Abstract
Mantle cell lymphoma (MCL) is a type of non-Hodgkins lymphoma (NHL) associated with poor progression-free and overall survival. There is a high relapse rate with conventional cytotoxic chemotherapy. Intensive combination chemotherapy including rituximab, dose intense CHOP- (cyclophosphamide-doxorubicin-vincristine-prednisone) like regimens, high dose cytarabine, and/or consolidation with autologous stem cell transplant (autoSCT) have shown promise in significantly prolonging remissions. Data from phase II studies show that even in patients with chemotherapy refractory MCL, allogeneic stem cell transplant (alloSCT) can lead to long term disease control. Most patients with MCL are not candidates for myeloablative alloSCT due to their age, comorbidities, and performance status. The advent of less toxic reduced intensity conditioning (RIC) regimens, which rely more on the graft-versus-lymphoma (GVL) effect, have expanded the population of patients who would be eligible for alloSCT. RIC regimens alter the balance of toxicity and efficacy favoring its use. Treatment decisions are complicated by introduction of novel agents which are attractive options for older, frail patients. Further studies are needed to determine the role and timing of alloSCT in MCL. Currently, for selected fit patients with chemotherapy resistant MCL or those who progress after autoSCT, alloSCT may provide long term survival.
Mantle cell lymphoma (MCL) is a unique subtype of mature B-cell non-Hodgkin’s lymphomas (NHL) with a high response rate but an equally high relapse rate and relatively poor median survival. MCL can exhibit a spectrum of clinical courses. At one end of the spectrum is a chronic lymphocytic leukemia (CLL) like disease with a true chronic course where observation alone is warranted.Citation1 At the other end is an aggressive disease that displays rapid growth, relative chemoresistance, and early relapse. Generally, when treatment is necessary MCL usually exhibits shorter progression-free intervals compared to other indolent NHL. The wide variability in clinical behavior has increased treatment and prognostic uncertainty. This variability is reflected in its inclusion as an aggressive lymphoma in the Revised European-American Lymphoma (REAL) classification,Citation2 yet it remains included with indolent lymphomas in many current European and some US studies.
MCL has recently emerged as a separate clinicopathologic entity critically dependent on the dysregulation of cyclin D. The cytogenetic translocation t(11;14) (q13;q32) between the cyclin D1 (BCL-1 locus) and the immunoglobulin heavy chain (IgH) locus, which results in cyclin D1 dysregulation, is found in the majority of MCL cases. In the few cyclin D1 negative MCL, cyclin D2, D3, or both are overexpressed. MCL shares the CD5+ B-cell immunophenotype of CLL, but is differentiated from CLL by having bright CD20 with high levels of surface immunoglobulin and lack of CD23 expression. Morphologically, the appearance of MCL can vary from a small mature lymphocyte to a more blastoid larger cell that mirrors its protean behavior.
Mantle cell biology and behavior
Attempts have been made to subclassify MCL based on its histopathology, ie, classic, small cell, pleomorphic, and blastoid variants.Citation3 Patients with the blastoid variant often have the worst outcomes and those with the small cell type of MCL seem to do the best, but this has not yet defined a treatment algorithm to address each subtype separately. Rosenwald et al used gene expression profiling by microarray to predict survival of patients with MCL. While no specific gene correlated with survival, expression of a panel of proliferation signature genes allowed separation into four quartiles of patients in which the first and fourth quartiles differed by more than 5 years in median survival.Citation4 Reports of other gene panels to predict MCL outcome are intriguing and require validation in other patient populations.Citation5
Proliferation assessed by Ki67 staining remains an effective, readily available prognostic indicator. More precise quantification may make it even more useful.Citation6 A Cancer and Leukemia Group B (CALGB) phase II trial showed that >35% Ki-67 expression was associated with a shorter progression-free survival (PFS) and event-free survival (EFS) and PIM1 (a cell cycle-related gene) expression was associated with a shorter PFS.Citation7 The German Low Grade Lymphoma study group (GLSG) developed the mantle cell lymphoma international prognostic index (MIPI) based on five prognostic factors: age, performance status, lactate dehydrogenase (LDH), leukocyte count, and Ki67 immunostaining indexCitation8 to classify MCL patients into three groups based on their overall survival (OS): low risk (median OS not reached), intermediate risk (51 months), and high risk groups (29 months).Citation9 MIPI has, however, been variably predictive when applied to other intensively treated subsets of patients.Citation10,Citation11
