%–85% of cases, with nearly 700,000 deaths occurring worldwide each year.Citation2,Citation3 Recurrence or metastasis is quite common in patients who have had a resection, and survival rate is only 30%–40% at five years after surgery.Citation4 A variety of risk factors may cause HCC which vary according to geographical region,Citation5 and susceptibility to disease depends upon the host’s genetic factors and pre-existing health conditions.Citation6
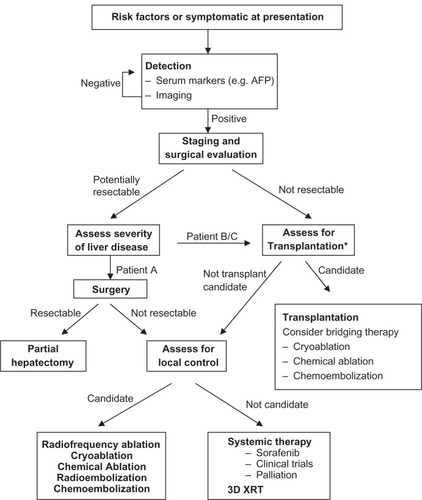
During the past decade, advances in imaging techniques such as magnetic resonance imaging and contrast-enhanced ultrasonography with real-time low mechanical index harmonic imaging ultrasound equipment have allowed us to perform noninvasive diagnosis of HCC and replaced the need for invasive procedures like ultrasound-guided biopsy or angiography.Citation7 Currently, in addition to standard chemotherapy, a number of treatment options are being used in the clinical setting.Citation8 They include surgical resections such as enucleation, segmentectomy and lobectomy, percutaneous interventions such as hyperthermia and cryotherapy, transarterial interventions, and various embolization methods (chemical, thermochemical, and others). The treatment regimen that is currently being administered is shown in . In addition to these treatment methods, efforts are being made to target cellular signaling pathways involved in HCC to suppress the tumors. Putative drug candidates for these studies include small-molecule protein kinase inhibitors, monoclonal antibodies, and antibiotics, either alone or in combinations.Citation8,Citation9 The most effective candidate among them is sorafenib, which showed a modest, three-month survival benefit in some patients.Citation9 At present, sorafenib is the only drug approved by the Food and Drug Administration in the US for liver cancer. Prior to this, over 100 clinical trials of intravenous (IV) chemotherapy failed to show any statistically significant survival benefit in patients with HCC.Citation10 For those patients fortunate enough to be identified, surgery is potentially curative. The shortage of donor livers for transplantation, however, has led to the development of techniques such as split liver transplantation and living donor transplantation.Citation11 Currently, efforts are geared towards developing cell-based therapies where hepatocytes and stem/progenitor cells will be used for transplantation into damaged and diseased livers. Such transplanted cells could proliferate and repopulate the liver and eventually restore its functions.
Figure 1 A general strategy for management of hepatocellular carcinoma based on tumor burden and the degree of liver disease. This algorithm depicts various treatment options that are used in the clinic depending upon the disease severity and the patient’s previous medical history.
Abbreviations: AFP, alfa-fetoprotein; 3D XRT, three-dimensional conformal radiotherapy.
Liver stem cells
The liver is the only organ in the human body that is capable of renewing itself following the loss of the natural tissue. Even after 70% hepatectomy, this remarkable regenerative capacity is achieved due to the proliferation of hepatocytes and cholangiocytes, and other hepatic cells such as stellate cells, macrophages, and endothelial cells.Citation12 Under special circumstances, stem/progenitor cells and bone marrow (BM) cells also contribute to this regeneration process.Citation13 Stem/progenitor cells are critical to the tissue restoration process because they are bipotent and can differentiate into the two primary cell types of the liver, ie, hepatocytes and biliary ductal cells (cholangiocytes). In addition to their role in liver regeneration, stem/progenitor cells are important for studies of organogenesis and liver development.Citation5,Citation14,Citation15
To date, a number of different types of stem/progenitor cells have been successfully isolated from healthy and injured livers (adult and fetal) as well as from liver tumors.Citation16–Citation32 These include cells of human, rodent, canine, swine, and simian origins (). One such population of human liver stem cells was recently shown to contribute to the generation of liver parenchyma in severe combined immunodeficient mice.Citation33 Among liver stem cells, the human hepatic progenitor cells (HPCs) are the best studied. Apart from adult and fetal livers,Citation15,Citation34–Citation36 they have been isolated and characterized from liver specimens with severe hepatocellular necrosis, chronic viral hepatitis, and chronic alcoholic liver disease.Citation37 In normal adult liver, these cells are localized in biliary ductules (canals of Hering).
Table 1 Liver stem/progenitor cells
The rodent counterparts of HPCs are termed “oval cells” because of their characteristic ovoid nucleus.Citation38–Citation41 They are bipotential,Citation42 and express cell surface markers such as Thy1.1, CD34, Flt3-receptor, and c-kit.Citation43 They also produce alfa-fetoprotein (AFP), cytokeratin 19, and γ-glutamyl-transferase, and stain positive for one-cut 2 transcription factor (OC2), ovalbumin (OV-6), and BD1 when treated with monoclonal antibodies. Oval cells are normally isolated from the liver following treatment with agents such as 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), or from animals fed on choline-deficient diets and treated with 2-acetylaminofluorene (2-AAF).Citation44,Citation45 They proliferate in vivo following liver damage when the hepatocytes can no longer divide.Citation46 In diseases such as alcoholic liver disease and during hepatitis C virus (HCV) infection, their numbers increase and correlate with the severity of the disease.Citation47
Recently, Liu et al isolated epithelial progenitors from fetal rat livers that were able to divide in cell culture and express liver epithelial and biliary-specific markers.Citation48 Upon differentiation in vitro, they also expressed albumin, CK-18 and AFP, and two weeks after transplantation into animals they were shown to display hepatocyte-like features in vivo.Citation48 It has been proposed that certain epithelial cells in fetal and injured adult livers undergo epithelial-mesenchymal transition and home to liver parenchyma, and some of these cells may be capable of reversing this transition and become hepatocytes or cholangiocytes.Citation49 This phenomenon of transition has been reported in a number of human diseases.Citation50
Extrahepatic sources of liver stem cells
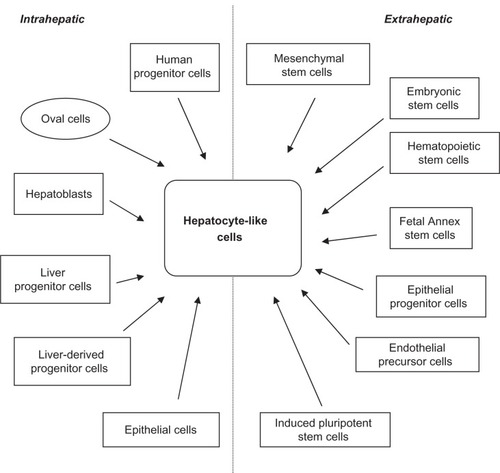
In addition to these liver-derived stem/progenitor cells, many studies have been published demonstrating that cells of nonliver origin could also differentiate into “hepatocyte-like” cells (). These cells could be a valuable source of hepatocytes and cholangiocytes. It is beyond the scope of this review to discuss every stem cell type that has been described, but those that have been well studied and successfully differentiated into hepatic cell types will be highlighted.
