Abstract
Imiquimod (IQ) is an immune-response modifying agent, first approved by FDA for the topical treatment of external genital and perianal warts in 1997. It induces, through stimulation of Toll-like receptors (TLRs) localized on the surface of antigen-presenting cells, synthesis and release of several endogenous pro-inflammatory cytokines such as interferon-α (IFN-α), tumor necrosis factor-α (TNF-α) and interleukins (IL) 6 and 12, which in turn stimulate both the innate and acquired immune pathways, resulting in upregulation of natural antiviral and antitumor activity. IQ 5% cream has been used for the treatment of a wide variety of dermatologic conditions in which the immune system is thought to play a role in regression of the disease. In some disorders, such as genital and perianal warts, actinic keratoses, basal cell carcinomas, Bowen’s disease and molluscum contagiosum, relative safety and efficacy are supported by randomized controlled trials of IQ. However, it is common for patients to experience local skin reactions, which can range from mild to severe in intensity, but usually resolve 1–2 weeks after interrupting treatment. Additional randomized trials are encouraged to assess safety and efficacy of IQ in the treatment of an even wider range of cutaneous disorders.
Keywords:
Introduction
Imiquimod (IQ) is a member of a class of immune-response modifying agents, first approved by FDA for the topical treatment of external genital and perianal warts in 1997.
The effect of IQ cream on immunity has suggested its possible use in the treatment of a wide variety of dermatologic conditions in which the immune system is thought to play a role in regression of the disease (CitationTyring et al 2002; CitationVender and Goldberg 2005).
In this review recent literature regarding mechanism of action, clinical applications and safety of IQ will be discussed.
Mechanism of action
IQ (1-[2-methylpropyl]-1H-imidazo[4,5-c]quinolin-4-amine) is a synthetic imidazoquinoline amine that enhances, through cytokine induction, both the innate and acquired immune pathways, resulting in immunomodulating, antiviral and antitumor effects.
The mechanism of action of IQ is mainly related to the binding and stimulation of Toll-like receptors (TLRs), that are located on the surface of antigen-presenting cells, and serve to recognize and defend against invading pathogens (CitationChang et al 2005; CitationCeilley and Del Rosso 2006; CitationPatel et al 2006). IQ is a potent TLR-7 agonist which induces synthesis and release of several endogenous pro-inflammatory cytokines from Langerhans cells, monocytes/macrophages and dendritic cells. These include interferon-α (IFN-α), tumor necrosis factor-α (TNF-α) and interleukins (IL) 1, 6, 8, 10 and 12, which in turn activate and perpetuate cell-mediated immune responses primarily mediated by lymphocytes CD4+ and CD8+ (CitationSauder 2003; CitationSchiller et al 2006). In particular, IQ stimulates natural killer cells of the innate immune system and activates T helper cell type 1 and cytotoxic T lymphocytes responsible for killing virus-infected and tumor cells, as well as establishing immunological memory (CitationGarland 2003).
Immune-system induction is believed to be responsible for both antiviral and antitumor activity of IQ. However, recent studies have demonstrated that IQ may directly stimulate production of pro-apoptotic signalling. As a result, virus-infected keratinocytes and/or neoplastic cells are driven into apoptosis and eliminated. This process is mediated via death receptors, including Fas Receptor (FasR), a member of the tumor necrosis factor receptor family (CitationBerman et al 2003). When FasR binds its ligand, namely Fas ligand (FasL), a cascade of events follows, including caspase activation, ultimately eventuating in cellular death. In a vehicle-controlled study, IQ induced expression of FasR in basal cell carcinoma (BCC) cells, leading to cell apoptosis. Normally, BCC cells fail to express FasR, thus preventing cell apoptosis via FasR-Fas ligand interaction (CitationBerman et al 2003; CitationCeilley and Del Rosso 2006).
Clinical trials
IQ has been reported to be safe and effective in the treatment of a number of skin disorders (); however, the clinical literature is replete with case reports, limited case series and open label trials. This review will focus on those conditions in which IQ efficacy is supported by at least one randomized controlled trial (RCT).