Current standard therapy
Analysis of outcomes data in MCL from trials conducted between 1975 to 1986 by the Kiel Lymphoma Study Group compared to trials conducted by the GLSG from 1996 to 2004 revealed an increase in median overall survival from 2.7 years to 4.8 years. Some of this improvement may be due to earlier and more specific diagnosis and better supportive care, but may also represent the improvements in MCL therapy with dose intense regimens and new therapeutic agents.Citation12
The development of more intensive induction regimens, such as rituximab (a chimeric monoclonal anti-CD20 antibody) plus hyperfractionated cyclophosphamide and vincristine-doxorubicin-dexamethasone (R-HyperCVAD) with alternating dose intense chemotherapy combinations using high dose cytarabine, increased the reported percentage of complete responses (CR) and median PFS.Citation13 Patient selection, however, may be an issue as two cooperative groups have been unable to effectively treat large numbers of patients with this regimen and there are no randomized data proving superior overall survival benefit compared to conventional anthracycline based chemotherapy (R-CHOP). Questions of improved long-term survival from dose intensification and addition of other cytotoxic agents remain unanswered, although recent evidence suggests there may be a subset of patients who gain a good long-term outlook with chemotherapy alone.Citation13
The addition of rituximab has improved overall and complete response rates,Citation14,Citation15 but not necessarily overall survival. Relatively small numbers of patients in recent trials may have limited statistical power to detect a difference, although in a relatively large MCL trial (122 patients) conducted by the GLSG, Lenz et al were unable to show a significant improvement in PFS from addition of rituximab to CHOP despite increased CR and overall response rates.Citation15 A Cochrane meta-analysis in 2007 led to researchers concluded that adding rituximab did improve OS, although there were only three trials included and these were heterogenous.Citation16,Citation17 Currently, the addition of rituximab has become a standard in the management of MCL first line in the United States ().
Table 1 First-line MCL regimens
High-dose therapy with autologous stem-cell support
A natural extension of the dose intensification approach is the use of high-dose therapy requiring stem cell support. This has now been studied in multiple trials, which have predominantly been single arm Phase II trials evaluating response rates (). The European MCL network’s randomized trial comparing consolidation with myeloablative radiochemotherapy followed by autologous stem cell support (ASCT) to interferon-α maintenance in first remission showed a median PFS benefit (39 mos versus 17 mos, P = 0.01) with ASCT.Citation21 The absence of minimal residual disease (MRD) after ASCT, assayed by quantitative real-time PCR of clonal IgH gene rearrangements, strongly predicted for longer failure free interval.Citation22 Autotransplant in CR1 (complete response after first-line therapy) following R-CHOP or similar regimens is currently adopted as one standard approach in patients fit to undergo high dose therapy.
Table 2 Trials employing autologous Stem cell transplant consolidation
In a large retrospective study of the Autologous Blood and Marrow Transplant Registry (ABMTR) and European Blood and Bone Marrow Transplant (EBMT) registry, transplanted patients had a median survival of 59 months, which is longer than historical (1990s) series of patients treated conventionally with a median survival of 36 months.Citation23 Patients who received autotransplant in CR1 clearly did better than partial responders in the study. The addition of radioimmunotherapy holds promise in overcoming this difference by improving the degree of response prior to autoSCT.Citation24 Biologic markers are clearly needed to help stratify patients among treatment options and especially transplant options.
Allogeneic transplant in indolent non-Hodgkin’s lymphomas
Indolent lymphomas are currently considered incurable by cytotoxic chemotherapy alone even at high doses. In selected patients allogeneic transplant may offer the potential for longer remission and potentially cure. The significant toxicity, both high early mortality rate and graft-versus-host disease (GVHD), with myeloablative regimens and allogeneic stem cell transplant (alloSCT) has limited the use of this modality. The development of less toxic (nonmyeloablative or reduced intensity conditioning, RIC) regimens and improved supportive care with reduced early mortality and morbidity has led to renewed interest in allogeneic transplantation in indolent lymphomas where the challenge is to select appropriate patients with high enough risk of their disease to warrant the transplant risks.