Figure 2 Intra- and extrahepatic sources of stem cells from different origins that have been demonstrated to differentiate into hepatocyte-like cells.
Bone-marrow derived cells
Mesenchymal stem cells (MSC) are multipotent stem cells derived from BM aspirates. They can be expanded readily in cell culture and can be induced to differentiate into many different cell types, including hepatic cells.Citation51 These in vitro differentiated cells can express hepatocyte markers and possess hepatocyte-specific biochemical activities such as albumin secretion, urea production, and glycogen storage.Citation52,Citation53 It has been suggested that these BM-derived cells fuse with damaged hepatocytes after transplantation and change their gene expression patterns to that of mature hepatocytes.Citation41,Citation54 Furthermore, implanted MSCs protect the liver by secreting soluble factors that possess antiapoptotic and promitotic properties, as shown in experiments with rats where hepatic failure was caused by D-galactosamine.Citation55 In support of these studies, Lagasse et al showed that transplanted MSCs could differentiate into mature hepatocytes in vivo and rescue fumarylacetoacetate hydrolase-deficient mice.Citation56 Transplanted MSCs were also demonstrated to restore liver function in albumin urokinase transgenic mice and in hepatitis B virus (HBV) transgenic mice.
Hematopoietic stem cells (HSC) are another type of stem cell present in the BM. They have the potential to differentiate into liver cells, both in vitro and in vivo, without fusion and could contribute to liver regeneration.Citation57 A comparative study of the hepatocyte differentiation capacity of HSCs and MSCs in a rat model has shown that MSCs are more potent than HSCs in differentiating into hepatocytes.Citation58
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from the inner mass of an early-stage embryo known as the blastocyst.Citation59 They are pluripotent and can be maintained in cell culture for prolonged periods of time without disturbing their developmental potential.Citation60 In the early studies, embryonic stem cells from murine embryos were shown to differentiate into functional hepatocytes in vitro.Citation61–Citation63 Later it was shown that both murine- and human BM-derived MSCs could differentiate into hepatocytes both in vitro and in vivo.Citation52,Citation64 Several studies have shown that ESCs could be induced to differentiate into liver cells. For example, Hay et al differentiated human ESCs into hepatocyte-like cells by treating them with hepatocyte growth factor,Citation65 and Ishizaka et al managed to differentiate mouse ESCs into hepatocytes by treating them with hepatocyte nuclear factor-3β.Citation66 Studies with BM transplant recipients have shown that these cells could home to liver and differentiate into normal hepatocytes.Citation67,Citation68 Because of major ethical concerns with the use and handling of ESCs, and due to difficulties in controlling their robust proliferative and differentiation potential, their use is currently limited to in vitro studies and to animal models.
Fetal annex stem cells
Umbilical cord blood contains multiple populations of pluripotent stem cells. Each of these populations can serve as a source of hepatocytes for liver regeneration. For instance, mesenchymal stromal cells isolated from the umbilical cord could be induced to differentiate into hepatocyte-like cells in cell culture after treating them with hepatocyte growth factor and fibroblast growth factor-4.Citation69 Such differentiated cells express CK-18, AFP, and albumin. Similarly, umbilical cord matrix stem cells could also differentiate into hepatocyte-like cells.Citation70 In addition to these cells, human mononuclear cells from umbilical cord blood could differentiate into functional hepatocytes when transplanted in utero into fetal ratsCitation71 and into carbon tetrachloride (CCl4)-injured mouse liver.Citation72
Placenta-derived stem cells are another source of hepatocytes. They can be expanded in cell culture for more than 20 populations.Citation73 Recently, Chien et al cultured these cells from human placentae, differentiated them into hepatocytes, and examined their hepatocyte-specific functions.Citation74 When compared with ESCs, there are no ethical issues involved in using these cells because the collection of placentae does not harm either the human mother or the infant.Citation73
Induced pluripotent stem cells
The induced pluripotent stem cell (iPS) is an inducible cell type that can be generated by epigenetic reprogramming following induced expression of certain transcription factors. Takahashi and Yamanaka demonstrated this in a landmark study where they overexpressed four transcription factors Oct-3/4, Sox2, c-Myc, and Klf4 in mouse fibroblasts using a retrovirus to generate first iPS cells.Citation75 In subsequent studies, Yamanaka et al and Yu et al demonstrated the generation of iPS cells from murine and human somatic cells.Citation76,Citation77 Recently, Si-Tayeb et al and Song et al reported the generation of human hepatocyte-like cells from iPS cells obtained from human ESCs.Citation78,Citation79 Hepatocyte-like cells produced in this procedure expressed various hepatic markers such as HNF-4a, FoxA2, AFP, and secreted albumin. Aoi et al isolated pluripotent stem cells from adult mouse liver and generated iPS cells by expressing these four transcription factors from a retrovirus in adult mouse hepatocytes and gastric epithelial cells.Citation80 This finding suggests that functional hepatocytes and other liver cells can be reprogrammed to generate stem cells.