Table 1 Topical imiquimod in the treatment of dermatologic disorders
External genital and perianal warts
IQ 5% cream is FDA approved for the treatment of external genital and perianal warts. Its efficacy in these disorders has been demonstrated in several RCTs (CitationBeutner et al 1998; CitationEdwards et al 1998; CitationPerry and Lamb 1999; CitationGarland et al 2001; CitationSauder et al 2003; CitationScheinfeld and Lehman 2006).
In the largest RCT (CitationEdwards et al 1998), 311 patients 18 years of age and older with external genital/perianal warts were randomized to 3 arms (109:102:100) IQ 5% cream, IQ 1% cream or vehicle 3 times per week for a maximum of 16 weeks. Complete clearance (absence of lesions) was achieved in 50% of IQ 5% patients, 21% of IQ 1% patients and 11% of placebo patients. There was a significantly better complete clearance response in female patients compared to males (72% vs 33% respectively) among the IQ 5% group. The mean time for complete clearance ranged from 8 weeks for women to 12 weeks for men. Recurrence rate of at least 1 wart for patients treated with IQ 5% was 13% (12 weeks after treatment was stopped).
In an international open-label multicentre phase IIIB trial (CitationGarland et al 2001) consisting of 943 patients from 114 clinic sites in 20 countries, IQ 5% cream was applied 3 times per week for up to 16 weeks with an overall complete clearance rate of nearly 48%. Recurrence rate at 6-months follow up was 23%. This study also confirmed a better clinical outcome in females (65%) compared to males (44%). The authors have hypothesized that the different outcome might be related to more incomplete keratinization of vulvar epithelium compared to that of male genitalia (CitationTyring et al 2002). The semi-occlusive effect of the foreskin has also played a role in the higher clearance rates observed among uncircumcised compared to circumcised individuals (CitationTyring et al 2002).
In another RCT (CitationBeutner et al 1998) IQ 5% cream was applied once daily for up to 16 weeks. Complete wart clearance occurred in 52% of patients, with 19% wart recurrence at a 12-week follow-up. These results are similar to those obtained with 3 applications/week (CitationEdwards et al 1998; CitationGarland et al 2001). Therefore, 3 times per week treatment schedule is recommended because of a relatively lower rate of side effects (CitationPerry and Lamb 1999; CitationGupta et al 2004; CitationChang et al 2005; CitationScheinfeld and Lehman 2006).
Topical IQ appears to be less effective in immunosuppressed patients. In a RCT, 97 male HIV-positive patients with genital warts and receiving no antiretroviral therapy, were treated with either IQ or vehicle: the complete response rates were 11% and 6% respectively (CitationGilson et al 1999).
Although further trials are required to compare the efficacy of IQ to other therapies, available data suggest that its efficacy is similar to that of other treatment modalities, including intralesional interferon and podophyllotoxin, but with lower recurrence rates (CitationPerry and Lamb 1999; CitationGarland 2003).
Actinic keratoses
IQ 5% cream is also FDA-approved for the treatment of actinic keratoses (AKs) of face and scalp in immunocompetent individuals and its efficacy has been demonstrated by several RCTs followed by three meta-analysis studies (CitationGupta et al 2005; CitationFalagas et al 2006; CitationHadley et al 2006).
The first meta-analysis study (CitationHadley et al 2006) included 5 RCTs with a total of 1293 patients. Complete clearance occurred in 50% of patients treated with IQ 5% cream compared to 5% in the placebo group. The number needed to treat (representing the number of patients needed to be treated with IQ compared to vehicle to obtain one additional beneficial event) was 2.2, showing that IQ 5% cream is an effective short term therapy.
Another meta-analysis study (CitationFalagas et al 2006) identified 4 RCTs including 1266 patients. In 2 studies IQ or vehicle cream was applied 3 times per week for 16 weeks, in one study applications were 3 times per week for 12 weeks, and in the fourth study, application rate was 2 times per week for 16 weeks. Meta-analysis for these studies showed complete clearance of AKs ranging from 45 to 84% in patients treated with IQ compared to 0%–7% in the vehicle group (p < 0.0001).