In the early 1990s the European Bone Marrow Transplant Group (EBMTG) conducted a case-controlled study in NHL matching 101 alloSCT patients with 101 autologous stem cell transplant (autoSCT) patients. Although progression-free survival was similar (49% alloSCT versus 46% autoSCT), there was a trend to lower relapse rate for the alloSCT patients (23% vs 38%). Patients with chronic GVHD had a significantly lower relapse rate than those without (0% versus 35% P = 0.02) suggesting a strong graft-versus-lymphoma (GVL) effect.Citation29 An advantage in long term survival for patients who underwent allogeneic bone manow transplant (BMT) was suggested by other nonrandomized trials.Citation30,Citation31 A Dutch trial involved 28 patients with recurrent or refractory low-grade NHL. The 18 patients with chemotherapy-sensitive disease underwent autoSCT and 10 patients, of whom seven were chemoresistant, underwent alloSCT. The 2 year PFS rates were 68% for alloSCT patients versus 22% for autoSCT patients (P = 0.049).Citation31
Later trials concentrated on reduced intensity conditioning regimens. A fludarabine/cyclophosphamide-based regimen developed at the MD Anderson Cancer Center showed an actuarial probability for being alive and in remission at 2 years of 84%.Citation32 An 8 year prospective study reported 47 patients treated with alloSCT using a fludarabine- cyclophosphamide-rituximab (FCR) conditioning regimen. The estimated PFS rate was 83% and survival of 85% at a median follow-up time of 60 months.Citation33 Other approaches with encouraging results include alemtuzumab-based regimens ( and ).Citation34,Citation35
Table 3 Comparison of transplant strategies in indolent NHL and MCL
Table 4 Studies of fully myeloablative regimens in mantle cell lymphoma
Allogeneic transplant in MCL
Patients with relapsed or refractory MCL following conventional intensive chemotherapy or autoSCT have limited therapeutic options. Allogeneic transplant using myeloablative regimens was initially studied as a potentially curative approach for these patients. Because of the risks of early mortality and GVHD, allogeneic transplant is generally reserved for MCL patients who have relapsed after an autologous transplant and other intensive regimens. In cases of somewhat indolent progression of MCL, the less toxic RIC regimens, in which the GVL effect is thought to be the chief therapeutic effect, are attractive. However, the wide proliferative variation in MCL results in many patients whose highly proliferative disease outpaces the GVL effect following RIC regimens.
Case reports of patients with chemotherapy refractory MCL having prolonged remission after allogeneic transplant suggested a GVL effect.Citation40 Khouri et al reported results in 16 patients bolstering the hypothesis. Of the 16 patients, 11 were previously treated; 14 were treated with myeloablative regimens – either Cy/TBI (High-dose cyclophosphamide 120 mg/kg and total body irradiation-12 Gy given in four daily fractions) or BEAM (BCNU/Etoposide/Cytarabine/Melphalan). Overall survival and freedom from progression at 3 years was 55% and the results were even more encouraging for patients with chemotherapy sensitive disease.Citation36 Molecular studies demonstrated that three patients with disease detectable by PCR following transplant converted to negative status several months later, suggesting a GVL effect.