Endothelial progenitor/precursor cells
Endothelial precursor cells (EPCs) constitute a cell type that has the potential to differentiate into mature functional endothelial cells that form a capillary or line the lumen of a blood vessel.Citation81 They were first isolated from a population of CD34+ peripheral mononuclear blood cells of the BM.Citation82 Subsequently, they have been isolated from sources other than BM, such as the vessel wall.Citation83 It has been reported that EPCs could differentiate into hepatocytes when cultured in hepatic differentiation media.Citation84
Others
The adipose tissue contains MSCs that are multipotent and can be differentiated into functional hepatocyte-like cells by treatment with a cocktail of cytokines.Citation85 In a recent study it was shown that, after transplantation, these human adipose tissue cells get incorporated into the liver parenchyma after transplantation into mice.Citation86 After differentiation, they express a number of hepatocyte markers and biologic activities of liver cells. Recently, Ikeda et al showed that tooth germ progenitor cells derived from adipose tissue could prevent liver fibrosis and improve liver function after transplantation in rodents with liver injury caused by CCl4.Citation87 Other adipose tissue cells, such as salivary gland progenitor cells, differentiate into hepatocyte-like cells and express alpha-1 antitrypsin and albumin after transplantation into the murine liver via the portal vein.Citation88 A novel cell type, the liver-derived progenitor cell, was recently discovered and isolated from healthy, uninjured rat livers.Citation19
Based on these numerous reports of successful in vitro and in vivo hepatocyte-like differentiation of stem/progenitor cells isolated from different organs, it is predictable that other stem cells, such as amniotic epithelial cellsCitation89 and very small embryonic-like stem cellsCitation90 might also be induced to produce cells of hepatocyte and biliary lineages.
Role of stem cells in liver cancer
In the normal adult liver, there is little proliferation. However, following partial hepatectomy or injury, hepatocytes proliferate rapidly and repopulate the liver to restore its physical mass and physiologic functions. Initial experiments with rodents have demonstrated the ability of hepatocytes to proliferate and to populate the liver mass after partial hepatectomyCitation91,Citation92 The data regarding the ability of human hepatocytes to undergo extensive cell division came from studies on chronic hepatitis with HCV and HBV In the livers of patients with chronic viral hepatitis, there is an ongoing excessive hepatocyte death and compensatory proliferation. Based on the staining for cell proliferation markers, such as proliferating cell nuclear antigen and Ki-67, it was estimated that about 1% to 3% of hepatocytes die daily in the diseased liver, as opposed to <0.01% in normal healthy livers. This excessive hepatocyte death obviously requires extensive proliferation to maintain a stable liver mass, suggesting that an individual hepatocyte would have to divide many times during the life of a patient with chronic hepatitis. Such prolonged self-replication in an inflammatory microenvironment could result in the accumulation of genetic lesions that cause cancer formation.Citation93–Citation95
Subsequent experiments with mouse urokinase plasmin ogen activator have shown that transplanted hepatocytes could regenerate the liver in its entirety after undergoing 12 rounds of replication.Citation96 Serial transplantation of hepatocytes into fumarylacetoacetase hydrolase-deficient mice indicated that transplanted hepatocytes could divide at least 69 times in vivo, and when normal hepatocytes from wild-type mice are injected into these mutant mice, they colonized the mutant liver effectivelyCitation97 In another study, Laconi et al transplanted dipeptidyl peptidase IV (DPPIV)-positive cells into DPPIV-deficient F344 rats, and showed that transplanted cells could repopulate 40% to 60% of female rat livers within a year and 98% to 99% of hepatic mass in male rats within nine months after transplantation.Citation98 Liu et al demonstrated that transplanted BM-derived EPCs could ameliorate the damage caused by CCl4 injury in rats.Citation99 They transplanted EPCs into portal veins of female rats 12 weeks after treating the animals with CCl4 and found that EPCs home to the liver and regenerate into hepatocytes. In a similar study, Nakamura et al have showed that transplanted EPCs derived from BM could improve the outcome in a cirrhotic liver rat model.Citation84 These findings demonstrate that the transplantation of hepatocytes and extrahepatic stem cells could be an effective treatment strategy to combat liver cancer.
Recent studies have demonstrated that the capacity to sustain tumor formation and growth resides in a small proportion of stem cells known as “cancer stem cells” (CSCs).Citation100,Citation101 They have a greater colony-forming efficiency, higher proliferation potential, and greater ability to form tumor in animal models. The identification of CSCs in a number of tissues including brain,Citation102–Citation104 prostate,Citation105 breast,Citation106 myeloid,Citation107 gastric,Citation108 colon,Citation109,Citation110 and lungCitation111 reinforced the notion that stem cells might also exist in the liver. CSCs were later identified and isolated from the liver.Citation112
The presence of CSCs and successful isolation of oval cells from cancerous tissue suggests that stem/progenitor cells play a key role in tumor formation in the liver. The clonality of HCC was later established, based on studies examining integration sites of HBV in tumor samples,Citation113–Citation115 as well as on the determination of restriction fragment length polymorphisms of X-linked genes such as the androgen receptor gene in tumor cells.Citation116 However, the cell type that gives rise to HCC is not well understood as yet.Citation117 In general, cell proliferation at the time of carcinogen exposure is a prerequisite for injury to the genome to take a heritable form. Therefore, it is logical to expect that the “target cell” has both the capacity to undergo extensive cell division and to remain viable for extended periods of time allowing for the accumulation of additional mutations needed for malignant transformation.