Most authors have observed that patients may experience an increase in the number of AKs within the treatment area at treatment initiation. This phenomenon is thought to be the result of the appearance of sub-clinical lesions rather than the formation of new ones (CitationFalagas et al 2006). Thus IQ’s ability to uncover and treat sub-clinical lesions may be considered an additional benefit of treatment with this drug (CitationFalagas et al 2006). Moreover, clearance of AKs is more frequent in patients who developed intense erythema or other local reactions at the application site, suggesting that inflammation is part of the mechanism of action for topical IQ (CitationFalagas et al 2006).
A third meta-analysis study (CitationGupta et al 2005) of 10 clinical trials (4 RCTs with topical IQ and 6 RCTs with topical fluorouracil) indicated that IQ may have relatively higher efficacy than topical fluorouracil for AKs located on the face and scalp. The mean efficacy rate for each drug (with 95% confidence interval) was 5-fluorouracil, 52 ± 18% (n = 6 studies, 145 subjects) and IQ, 70 ± 12% (n = 4 studies, 393 subjects).
Long-term effectiveness of topical IQ in the treatment of AKs remains to be determined; only one RCT consisted of “long-term” follow-up (25 patients with 1-year follow-up had 10% recurrence rate) (CitationStockfleth et al 2002). Moreover, optimal frequency of application and duration of treatment need to be elucidated by further studies.
Basal cell carcinoma
Topical IQ 5% cream is FDA-approved for the treatment of small superficial BCCs.
A Cochrane Skin Group analysis of 7 RCTs demonstrated IQ to be effective and well-tolerated in the treatment of both superficial and nodular BCC ( and ), although response rates varied according to number of applications and BCC clinical type (superficial vs nodular) (Bath-Hextal et al 2006).
Figure 1 Superficial BCC of the scalp before (a) and after (b) 8 weeks of treatment with IQ 5% cream 5 applications/week: complete clearance.
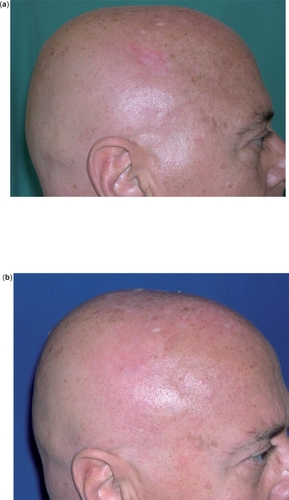
Figure 2 Nodular BCC of the nose before (a) and after (b) 6 weeks of treatment with IQ 5% cream 5 applications/week: complete clearance.
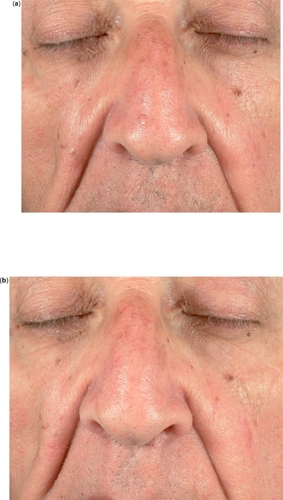
A multicenter 6-week dose-response trial (CitationMarks et al 2001) on 99 patients with primary superficial BCCs comparing different application regimens of IQ 5% cream showed histological clearance rates of 100%, 88%, 73% and 70% for twice daily, once daily, 6 times weekly and 3 times weekly regimens, respectively. Another RCT of 128 patients (CitationGeisse et al 2002) compared IQ in different dose regimens versus vehicle for superficial BCCs ranging in size between 0.5 and 2 cm2 for 12 weeks. Clearance rates were respectively 100%, 87%, 81% and 52% for twice daily, once daily, 5 days per week and 3 days per week applications; clearance rate for vehicle was 19%.