Berdeja et al reported results in 35 patients with MCL and low grade lymphoma showing a 50% event free survival at 25 months median follow-up. All grafts were from matched sibling donors and were T-cell depleted to reduce GVHD.Citation37 This strategy had encouraging results in patients with chemotherapy-sensitive disease showing transplant related mortality (TRM) of 14% and chronic GVHD of just 6%, but fared poorly in patients with resistant disease (TRM 86% and EFS of 0%). T-cell depletion, although effective for prevention of GVHD, severely compromises the beneficial GVL effect. A promising technique under investigation to control GVH without necessarily compromising GVL involves the use of suicide gene-modified human T-lymphocytes. Herpes simplex virus-thymidylate kinase modified donor T cells, which play a central role in the causation of GVHD, could potentially be controlled by the addition of an antiviral drug: ganciclovir.Citation41
The University of Nebraska reported data on outcomes in patients with chemotherapy sensitive MCL undergoing autologous (n = 80) versus allogeneic (n = 17) stem cell transplant. Five year estimated event free survival (44% versus 39%) and overall survival (49% versus 47%) were similar in both groups. The five year relapse rate was lower at 21% in the alloSCT group, compared with 56% in the autoSCT group. This was balanced, however, by higher day 100 mortality rate in patients receiving allotransplant (19%). These data emphasize the dilemma of weighing the competing risks of progressive lymphoma versus treatment-related toxicity. The high early mortality limits the possibility of including allogeneic transplant in the initial treatment regimen for patients with chemotherapy sensitive MCL ().Citation39
Table 5 Results of alloSCT using reduced intensity regimens in mantle cell lymphoma
Fludarabine-based RIC regimens have gained precedence over myeloablative regimens in the last decade. Sorror et al updated the Seattle experience in 2008 with 33 patients with median follow up of 63 months and an additional 20 patients with shorter follow up. All patients received a fludarabine and 200 cGy TBI RIC regimen. This was a heavily pretreated population in which 40% of the patients had disease progression after prior autoSCT and an additional 11% had undergone planned autoSCT before alloSCT. Five year overall and progression free survival rates were 58% and 52% respectively, and there were no major differences between the use of related and matched unrelated donors.Citation46 At 5 years 44% were alive without GVHD, 14% with chronic GVHD requiring immunosuppression, and continued resolution of chronic GVHD was observed with time. Long term survival was achieved even in some chemotherapy-refractory patients, but those with bulky lymphadenopathy (≥5 cm) at the time of transplant invariably did poorly.
Tam et al published updated results of a risk adapted strategy at the MD Anderson Cancer Center. Of a total of 121 patients enrolled in sequential transplant protocols over a 17 year study period, 86 underwent autoSCT. The addition of rituximab resulted in a marked PFS improvement for those getting autoSCT in CR1. There were 35 patients (median age 58), all with relapsed or refractory MCL, who underwent nonmyeloablative allogeneic stem cell transplant. All patients were Stage 3 or 4 and 83% had chemosensitive disease at the time of transplant (46% in CR). With a median follow-up of 56 months the median PFS duration was 60 months, and the median OS had not yet been reached. Major determinants of disease control were use of peripheral blood stem cells (PBSC) versus bone marrow stem cells and achievement of >95% donor chimerism. Among 24 patients meeting both criteria, no lymphoma relapses had occurred at a median follow-up of 60 months.Citation47
In a retrospective study of 279 patients reported to the EBMT registry between 1998 and 2007 who had received RIC regimens in MCL, the Kaplan-Meier estimate of the PFS at 1 and 3 years was 49% and 29% respectively. The overall survival at 1 and 3 years was 60% and 43% respectively ().Citation48
Table 6 Characteristics of patients with mantle cell lymphoma receiving allogeneic hematopoietic cell transplants from 1998 to 2007 and registered to the CIBMTR
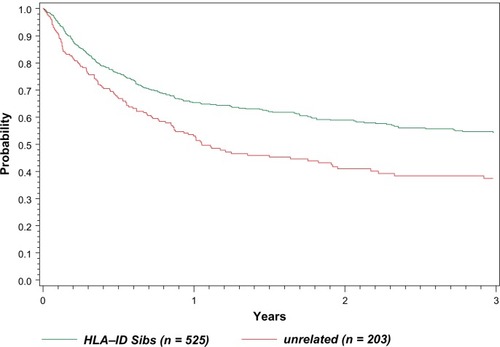
Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1998 to 2007 shows a steady use of related donors and increasing use of MUD donors in allogeneic transplants for MCL (written communication, January 2010). Roughly half of the 525 patients in the registry used RIC regimens for both related sibling and matched unrelated donors (MUD) donors and a major increase in the use of RIC regimens was seen in 2000 with roughly equal ablative and RIC regimens since that time. The median age for HLA-identical sibling donors was 53 (range 26–93) and for matched unrelated donors was 56 (range 23–72). The overall survival curves for this entire cohort are shown in .