Additional evidence regarding the origin of HCC from hepatocytes comes from several studies done in rodents. In one such experiment, Gournay et al labeled hepatocytes with a retroviral vector expressing the β-galactosidase gene after two-thirds hepatectomy and fed the animals with 2-AAF to induce HCC.Citation118 They later observed that some of the neoplastic nodules in the liver samples of these animals contained cells expressing β-galactosidase, indicating that they were directly derived from retrovirally-labeled hepatocytes. In another study, using similar retroviral marking of hepatocytes with β-galactosidase gene, Bralet et al showed that following chronic administration of diethyl-nitrosamine (DENA), some of the tumors that developed in the liver contained cells expressing β-galactosidase. confirming that mature hepatocytes can give rise to HCC in a clonal manner.Citation119 While these studies demonstrated the capacity of resting hepatocytes to re-enter the cell cycle and divide in response to injury, others have showed that stem cells in the liver are also activated by injury.Citation120,Citation121 In fact, oval cells can generate hepatocytes and cholangiocytes when hepatocytes fail to respond after injury.Citation42,Citation122
The concept that oval cells and HPCs are involved in the development of HCC is based on numerous studies performed with animal models and with human clinical liver tumor specimens. Most models of hepatocarcinogenesis are characterized by very prominent proliferation of oval cells. One well-known example is the “Solt-Farber” model.Citation123 In this model, DENA is administered first as an initiator, and after two weeks the animals were fed with 2-AAF to inhibit the proliferation of no initiated hepatocytes. Two-thirds partial hepatectomy was then performed in these animals to stimulate proliferation of cells that were initiated with DENA. The most prominent histologic feature observed in this model is proliferation of oval cells within biliary ductular structures, which peaked at 1–3 weeks, followed by the appearance of dysplastic nodules where oval cells surrounded the nodules, and migration into them. The development of HCC then occurred within 14 months in these rats. The comparison of the phenotypes between various cell populations has shown that tumor cells have a phenotype similar to that of oval cells but not hepatocytes.Citation123
Another example of the experimental models supporting the involvement of progenitor/stem cells in hepatocarcinogenesis is the choline-deficient diet model.Citation45 In this model, administration of the carcinogens ethionine or AAF to animals on a choline-deficient diet results in a rapid proliferation of oval cells beginning 1–2 days after the administration of the diet and results in tumor formation. Even though it is not clear whether oval cells or periductular progenitor cells are ultimately responsible for cancer formation, it is apparent that some type of hepatic progenitor cell, and not the mature hepatocyte, is the target for malignant transformation. The most direct evidence for the involvement of oval cells in hepatocarcinogenesis was provided by Dumble et al.Citation124 This group isolated oval cells from p53 null mice and then transplanted them into athymic nude mice where the p53-deficient oval cells produced hepatocellular carcinomas, conclusively demonstrating the ability of oval cells to serve as the target cells of malignant transformation in HCC.
In another study, Yang et al used CD90 expression as a marker to characterize CSCs in HCC cell lines, tumor specimens, and blood samples.Citation125 They showed that CD45−and CD90+ cells, but not CD90− cells, had tumorigenic potential in cell lines. All tumor specimens and most blood samples from HCC patients contained a population of CD90+ and CD44− cells which were capable of generating tumor nodules when transplanted in immunodeficient mice. The CD90+CD44+ cells demonstrated a more aggressive phenotype than the CD90+CD44− cells and formed metastatic lesions in the lungs of these mice. The gene expression profile of CD45−CD90+ cells indicated a stem cell-like phenotype, and blockage of CD44 expression prevented the formation of local and metastatic tumor nodules, suggesting that CD44 be a viable target in the treatment for HCC.
HCC originating from hematopoietic progenitor cells was inferred from the observation that many HCC specimens contain a mixture of mature cells that were phenotypically similar to HPCs expressing OV-6, CK7, CK19, and chromogranin-A. This would suggest an origin from stem cells with partially and fully differentiated malignant progeny existing within the same tumor sample.Citation126 Cells resembling HPCs have also been described in hepatoblastoma.Citation32,Citation127 Hepatoblastoma is generally thought to be stem cell-derived because both epithelial and mesenchymal tissue components can be found in such tumor specimens.Citation128 Other studies have identified cells with an HPC phenotype in a rare subset of hepatic cancers. These tumors have two major components, ie, an HCC component and a cholangiocarcinoma component, suggesting that the tumor could originate from a bipotential progenitor cell.Citation129 More recently, Zhang et al studied 12 cases of combined hepatocellular cholangiocarcinoma where the majority of the tumor cells expressed hepatic, biliary, as well as stem cell markers simultaneously, suggesting that these tumors were derived from HPCs.Citation130
Stem cell therapy for treatment of HCC
Due to the problems associated with orthotopic liver transplantation, transplantation of hepatocytes has been proposed as an alternative treatment option for liver disease. However, widespread use of this approach is severely limited due to the shortage of reproducible sources of hepatocytes.Citation131 As a result of this, as well as the emergence of the stem cell field, stem/progenitor cells with the capacity to differentiate into hepatocyte-like cells appear to be a promising curative option in liver disease. These cells could regenerate the liver mass because they can proliferate for prolonged periods of time and differentiate into hepatic cells after transplantation.
The most important implication of liver CSCs is their potential clinical impact in developing novel therapeutic approaches for HCC. Recently, several groups have reported isolation and characterization of human HCC stem cells. For example, CD133 has been reported to be a marker of CSCs in various tissues (brain, pancreas, prostate, colon) and to identify CSCs in hepatocellular carcinoma cell lines.Citation27 They found that HCC was hierarchically organized and originated from a population of progenitor cells that expressed CD133+. These progenitor cells also possessed characteristics similar to that of normal stem cells and the ability to self-renew and differentiate. In a follow-up study by the same researchers, it has been shown that CD133+ HCC stem cells were the cell population responsible for the chemotherapy (doxorubicin and 5-fluorouracil) resistance seen in HCC, and could be the source of tumor recurrence after chemotherapy.Citation132 They also demonstrated that CD133+ HCC cells survived chemotherapy significantly better than most tumor cells which did not express CD133, and that the underlying mechanism was the constitutive activation of the serine/threonine protein kinase Akt and Bcl-2 cell survival pathways. An obvious clinical implication of this finding is the fact that specific inhibitors of these pathways would potentially be useful in the treatment of HCC.