A European multicenter study (CitationSchulze et al 2005) of 166 subjects evaluated the clinical efficacy of IQ vs vehicle applied 7 times per weeks for 6 weeks: composite clearance (based on both clinical and histological clearance) was demonstrated in 77% and 6% of cases respectively (p < 0.001), while histological clearance occurred in 80% and 6% of cases, respectively (p < 0.001). However, in comparing 5 vs 7 applications per week for 6 weeks (CitationGeisse et al 2004), there was no statistically significant or clinically meaningful difference in complete clearance rate between the 2 regimens (75% vs 73% composite clearance, based on both clinical and histological clearance). Therefore, the 5-times-per-week regimen is preferred, as it is able to provide a balance of efficacy and safety, with fewer treatment-emergent side effects.
Nodular BCCs are more difficult to treat with topical IQ, most likely because of the skin barrier effect and the deeper localization of tumor cells (CitationChang et al 2005). In a RCT of 92 patients (CitationShumack et al 2002), the rates of subjects having no histological evidence of BCC in post-treatment excision specimens were 75%, 76%, 70% and 60% after 12 weeks for twice daily, once daily, five days per week or three days per week respectively; treatment failure for vehicle was 87%. These results were confirmed by a 6-week, randomized, open-label, dose-response study evaluating 4 dosing regimens on 99 patients, in which topical IQ application once daily for 7 days per week resulted in the highest clearance rate (71%) (CitationShumack et al 2002).
Further, two studies comparing the use of topical IQ 5% cream with and without occlusion in the treatment of superficial and nodular BCCs (CitationSterry et al 2002) showed no significant difference in early treatment failure for the two application modalities.
Finally, some case reports (no RCTs) have claimed positive responses to topical IQ 5% in large superficial BCCs (CitationShumack et al 2004; CitationMicali et al 2005) and multiple BCCs as observed in Gorlin syndrome (CitationKagy and Amonette 2000; CitationMicali, Lacarrubba, et al 2003).
Indications for the use of topical IQ in the treatment of BCCs are summarized in . Further long-term studies on the efficacy of topical IQ compared to surgery for BCCs are needed.
Table 2 Topical imiquimod in the treatment of BCCs: indications
Bowen’s disease
A recent RCT has demonstrated the efficacy of topical IQ 5% cream in the treatment of Bowen’s disease (CitationPatel et al 2006). Thirty-one patients with biopsy-proven Bowen’s disease were randomly assigned to receive in a double-blind fashion either IQ 5% cream (Pt =15) or vehicle (Pt =16) daily for 16 weeks. At the end of the study, 9 of the 12 patients completing the study in the IQ group (75%) showed clinical and histological resolution, with no relapse during a 9-month follow-up period, while no improvement was recorded in the placebo group (p < 0.001). The authors concluded that the lack of response in the 3 patients treated with IQ was associated with the presence of thick, hyperkeratotic lesions. The results from this single RCT are in agreement with several case reports and non-randomized clinical trials (CitationMackenzie-Wood et al 2001; CitationSmith et al 2001), but further studies are required.
Molluscum contagiosum
The efficacy of topical IQ 5% cream in childhood molluscum contagiosum has been evaluated in a double-blind RCT (CitationTheos et al 2004). Twenty-three children (age 1–9 years) were randomized to receive either IQ cream 5% (Pt = 12) or vehicle (Pt = 11) 3 times a week for 12 weeks. At the end of the study partial clinical clearance (≥ 30% clearance of lesions) was noted in 67% of IQ patients vs 18% of controls; also, complete clinical clearance was noted in 33.3% vs 9.1% of patients, respectively. The mean percentage change in lesion count at week 12 was −46% in the IQ group and +27% in the vehicle group.
Further, two case series have reported some positive outcomes with topical IQ in treating molluscum contagiosum in both children and adults (CitationBayerl et al 2003; CitationHengge and Cusini 2003).
Safety
In all the RCTs examined in this review topical IQ 5% cream has demonstrated an acceptable safety profile.