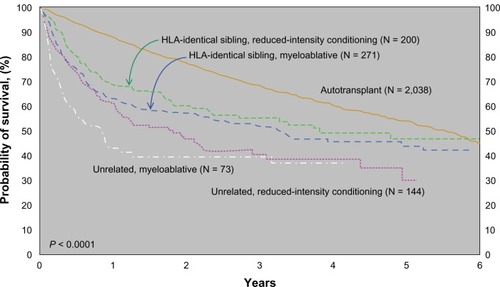
The CIBMTR published outcomes of stem cell transplantation subdivided by donor type and conditioning regimen as shown in .Citation49 Although it is difficult to make direct comparisons between the groups of patients who underwent auto versus allotransplant (selection bias – the allotransplant arm having more relapsed/refractory patients), it does give us an idea of real world outcomes. Patients who underwent autotransplant did better in the short run but seemed to have a continuing risk of relapse years post therapy. Patients undergoing allotransplant had worse survival immediately posttransplant due to short-term toxicity, but this early risk is balanced by the fact that the survival curves seem to plateau approximately 2 years later.
Figure 2 Probability of survival after transplants for mantle cell lymphoma, 1998–2007 by donor type and conditioning regimen.Citation49
Conclusions
The protean nature of MCL demands individualized treatment considerations. A small subset of MCL patients can be monitored for some years without therapy. It is unclear whether indicators such as a low MIPI score or low proliferation, whether by Ki67 or gene signature, can be a guide for this most indolent population. For most patients requiring treatment the current standard, if the patient is relatively young and fit, is a dose-intense or high-dose regimen. The optimal regimen, however, is currently unknown, though most current studies include a CHOP-like regimen as well as high dose cytarabine and rituximab. With the newer intensive regimens the place of high-dose therapy with autologous stem cell support continues to be debated, though there seems to be a progression-free survival advantage in most trials. Conclusions about the place of allogeneic transplant are complicated by the paucity of data, with only a few small phase II trials directing the field. Patients whose disease progressed after autoSCT have a very poor prognosis and, if they are suitable candidates, will likely benefit from an allogeneic approach. The current data, while encouraging, are limited by small numbers of highly selected patients, and whether there will be true long-term plateau suggesting cure is unclear in the current data from the CIBMTR and others.
Though it makes intuitive sense to consider patients with high MIPI scores as candidates for allogeneic transplant upfront, especially if they are relatively young and fit and have a sibling donor, this has not been tested in comparison to autologous transplant or intensive chemotherapy (such as R-HyperCVAD) in clinical trials to date. A very high risk population in which to consider allogeneic transplant would be patients with chemoresistant disease (ideally without bulky lymphadenopathy) where autologous transplant would not likely be of benefit.
In patients with relapsed MCL with lower proliferative behavior there may be time posttransplant for the GVL effect of a RIC allogeneic transplant to develop to overcome chemoresistance. Better biologic stratification of MCL in terms of proliferative rate will be important to define this group. Certain patients, particularly those with more rapid growth and higher tumor burdens, may require more intensive conditioning regimens to optimize disease control or even a planned tandem procedure of autoSCT followed soon after with an RIC allogeneic transplant, as reported in multiple myeloma.
The way forward
Therapeutic approaches in MCL must be individualized based on both host and disease characteristics. Clear definition of the lowest risk, indolent MCL subset are needed and recommended monitoring intervals defined for this indolent group. The optimal initial therapy of MCL for those requiring treatment has not been defined in clinical trials. This is now complicated by the many new agents available for trial including bendamustine, bortezomib, lenalidomide, and radioimmunotherapy. The evolving approach is to have separate trials for older, more frail patients that incorporate novel agents. These trials will test whether these new agents can be combined with, or replace, currently used chemotherapy combinations.