The notion of CSCs initiating and advancing tumors is now well accepted in the scientific community. Unlike the great majority of cells in a given organ, only a small number of CSCs possess tumorigenic potential and the necessary phenotype of “stemness” to initiate tumors.Citation133 Therefore, a critical step towards developing effective cancer treatments is to understand cellular, molecular, and biochemical differences between normal stem cells and CSCs. Knowledge of such differences would allow us to identify key targets for therapeutic applications. One common method that is being used to distinguish CSCs from other cells is identifying their expression of cell surface markers. Unfortunately, this approach has not been very useful because several of these surface markers are shared both by CSCs as well as normal stem cells of the corresponding tissue. For instance, both CSCs and normal stem cells of the liver express CD133.Citation27,Citation134–Citation136 With the improvement in assays to determine gene expression profiles, it should be possible to identify the differences between the two cell types in the near future. Other approaches, such as studies of heritable epigenetic gene silencing, genetic alterations, and mutations in specific oncogenes of these cells, as well as the resident microenvironment of tumors, may allow us to understand the conditions that give rise to CSCs. Similarly, targeting specific cellular signaling pathways involved in HCC is another approach towards finding new treatments.Citation5,Citation8 Furthermore, removing the stemness of CSCs to eliminate their ability to proliferate and differentiate, and inhibiting their maintenance of stem cell state, is another approach that might provide us with important breakthroughs.Citation137 It has been shown in a number of cancers that generation of tumors can be achieved by transplanting CSCs into animals. Therefore, transplantation of CSCs and stem/progenitor cells expressing oncogenes such as c-Myc might allow us to develop animal models to study the disease process and to develop novel cancer therapies.
As discussed above, stem/progenitor cells have been isolated from almost every mammalian organ, and shown to differentiate into hepatocytes and other cell types. The important question is: are all these stem cells created equal? In other words, can we use stem cells from any organ for transplantation into livers for therapeutic purposes? To address this question, we need a clear understanding of the similarities and differences between various stem cells prior to and after their differentiation into the desired cell type. A couple of recent investigations have dealt with this issue. In one study, Sharova et al looked at the similarities and differences among mouse stem cells of different origins and strains using gene expression profiling.Citation138 They found that ESCs and embryonic germ cells were indistinguishable in their gene expression pattern. Very few differences were observed when these cells were grown in normal growth medium and in the medium that promotes differentiation. However, using stringent statistical analysis and quantitative real-time polymerase chain reaction, the authors were able to identify a signature profile containing 20 genes that distinguish each cell type. Interestingly, the variation in gene expression was greater between ESCs and embryonic germ cells of different mouse strains than within a single strain. In another study, Noël et al evaluated global gene and protein expression profiling of human adipose tissue-derived and multipotential stromal cells using microarrays, 2D gel electrophoresis, and functional assays. They found that cell type-specific differences do exist between these cells, although they possess similar differentiation potentials.Citation139 Their data suggest that stem cells are different and express genes depending upon their tissue origin. Further studies are needed to understand molecular mechanisms involved in the regulation of stemness and differentiation.
Conclusions
Advances in stem cell technology provide opportunities to develop novel approaches with an ability to reduce the morbidity and mortality associated with liver cancer. Liver cancer is a multifactorial disease with many different underlying pathogenic mechanisms caused by a variety of risk factors. Despite enormous progress during the past several decades, patient survival remains very low. Prospective, randomized human clinical trials are expensive, time-consuming, and very difficult to perform. A severe shortage of livers for orthotopic transplantation has compounded this problem even further. Unfortunately, hepatocyte transplantation has achieved little success in humans so far and there is a greater need to isolate and enrich stem cells with greater clonogenic potential to use them for therapy.Citation117 However, stem cell therapy for the treatment of liver cancer is a long way away from reality. Rather than using treatments such as tetrosine, which are clinically unacceptable, enhancing the clonogenic potential of stem/progenitor cells isolated from healthy livers might prove critical for creating novel cancer therapeutics. Towards this goal, a better understanding of the molecular mechanisms involved in tumor formation and progression, development of new antifibrotic agents, experimental animal models that closely mimic the human disease, and antiviral agents are critical for the success of cell-based therapies for liver cancer.
Disclosure
The author reports no conflict of interest in this work.