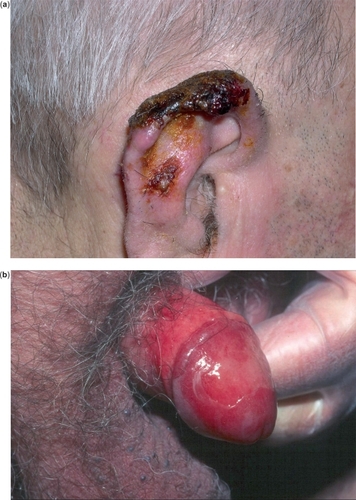
During treatment with topical IQ it is common for patients to experience local skin reactions that range from mild to severe, and may extend beyond the application site onto the surrounding skin. Such reactions include burning, pruritus, pain, tenderness, erythema, oedema, vesicles, erosions, ulcerations, excoriations, exudation and crusting (–). In cases of acute reactions temporary discontinuation (generally 1–2 weeks) and application of cold compresses is suggested. Superficial scarring, pigmentary changes, and, rarely, induction of other dermatoses (psoriasis, pemphigus foliaceus, aphthosis, vitiligo, angioedema, eruptive epidermoid cysts) have also been reported (CitationBarton 2004; CitationGilliet et al 2004; CitationBrown et al 2005; CitationMarty et al 2005; CitationMashiah and Brenner 2005; CitationZalaudek et al 2005).
Figure 3 Local skin reactions during treatment with IQ 5% cream: marked erythema (a), erosion, exudation, and crusting (b).
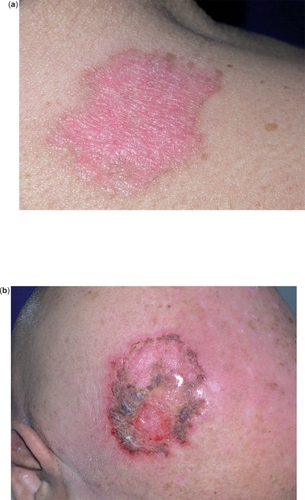
Figure 4 Local skin reactions during treatment with IQ 5% cream: crusting (a), marked erythema, erosion, and exudation (b).
Systemic absorption appears to be minimal and influenza-like or gastrointestinal symptoms (fatigue, fever and chills, arthralgias, myalgias, nausea, diarrhea) or induction of non-dermatologic disorders (chronic neuropathic pain, autoimmune spondyloarthropathy) are rare (CitationBenson 2004; CitationChang et al 2005; CitationYi et al 2005).
Exposure to sunlight (including sunlamps) should be avoided or minimized during use of topical IQ because of concern for heightened sunburn susceptibility, therefore the use of sunscreen might be encouraged.
Safety of topical IQ during pregnancy is not established and thus its use is contraindicated. Animal studies did not show any evidence of teratogenicity or fetotoxicity. However, contraception is recommended for women of childbearing age using topical IQ (CitationScheinfeld and Lehman 2006).
Conclusions
IQ is a topically active immunomodulatory agent that is currently available as a 5% cream in single-use sachets for the treatment of some viral and non-viral conditions. It is generally applied sparingly at bedtime from 3 to 5 times per week and washed off in the morning (CitationChang et al 2005; CitationScheinfeld and Lehman 2006). For AKs and superficial BCCs, it may be possible to reduce treatment cost by repeated use of a sachet, which is not advised when treating genital warts because of the potential risk of transferring infectious material into the sachet (CitationPatel et al 2006).
The use of IQ in dermatologic disorders presents some advantages: the cream is self-applied by the patient, compared to other therapeutic strategies, avoiding more costly and painful procedures (CitationPerry and Lamb 1999; CitationBath-Hextal et al 2006); in addition, it provides a non-scarring cosmetic outcome, when compared to surgical procedures. Also, side effects are usually localized to the treatment site and resolve after treatment cessation.