For young, fit patients trials will involve dose-dense or dose-intense regimens often requiring stem cell support. Autologous SCT is currently a favored approach as consolidation of first remission or, if not utilized until that point, as part of therapy for first relapse in appropriately selected patients. Trials should be designed to optimize conditioning regimens and investigate post transplant maintenance therapy, similar to evolving trials for multiple myeloma. Radioimmunotherapy is currently being investigated as part of the conditioning regimens for both autologous and allogeneic transplantation. Furthermore, the importance of disease burden at the time of transplant should be carefully defined systematically through imaging techniques. This would allow for stratification by MIPI or other prognostic risk groups and disease burden to permit comparison of autoSCT results. The prognostic importance of achieving a minimal residual disease state in the bone marrow, assayed by flow cytometric or PCR techniques, also needs to be evaluated, perhaps serving as a surrogate marker of outcome. While it is difficult to foresee use of even RIC alloSCT as consolidation therapy of first remission MCL until toxicity and early mortality is significantly reduced, the role of alloSCT in the relapsed setting merits further exploration. Once there is a greater consensus about the optimal autoSCT regimen for MCL, a randomized trial comparing autologous to allogeneic transplant using a RIC regimen could be undertaken in selected patients who have relapsed after their initial therapy. Better definition of high risk features that predict poor outcome with autoSCT would be very useful. At present, allogeneic transplant trials for MCL should be restricted to appropriately selected high risk patients whose disease is relapsed/refractory post autoSCT, or demonstrates chemotherapy resistance making autoSCT a poor option.
Disclosure
The authors report no conflicts of interest in this work.
References
- MartinPChadburnAChristosPOutcome of deferred initial therapy in mantle-cell lymphomaJ Clin Oncol2009271209121319188674
- HiddemannWLongoDLCoiffierBLymphoma classification-the gap between biology and clinical management is closingBlood199688408540898943841
- TiemannMSchraderCKlapperWHistopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL NetworkBr J Haematol2005131293816173960
- RosenwaldAWrightGWiestnerAThe proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphomaCancer Cell2003318519712620412
- Rubio-MoscardoFClimentJSiebertRMantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcomeBlood20051054445445415718413
- SchaffelRHedvatCVTeruya-FeldsteinJPrognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphomaAnn Oncol201021113313920019090
- HsiEDJungSHLaiRKi67 and PIM1 expression predict outcome in mantle cell lymphoma treated with high dose therapy, stem cell transplantation and rituximab: a Cancer and Leukemia Group B 59909 correlative science studyLeuk Lymphoma2008492081209019021050
- KlapperWHosterEDetermannOKi-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL NetworkJ Hematop2009210311119669190
- HosterEDreylingMKlapperWGisselbrechtCA new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphomaBlood200811155856517962512
- ShahJJFayadLRomagueraJMantle Cell International Prognostic Index (MIPI) not prognostic after R-hyper-CVADBlood2008112258318779406
- GeislerCHKolstadALaurellAThe Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive firstline immunochemotherapy and autologous stem cell transplantation (ASCT)Blood20101151530153320032504
- HerrmannAHosterEZwingersTImprovement of overall survival in advanced stage mantle cell lymphomaJ Clin Oncol20092751151819075279
- RomagueraJEFayadLRodriguezMAHigh rate of durable remissions after treatment of newly diagnosed aggressive mantlecell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabineJ Clin Oncol2005237013702316145068
- HowardOMGribbenJGNeubergDSRituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survivalJ Clin Oncol2002201288129411870171
- LenzGDreylingMHosterEImmunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG)J Clin Oncol2005231984199215668467
- SchulzHBohliusJSkoetzNChemotherapy plus Rituximab versus chemotherapy alone for B-cell non-Hodgkin’s lymphomaCochrane Database Syst Rev200717CD00380517943799
- SchulzHBohliusJFTrelleSImmunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysisJ Natl Cancer Inst20079970671417470738
- MeusersPEngelhardMBartelsHMulticentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosisHematol Oncol198973653802670728
- NickenigCDreylingMHosterECombined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study GroupCancer20061071014102216878325