References
- LeendersMWNijkampMWBorel RinkesIHMouse models in liver cancer research: A review of current literatureWorld J Gastroenterol2008146915692319058325
- El-SeragHBRudolphKLHepatocellular carcinoma: Epidemiology and molecular carcinogenesisGastroenterology20071322557257617570226
- FaraziPADePinhoRAHepatocellular carcinoma pathogenesis: From genes to environmentNat Rev Cancer2006667468716929323
- BlumHEHepatocellular carcinoma: Therapy and preventionWorld J Gastroenterol2005117391740016437707
- AravalliRNSteerCJCressmanENKMolecular mechanisms of hepatocellular carcinomaHepatology2008482047206319003900
- El-SeragHRudolphKLHepatocellular carcinoma: Epidemiology and molecular carcinogenesisGastroenterology20071322557257617570226
- PiscagliaFBolondiLRecent advances in the diagnosis of hepatocellular carcinomaHepatol Res200737S178S19217877481
- AravalliRNCressmanENKMolecular signaling in hepatocellular carcinomaCancer Chemother Rev20094157164
- SkeltonMO’NeillBTargeted therapies for hepatocellular carcinomaClin Adv Hematol Oncol2008620921818391920
- ThomasMO’BeirneJPFuruseJChanATCAbou-AlfaGJohnsonPSystemic therapy for hepatocellular carcinoma: Cytotoxic chemotherapy, targeted therapy and immunotherapyAnn Surg Oncol2008151008101418236117
- GruessnerBLiving Donor Organ TransplantationNew York, NYMcGraw Hill2008
- MichalopoulosGKLiver regeneration: Alternative epithelial pathwaysInt J Biochem Cell Biol9272009 [Epub ahead of print]
- FaustoNLiver regeneration and repair: Hepatocytes, progenitor cells, and stem cellsHepatology2004391477148715185286
- AravalliRNSteerCJSahinMBCressmanENKStem cell origins and animal models of hepatocellular carcinomaDig Dis Sci6102010 [Epub ahead of print]
- ZhouQJXiangLXShaoJZIn vitro differentiation of hepatic progenitor cells from mouse embryonic stem cells induced by sodium butyrateJ Cell Biochem2007100294216888815
- AllainJEDagherIMahieu-CaputoDImmortalization of a primate bipotent epithelial liver stem cellProc Natl Acad Sci U S A2002993639364411904425
- LeeJHRimHJSellSHeterogeneity of the “oval-cell” response in the hamster liver during cholangiocarcinogenesis following Clonor-chis sinensis infection and dimethylnitrosamine treatmentJ Hepatol199726131313239210619
- MassonNMCurrieISTerraceJDGardenOJParksRWRossJAHepatic progenitor cells in human fetal liver express the oval cell marker Thy-1Am J Physiol Gastrointest Liver Physiol2006291G45G5416769813
- SahinMBSchwartzREBuckleySMIsolation and characterization of a novel population of progenitor cells from unmanipulated rat liverLiver Transpl20081433334518306374
- ArendsBVankelecomHVander BorghtSThe dog liver contains a “side population” of cells with hepatic progenitor-like characteristicsStem Cells Dev20091834335018680393
- HirataMAmanoKMiyashitaAYasunagaMNakanishiTSatoKEstablishment and characterization of hepatic stem-like cell lines from normal adult rat liverJ Biochem2009145515818977772
- SpagnoliFMAmiconeLTripodiMWeissMCIdentification of a bipotential precursor cell in hepatic cell lines derived from transgenic mice expressing cyto-Met in the liverJ Cell Biol1998143110111129817765
- DanYYRiehleKJLazaroCIsolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineagesProc Natl Acad Sci U S A20061039912991716782807
- DuretCGerbal-ChaloinSRamosJIsolation, characterization, and differentiation to hepatocyte-like cells of nonparenchymal epithelial cells from adult human liverStem Cells2007251779179017412893
- Fougère-DeschatretteCImaizumi-ScherrerTStrick-MarchandHPlasticity of hepatic cell differentiation: Bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivoStem Cells2006242098210916946000
- NowakGEriczonBGNavaSIdentification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivoGut20055497297915951545
- MaSChanKWHuLIdentification and characterization of tumorigenic liver cancer stem/progenitor cellsGastroenterology20071322542255617570225
- Strick-MarchandHMorosanSCharneauPKremsdorfDWeissMCBipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytesProc Natl Acad Sci U S A20041018360836515155906
- ZhouHRoglerLETepermanLMorganGRoglerCEIdentification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liverHepatology20074571672417326146
- HarunaYSaitoKSpauldingSNalesnikMAGerberMAIdentification of bipotential progenitor cells in human liver developmentHepatology1996234764818617427
- TokiwaTYamazakiTOnoMEnosawaSTsukiyamaTCloning and characterization of liver progenitor cells from the scattered cell clusters in primary culture of porcine liversCell Transplant20081717918618468248
- FiegelHCGluerSRothBStem-like cells in human hepatoblastomaJ Histochem Cytochem2004521495150115505344
- HerreraMBBrunoSButtiglieriSIsolation and characterization of a stem cell population from adult human liverStem Cells2006242840285016945998
- HaqueSHarunaYSaitoKIdentification of bipotential progenitor cells in human liver regenerationLab Invest1996756997058941215
- WeissTSLichtenauerMKirchnerSHepatic progenitor cells from adult human livers for cell transplantationGut2008571129113818417531
- RaoMSKhanAAParveenNHabeebMAHabibullahCMPandeGCharacterization of hepatic progenitors from human fetal liver during second trimesterWorld J Gastroenterol2008145730573718837092
- RoskamsTALibbrechtLDesmetVJProgenitor cells in diseased human liverSemin Liver Dis20032338539614722815
- MenthenaADebNOertelMBone marrow progenitors are not the source of expanding oval cells in injured liverStem Cells2004221049106115536195
- PetersenBEBowenWCPatreneKDBone marrow as a potential source of hepatic oval cellsScience19992841168117010325227
- SiricaAEMathisGASanoNElmoreLWIsolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cellsPathobiology19905844642187478
- WangXWillenbringHAkkariYCell fusion is the principal source of bone-marrow-derived hepatocytesNature200342289790112665832
- LázaroCARhimJAYamadaYFaustoNGeneration of hepatocytes from oval cell precursors in cultureCancer Res199958551455229850088
- PetersenBGoffJPGreenbergerJSMichalopoulosGKHepatic oval cells express the hematopoietic stem cell marker Thy-1 in the ratHepatology1998274334459462642
- SellSLiver stem cellsMod Pathol199471051128159639
- ShinozukaHLombardiBSellSIammarinoREEarly histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient dietCancer Res1978381092109876508