Because of the potential properties of topical IQ, additional RCTs are encouraged to assess its efficacy in the treatment of other cutaneous disorders. Moreover, as the relatively reduced response rate in patients treated with topical IQ is apparently associated with fully keratinized skin, the use of adjuvant therapies to decrease the stratum corneum/epidermis barrier and improve drug penetration and efficacy warrant further investigation (CitationPatel et al 2006).
References
- BadavanisGMonastirliAPasmatziESuccessful treatment of granuloma annulare with imiquimod cream 5%: a report of four casesActa Derm Venereol200585547816396814
- BartonJCAngioedema associated with imiquimodJ Am Acad Dermatol200451477815338000
- Bath-HextallFJBongJPerkinsWInterventions for basal cell carcinoma of the skin (Cochrane review)Cochrane Database Syst Rev2006I
- BayerlCFellerGGoerdtSExperience in treating molluscum contagiosum in children with imiquimod 5% creamBr J Dermatol2003149Suppl 6625914616342
- BaumannLSHalemMLLip silicone granulomatous foreign body reaction treated with aldara (Imiquimod 5%)Dermatol Surg2003294293212656829
- BensonEImiquimod: potential risk of an immunostimulantAustralas J Dermatol200445123415068461
- BermanBSullivanTDe AraujoTExpression of Fas-receptor on basal cell carcinomas after treatment with imiquimod 5% cream or vehicleBr J Dermatol2003149Suppl 66596114616354
- BeutnerKRTyringSKTrofatterKFJrImiquimod, a patient-applied immune-response modifier for treatment of external genital wartsAntimicrob Agents Chemother199842789949559784
- BrownTZirviMCotsarelisGVitiligo-like hypopigmentation associated with imiquimod treatment of genital wartsJ Am Acad Dermatol2005527151615793538
- BrummittCFImiquimod 5% cream for the treatment of recurrent, acyclovir-resistant genital herpesClin Infect Dis20064257516421805
- CeilleyRIDel RossoJQCurrent modalities and new advances in the treatment of basal cell carcinomaInt J Dermatol2006454899816700779
- ChangYCMadkanVCook-NorrisRCurrent and potential uses of imiquimodSouth Med J2005989142016217984
- CohenPRSchulzeKETschenJATreatment of extramammary Paget disease with topical imiquimod cream: case report and literature reviewSouth Med J20069939640216634252
- DeethsMJChapmanJTDellavalleRPTreatment of patch and plaque stage mycosis fungoides with imiquimod 5% creamJ Am Acad Dermatol2005522758015692473
- DidonaBBenucciRAmerioPPrimary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimodBr J Dermatol2004150119820115214911
- Di LerniaVRicciCAlbertiniGSpontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod creamJ Eur Acad Dermatol Venereol200418626915324413
- DytocMTingPTManJFirst case series on the use of imiquimod for morphoeaBr J Dermatol20051538152016181467
- EdwardsLFerenczyAEronLSelf-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human PapillomaVirusArch Dermatol199813425309449906
- ErbagciZErbagciIAlmila TuncelARapid improvement of human orf (ecthyma contagiosum) with topical imiquimod cream: report of four complicated casesJ Dermatolog Treat200516353616428161
- ErbagciZTuncelAAErkilicSSuccessful treatment of antifungal- and cryotherapy-resistant subcutaneous hyalohyphomycosis in an immunocompetent case with topical 5% imiquimod creamMycopathologia2005159521615983738
- FalagasMEAngelousiAGPeppasGImiquimod in the treatment of actinic keratosis. A meta-analysis of randomized controlled trialsJ Am Acad Dermatol200655537816908371
- GarlandSMImiquimodCurr Opin Infect Dis20031685912734440
- GarlandSMSellorsJWWikstromAImiquimod Study Group. Imiquimod 5% cream is a safe and effective self-applied treatment for anogenital warts – results of an open-label, multicentre Phase IIIB trialInt J STD AIDS200112722911589811
- GeisseJCaroILindholmJImiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studiesJ Am Acad Dermatol2004507223315097956
- GeisseJKRichPPandyaAImiquimod 5% cream for the treatment of superficial basal cell carcinoma: a double-blind, randomized vehicle-controlled studyJ Am Acad Dermatol200247390812196749
- GillietMConradCGeigesMPsoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursorsArch Dermatol20041401490515611427
- GilsonRJShupackJLFriedman-KienAEA randomized, controlled, safety study using imiquimod for the topical treatment of anogenital warts in HIV-infected patients. Imiquimod Study GroupAIDS199913239740410597781
- GoldenbergGKrowchukDPJorizzoJLSuccessful treatment of a therapy-resistant pyogenic granuloma with topical imiquimod 5% creamJ Dermatolog Treat200617121316766338
- GoorneyBPPoloriRA case of Bowenoid papulosis of the penis successfully treated with topical imiquimod cream 5%Int J STD AIDS200415833515601490
- GuptaAKChermanAMTyringSKViral and nonviral uses of imiquimod: a reviewJ Cutan Med Surg200483385215868314
- GuptaAKDaveyVMcPhailHEvaluation of the effectiveness of imiquimod and 5-fluorouracil for the treatment of actinic keratoses: critical review and meta-analysis of efficacy studiesJ Cutan Med Surg200592091416502198
- HadleyGDerrySMooreRAImiquimod for actinic keratoses: systematic review and meta-analysisJ Invest Dermatol20061261251516557235
- HazenPGCarneyJFEngstromCWProliferating hemangioma of infancy: successful treatment with topical 5% imiquimod creamPediatr Dermatol200522254615916578
- HenggeURCusiniMTopical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosumBr J Dermatol2003149Suppl 66151914616340
- HughesPSTreatment of lymphomatoid papulosis with imiquimod 5% creamJ Am Acad Dermatol200654546716488317
- JainSSuccessful treatment of porokeratosis of Mibelli with imiquimod 5% creamClin Exp Dermatol200631302316487129
- JoJHKoHCJangHSInfiltrative trichilemmal carcinoma treated with 5% imiquimod creamDermatol Surg200531973616042947
- JoJHChinHWKimMBA case of eccrine poroma treated with 5% imiquimod creamJ Dermatol200532691316334877
- KagyMKAmonetteRThe use of imiquimod 5% cream for the treatment of superficial basal cell carcinomas in a basal cell nevus syndrome patientDermatol Surg200026577810848940
- KellySCPurcellSMImiquimod therapy for elastosis perforans serpiginosaArch Dermatol20061428293016847197
- LeTHicksWMenardCPreliminary results of 5% imiquimod cream in the primary treatment of vulva intraepithelial neoplasia grade 2/3Am J Obstet Gynecol20061943778016458632
- Mackenzie-WoodAKossardSde LauneyJImiquimod 5% cream in the treatment of Bowen’s diseaseJ Am Acad Dermatol2001444627011209116
- MarksRGebauerKShumackSAustralasian Multicentre Trial Group. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trialJ Am Acad Dermatol2001448071311312429
- Martin-GarciaRFImiquimod: an effective alternative for the treatment of invasive cutaneous squamous cell carcinomaDermatol Surg200531371415841646
- MartyCLRandleHWWalshJSEruptive epidermoid cysts resulting from treatment with imiquimodDermatol Surg200531780216029707
- MashiahJBrennerSPossible mechanisms in the induction of pemphigus foliaceus by topical imiquimod treatmentArch Dermatol2005141908916027316
- MicaliGDall’OglioFNascaMRAn open-label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous wartsJ Dermatol Treat2003142336
- MicaliGLacarrubbaFNascaMRThe use of imiquimod 5% cream for the treatment of basal cell carcinoma as observed in Gorlin’s syndromeClin Exp Dermatol200328S1192314616807
- MicaliMNascaMRMusumeciMLTreatment of an extensive superficial basal cell carcinoma of the face with imiquimod 5% creamInt J Tissue React2005271111416372477