- SmithMRChenHGordonLPhase II study of R-CHOP followed by 90Y-ibritumomab tiuxetan in untreated mantle cell lymphoma: Eastern Cooperative Oncology Group study E1499J Clin Oncol2007247503
- DreylingMLenzGHosterEEarly consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL NetworkBlood20051052677268415591112
- PottCSchraderCGeskSQuantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphomaBlood20061072271227816332971
- VandenbergheERuiz de ElviraCLoberizaFROutcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant RegistriesBr J Haematol200312079380012614212
- KolstadALaurellAAndersenNS90y-Ibritumumab Tiuxetan (Zevalin (R))-BEAM/C with Autologous Stem Cell Support as Frontline Therapy for Advanced Mantle Cell Lymphoma – Preliminary Results From the Third Nordic MCL Phase II Study (MCL3)Blood200911493219643993
- KhouriIFRomagueraJKantarjianHHyper-CVAD and highdose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphomaJ Clin Oncol199816380338099850025
- VoseJMBiermanPJWeisenburgerDDAutologous hematopoietic stem cell transplantation for mantle cell lymphomaBiol Blood Marrow Trans20006640645
- GopalAKRajendranJGPetersdorfSHHigh-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphomaBlood2002993158316211964278
- GeislerCHKolstadALaurellALong-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma GroupBlood20081122687269318625886
- ChopraRGoldstoneAHPearceRAutologous versus allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma: a casecontrolled analysis of the European Bone Marrow Transplant Group Registry dataJ Clin Oncol199210169016951403052
- RatanatharathornVUbertiJKaranesCProspective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphomaBlood199484105010558049425
- VerdonckLFDekkerAWLokhorstHMPetersenEJNieuwenhuisHKAllogeneic versus autologous bone marrow transplantation for refractory and recurrent low-grade non-Hodgkin’s lymphomaBlood199790420142059354692
- KhouriIFSalibaRMGiraltSANonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortalityBlood2001983595359911739162
- KhouriIFMcLaughlinPSalibaRMEight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximabBlood20081115530553618411419
- FaulknerRDCraddockCByrneJLBEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patientsBlood200410342843412969983
- KhouriIFAlbitarMSalibaRMLow-dose alemtuzumab (Campath) in myeloablative allogeneic stem cell transplantation for CD52-positive malignancies: decreased incidence of acute graft-versus-host-disease with unique pharmacokineticsBone Marrow Trans200433833837
- KhouriIFLeeMSRomagueraJAllogeneic hematopoietic transplantation for mantle-cell lymphoma: molecular remissions and evidence of graft-versus-malignancyAnn Oncol1999101293129910631455
- BerdejaJGJonesRJZahurakMLAllogeneic bone marrow transplantation in patients with sensitive low-grade lymphoma or mantle cell lymphomaBiol Blood Marrow Trans20017561567
- RifkindJMolleePMessnerHALiptonJHAllogeneic stem cell transplantation for mantle cell lymphoma – does it deserve a better look?Leuk Lymphoma20054621722315621804
- GantiAKBiermanPJLynchJCBociekRGVoseJMArmitageJOHematopoietic stem cell transplantation in mantle cell lymphomaAnn Oncol20051661862415781489
- AdkinsDBrownRGoodnoughLTKhouryHPopovicWDiPersioJTreatment of resistant mantle cell lymphoma with allogeneic bone marrow transplantationBone Marrow Trans1998219799
- BondanzaAValtolinaVMagnaniZSuicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytesBlood20061071828183616293601
- RobinsonSPGoldstoneAHMackinnonSChemoresistant or aggressive lymphoma predicts for a poor outcome following reducedintensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow TransplantationBlood20021004310431612393626
- KhouriIFLeeMSSalibaRMNonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphomaJ Clin Oncol2003214407441214645431
- MorrisEThomsonKCraddockCOutcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphomaBlood20041043865387115304395
- MarisMBSandmaierBMStorerBEAllogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphomaBlood20041043535354215304387
- SorrorMLStorerBSandmaierBMSustained graft-versus-lymphoma effect among patients (pts) with Mantle Cell Lymphoma (MCL) given nonmyeloablative allogeneic Hematopoietic Cell Transplantation (HCT)Blood20081122147
- TamCSBassettRLedesmaCMature results of the M.D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphomaBlood20091134144415219168784
- RobinsonSPSuredaACanalsCSrIdentification of prognostic factors predicting the outcome of reduced intensity allogeneic stem cell transplantation in mantle cell lymphoma. An analysis from the Lymphoma Working Party of the EBMTBlood200811245718650457
- PasquiniMCWangZCurrent use and outcome of hematopoietic stem cell transplantation: part II- CIBMTR summary slidesCIBMTR Newsletter20091459