- SellSThe role of progenitor cells in repair of liver injury and in liver transplantationWound Repair Regen2001946748211896989
- LowesKNBrennanBAYeohGCOlynykJKOval cell numbers in human chronic liver diseases are directly related to disease severityAm J Pathol199915453754110027411
- LiuYNZhangJHeQHDaiXShenLIsolation and characterization of epithelial progenitor cells from human fetal liverHepatol Res20083810311317760874
- ChoiSSDiehlAMEpithelial-to-mesenchymal transitions in the liverHepatology2009502007201319824076
- BaumBSettlemanJQuinlanMPTransitions between epithelial and mesenchymal states in development and diseaseSemin Cell Dev Biol20081929430818343170
- JiangYJahagirdarBNReinhardtRLPluripotency of mesenchymal stem cells derived from adult marrowNature2002418414912077603
- LeeKDKuoTKWhang-PengJIn vitro hepatic differentiation of human mesenchymal stem cellsHepatology2004401275128415562440
- SchwartzREReyesMKoodieLMultipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cellsJ Clin Invest20021091291130212021244
- VassilopoulosGWangPRRussellDWTransplanted bone marrow regenerates liver by cell fusionNature200342290190412665833
- van PollDParekkadanBChoCHMesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivoHepatology2008471634164318395843
- LagasseEConnorsHAl-DhalimyMPurified hematopoietic stem cells can differentiate into hepatocytes in vivoNat Med200061229123411062533
- BrulportMSchormannWBauerAFate of extrahepatic human stem and precursor cells after transplantation into mouse liversHepatology20074686187017668884
- SatoYArakiHKatoJHuman mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusionBlood200510675676315817682
- MartinGRIsolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cellsProc Natl Acad Sci U S A198178763476386950406
- AmitMCarpenterMKInokumaMSClonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of cultureDev Biol200022727127811071754
- ChinzeiRTanakaYShimizu-SaitoKEmbryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytesHepatology200236222912085345
- HamazakiTIiboshiYOkaMHepatic maturation in differentiating embryonic stem cells in vitroFEBS Lett2001497151911376655
- HeoJFactorVMUrenTHepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liverHepatology2006441478148617133486
- Anjos-AfonsoFSiapatiEKBonnetDIn vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditionsJ Cell Sci20041175655566415494370
- HayDCZhaoDRossAMandalamRLebkowskiJCuiWDirect differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activitiesCloning Stem Cells20079516217386014
- IshizakaSOujiYYoshikawaMNakataniKDerivation and characterization of hepatocytes from embryonic stem cells in vitroMethods Mol Biol200633038739916846038
- AlisonMRPoulsomRJefferyRHepatocytes from non-hepatic adult stem cellsNature200040625710917519
- TheiseNDNimmakayaluMGardnerRLiver from bone marrow in humansHepatology200132111610869283
- ZhangYNLiePCWeiXDifferentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cellsCytotherapy20091154855819657806
- CampardDLysyPANajimiMSokalEMNative umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cellsGastroenterology200813483384818243183
- Sáez-LaraMJFrechaCMartínFTransplantation of human CD34+ stem cells from umbilical cord blood to rats with thioacetamide-induced liver cirrhosisXenotransplantation20061352953517059580
- QianHWangJWangSGongZChenMRenZHuangSIn utero transplantation of human hematopoietic stem/progenitor cells partially repairs injured liver in miceInt J Mol Med20061863364216964416
- LorenziniSGittoSGrandiniEAndreonePBernardiMStem cells for end stage liver disease: How far have we got?World J Gastroenterol2008144593459918698672
- ChienCCYenBLLeeFKIn vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cellsStem Cells2006241759176816822884
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell200612666367616904174
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell200713186187218035408
- YuJVodyanikMASmuga-OttoKInduced pluripotent stem cell lines derived from human somatic cellsScience20073181917192018029452
- Si-TayebKNotoFKNagaokaMHighly efficient generation of human hepatocyte-like cells from induced pluripotent stem cellsHepatology20105129730519998274
- SongZCaiJLiuYEfficient generation of hepatocyte-like cells from human induced pluripotent stem cellsCell Res2009191233124219736565
- AoiTYaeKNakagawaMGeneration of pluripotent stem cells from adult mouse liver and stomach cellsScience200832169970218276851
- UrbichCDimmelerSEndothelial progenitor cells: Characterization and role in vascular biologyCirc Res20049534335315321944
- AsaharaTMuroharaTSullivanAIsolation of putative progenitor endothelial cells for angiogenesisScience19972759649679020076
- ZenginEChalajourFGehlingUMVascular wall resident progenitor cells: A source for postnatal vasculogenesisDevelopment20061331543155116524930
- NakamuraTTorimuraTSakamotoMSignificance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat modelGastroenterology20071339110717631135
- BanasATerataniTYamamotoYAdipose tissue-derived mesenchymal stem cells as a source of human hepatocytesHepatology20074621922817596885
- BanasATerataniTYamamotoYIFAT S collection: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injuryStem Cells2008262705271218535155
- IkedaEYagiKKojimaMMultipotent cells from the human third molar: Feasibility of cell-based therapy for liver diseaseDifferentiation20087649550518093227
- HisatomiYOkumuraKNakamuraKFlow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineagesHepatology20043966767514999685
- MikiTStromSCAmnion-derived pluripotent/multipotent stem cellsStem Cell Rev2006213314217237552
- RatajczakMZuba-SurmaEKWysoczynskiMRatajczakJKuciaMVery small embryonic-like stem cells: Characterization, developmental origin, and biological significanceExp Hematol20083674275118474305
- HarknessRDChanges in the liver of the rat after partial hepatectomyJ Physiol195211726727714946734
- TrotterNLA fine structure study of lipid in mouse liver regenerating after partial hepatectomyJ Cell Biol19642123324414153484
- DonatoMFArosioEMontiVProliferating cell nuclear antigen assessed by a computer-assisted image analysis system in patients with chronic viral hepatitis and cirrhosisDig Liver Dis20023419720311990392
- FreemanAHamidSMorrisLImproved detection of hepatocyte proliferation using antibody to the pre-replication complex: An association with hepatic fibrosis and viral replication in chronic hepatitis C virus infectionJ Viral Hepat20031034535012969185