- MicaliGNascaMRTedeschiATopical treatment of intraepithelial penile carcinoma with imiquimodClin Exp Dermatol200328S14614616802
- Miranda-VerasteguiCLlanos-CuentasAArevaloIRandomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in PeruClin Infect Dis200540139540315844060
- NaylorMFCrowsonNKuwaharaRTreatment of lentigo maligna with topical imiquimodBr J Dermatol2003149Suppl 66667014616356
- PatelGKGoodwinRChawlaMImiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen’s disease): a randomized, double blind, placebo-controlled trialJ Am Acad Dermatol20065410253216713457
- PatelPJSkinnerRBJrExperience with keloids after excision and application of 5% imiquimod creamDermatol Surg20063246216640700
- PerryCMLambHMTopical imiquimod: a review of its use in genital wartsDrugs1999583759010473026
- RedondoPDel OlmoJIdoateMAngiolymphoid hyperplasia with eosinophilia successfully treated with imiquimodBr J Dermatol200415111101115541101
- RinneDLinhartCSchoferHLip papillomatosis in immunodeficiency: therapy with imiquimodBr J Dermatol2000142196710819559
- SauderDNSkinnerRBFoxTLTopical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populationsSex Transm Dis200330124812567169
- SauderDNImiquimod: modes of actionBr J Dermatol2003149Suppl 665814616337
- SchalockPCKornikRIBaughmanRDTreatment of verrucous carcinoma with topical imiquimodJ Am Acad Dermatol2006545 SupplS233516631950
- ScheinfeldNLehmanDSAn evidence-based review of medical and surgical treatments of genital wartsDermatol Online J12316638371
- SchillerMMetzeDLugerTAImmune response modifiers – mode of actionExp Dermatol2006153314116630072
- SchulzeHJCribierBRequenaLImiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in EuropeBr J Dermatol20051529394715888150
- ShumackSGebaeuerKQuirkC5% imiquimod cream for the treatment of a large superficial basal cell carcinomaArch Dermatol2004140128615492202
- ShumackSRobinsonJKossardSEfficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma: comparison of dosing regimensArch Dermatol200213811657112224977
- SmithKJGermainMSkeltonHSquamous cell carcinoma in situ (Bowen’s disease) in renal transplant patients treated with 5% imiquimod and 5% 5-fluorouracil therapyDermatol Surg200127561411442593
- SmithKJGermainMYeagerJTopical 5% imiquimod for the therapy of actinic cheilitisJ Am Acad Dermatol20024749750112271290
- SterryWRuzickaTHerreraEImiquimod 5% cream for the treatment of superficial and nodular basal cell carcinoma: randomized studies comparing low-frequency dosing with and without occlusionBr J Dermatol200214712273612452875
- StockflethEMeyerTBenninghoffBA randomized, double-blind, vehicle-controlled study to assess 5% imiquimod cream for the treatment of multiple actinic keratosesArch Dermatol2002138149850212437457
- StockflethERowertJArndtRDetection of human papillomavirus and response to topical 5% imiquimod in a case of stucco keratosisBr J Dermatol20001438465011069470
- TavernaJAStefanatoCMWaxFDAdult cutaneous Langerhans cell histiocytosis responsive to topical imiquimodJ Am Acad Dermatol2006549111316635684
- TheosAUCumminsRSilverbergNBEffectiveness of imiquimod cream 5% cream for treating childhood molluscum contagiosum in a double-blind, randomized pilot trialCutis2004741348141215379366
- TyringSConantMMariniMImiquimod; an international update on therapeutic uses in dermatologyInt J Dermatol2002418101612453012
- VenderRBGoldbergOInnovative uses of imiquimodJ Drugs Dermatol20054586315696986
- YiBANirenbergMJGoldsteinSMChronic neuropathic pain associated with imiquimod: report of 2 casesJ Am Acad Dermatol20055257815692517
- WolfIHSmolleJBinderBTopical imiquimod in the treatment of metastatic melanoma to skinArch Dermatol2003139273612622616
- ZalaudekIPetrilloGArgenzianoGAphthous ulcers and imiquimodJ Am Acad Dermatol200553360116021146