- NowakMABonhoefferSHillAMBoehmeRThomasHCMcDadeHViral dynamics in hepatitis B virus infectionProc Natl Acad Sci U S A199693439844028633078
- RhimJA SEDegenJLPalmiterRDBrinsterRLReplacement of diseased mouse liver by hepatic cell transplantationScience1994263114911528108734
- OverturfKal-DhalimyMOuCNFinegoldMGrompeMSerial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytesAm J Pathol1997151127312809358753
- LaconiEOrenRMukhopadhyayDKLong-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsineAm J Pathol19981533193299665494
- LiYWanDFSuJJDifferential expression of genes during aflatoxin B(1)-induced hepatocarcinogenesis in tree shrewsWorld J Gastroenterol20051049750414966905
- PardalRClarkeMFMorrisonSJApplying the principles of stemcell biology to cancerNat Rev Cancer2003389590214737120
- ReyaTMorrisonSJClarkeMFWeissmanILStem cells, cancer, and cancer stem cellsNature200141410511111689955
- HemmatiHDNakanoILazareffJACancerous stem cells can arise from pediatric brain tumorsProc Natl Acad Sci U S A2003100151781518314645703
- SinghSKClarkeIDTerasakiMIdentification of a cancer stem cell in human brain tumorsCancer Res2003635821582814522905
- SinghSKHawkinsCClarkeIDIdentification of human brain tumour initiating cellsNature200443239640115549107
- CollinsATBerryPAHydeCStowerMJMaitlandNJProspective identification of tumorigenic prostate cancer stem cellsCancer Res200565109461095116322242
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A20031003983398812629218
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature19943676456487509044
- HoughtonJStoicovCNomuraSGastric cancer originating from bone marrow-derived cellsScience20043061568157115567866
- O’BrienCAPollettAGallingerSDickJEA human colon cancer cell capable of initiating tumour growth in immunodeficient miceNature200744510611017122772
- Ricci-VitianiLLombardiDGPilozziEIdentification and expansion of human colon-cancer-initiating cellsNature200744511111517122771
- KimCFJacksonELWoolfendenAEIdentification of bronchioalveolar stem cells in normal lung and lung cancerCell200512182383515960971
- RoskamsTLiver stem cells and their implication in hepatocellular and cholangiocarcinomaOncogene2006253818382216799623
- EsumiMAritakaTAriiMClonal origin of human hepatoma determined by integration of hepatitis B virus DNACancer Res198646576757713019535
- NgIGuanXYPoonRTFanSTLeeJMDetermination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multi centric origin from intrahepatic metastasisJ Pathol200319934535312579536
- YamamotoTKajinoKKudoMSasakiYArakawaYHinoODetermination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNAHepatology1999291446145210216128
- ZhangSHCongWMWuMCFocal nodular hyperplasia with concomitant hepatocellular carcinoma: A case report and clonal analysisJ Clin Pathol20045755655915113871
- AlisonMRLiver stem cells: implications for hepatocarcinogenesisStem Cell Rev2005125326017142862
- GournayJAuvigneIPichardVLigezaCBraletMPFerryNIn vivo cell lineage analysis during chemical hepatocarcinogenesis in rats using retroviral-mediated gene transfer: evidence for dedifferentiation of mature hepatocytesLab Invest20028278178812065689
- BraletMPPichardVFerryNDemonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosaminetreated ratsHepatology20023662363012198654
- SuzukiAZhengYWKanekoSClonal identification and characterization of self-renewing pluripotent stem cells in the developing liverJ Cell Biol200215617318411781341
- LowesKNCroagerEJOlynykJKAbrahamLJYeohGCOval cell-mediated liver regeneration: Role of cytokines and growth factorsJ Gastroenterol Hepatol20031841212519217
- EvartsRPHuZOmoriNOmoriMMarsdenERThorgeirssonSSPrecursor-product relationship between oval cells and hepatocytes: Comparison between tritiated thymidine and bromodeoxyuridine as tracersCarcinogenesis199617214321518895481
- SoltDMedlineAFarberERapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesisAm J Pathol19778859560918937
- DumbleMCroagerEJYeohGCQuailEAGeneration and characterization of p53 null transformed hepatic progenitor cells: Oval cells give rise to hepatocellular carcinomaCarcinogenesis20022343544511895858
- YangZHoDWNgMNSignificance of CD90+ cancer stem cells in human liver cancerCancer Cell20081315316618242515
- LibbrechtLRoskamsTHepatic progenitor cells in human liver diseasesSemin Cell Dev Biol20021338939612468238
- RuckPXiaoJCPietschTVon SchweinitzDKaiserlingEHepatic stem-like cells in hepatoblastoma: Expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6Histopathol199731324329
- LibbrechtLDesmetVRoskamsTStages of normal and aberrant intrahepatic bile duct development in a mixed hepatoblastomaHistopathology20034261862012786902
- TheiseNDYaoJLHaradaKHepatic ‘stem cell’ malignancies in adults: Four casesHistopathology20034326327112940779
- ZhangFChenX-PZhangWCombined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: Immunohistochemical and double-fluorescence immunostaining evidenceHistopathology20085222423218184271
- DrobinskayaILinnTSaricTScalable selection of hepatocyteand hepatocyte precursor-like cells from culture of differentiating transgenically modified murine embryonic stem cellsStem Cells2008262245225618556507
- MaSLeeTKZhengBJChanKWGuanXYCD133þ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathwayOncogene2008271749175817891174
- HuffCAMatsuiWHDouglas SmithBJonesRJStrategies to eliminate cancer stem cells: clinical implicationsEur J Cancer2006421293129716644203
- SchmelzerEZhangLBruceAHuman hepatic stem cells from fetal and postnatal donorsJ Exp Med20072041973198717664288
- RountreeCBBarskyLGeSZhuJSenadheeraSCrooksGMA CD133-expressing murine liver oval cell population with bilineage potentialStem Cells2007252419242917585168
- RountreeCBDingWHeLStilesBExpansion of CD133 expressing liver cancer stem cells in liver specific PTEN deleted miceStem Cells20082729029919008348
- SpiraAICarducciMADifferentiation therapyCurr Opin Pharmacol2003333834312901941
- SharovaLVSharovAAPiaoYGlobal gene expression profiling reveals similarities and differences among mouse pluripotent stem cells of different origins and strainsDev Biol200730744645917560561
- NoëlDCatonDRocheSCell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentialsExp Cell Res20083141575158418325494