Abstract
Neurotoxicity is the most frequent dose-limiting toxicity of oxaliplatin. Acute sensory neurotoxicity manifests as rapid onset of cold-induced distal dysesthesia and/or paresthesia, sometimes accompanied by cold-dependent muscular contractions of the extremities or the jaw. The symptoms, often occurring during or shortly after infusion, are usually transient and mild. A cumulative sensory peripheral neuropathy may also develop with prolonged treatment with oxaliplatin, eventually causing superficial and deep sensory loss, sensory ataxia, and functional impairment. Studies have shown patients with acute sensory symptoms to display little or no axonal degeneration. The similarity of acute symptoms induced by oxaliplatin to those caused by several drugs or toxins acting on neuronal or muscular ion channels suggests that these symptoms may result from a specific interaction of oxaliplatin with voltage-gated sodium (Na+) channels. The current recommendations for the management of the acute and cumulative neurotoxicity from oxaliplatin include education about exposure to cold, dose modification, “stop and go”, and use of neuromodulatory agents, in particular, intravenous calcium and magnesium infusion. Upon the approval of oxaliplatin-based regimens both for adjuvant and metastatic treatment of colon cancer, it is crucial to compile knowledge about the recognition and management of neurotoxicity from oxaliplatin.
Introduction
Nearly 150 000 Americans were diagnosed with colorectal cancer in 2004. This disease accounts for > 50 000 deaths per year and ranks second in cancer-related deaths in the United States (CitationJemal et al 2004). Among these cases of colorectal cancer, approximately 40% of patients had locally advanced disease or metastases not amenable to surgical resection.
5-fluorouracil (5-FU) has been a mainstay in the treatment of advanced colorectal cancer since the late 1950s (CitationMidgley and Kerr 1999; CitationMeyerhardt and Mayer 2005). Along the way, there have been improvements in clinical outcomes with altering dosing regiments and modulating 5-FU with leucovorin (LV) (CitationPetrelli et al 1989; CitationPoon et al 1989). Combination therapy with 5-FU-LV was considered standard of care up until the last decade. More recently, irinotecan and oxaliplatin were found to have activity in advanced colorectal cancer (CitationPetrelli et al 1989; CitationPoon et al 1989; CitationHoff and Pazdur 2004). Irinotecan inhibits topoisomerase I, a nuclear enzyme involved in the unwinding of DNA during replication (CitationHsiang et al 1989). Irinotecan has demonstrated activity against metastatic colorectal cancers when used as monotherapy (CitationShimada et al 1993; CitationConti et al 1996) or in conjunction with 5-FU-LV (CitationSaltz et al 2000).
Oxaliplatin is the only platinum derivative with activity against advanced colorectal cancer. It binds and cross-links strands of DNA, forming DNA adducts thus inhibiting DNA replication and transcription (Citationde Gramont et al 2000). Citationde Gramont and colleagues (2000) published one of the initial trials showing the success of using oxaliplatin in combination with 5-FU-LV as first-line therapy in advanced colorectal cancer. In that study, 420 previously untreated patients with colorectal cancer deemed unresectable were randomized to receive LV followed by bolus and infusion of 5-FU every 2 weeks (LV5FU2) either alone or with oxaliplatin 85 mg/m2 (FOLFOX4). Patients receiving the FOLFOX4 regimen had significantly longer progression-free survival (median, 9.0 vs 6.2 months, p = 0.0003) and better response rate (50.7% vs 22.3%, p = 0.0001), but the difference in median overall survival did not reach clinical significance when compared to LV5FU2 alone (Citationde Gramont et al 2000).
Not until recently had there been a head to head comparison of oxaliplatin-and-irinotecan-containing regimens in advanced colorectal cancer. CitationTournigand and colleagues (2004) provided one of the first published trials comparing oxaliplatin-and-irinotecan-based regimens. They set out to investigate two sequences: (1) folinic acid, 5-FU, and irinotecan (FOLFIRI), followed by folinic acid, 5-FU, and oxaliplatin (FOLFOX6), and (2) FOLFOX6 followed by FOLFIRI. The irinotecan-and-oxaliplatin-based regimens each used bolus 5-FU followed by a 46-hour continuous infusion. Their results showed that both sequences were similar in efficacy with median survival being greater than 20 months in both arms (p = 0.99) (CitationTournigand et al 2004). In another study by CitationGoldberg et al (2004), 795 patients were randomly assigned to receive FOLFOX4, irinotecan, and oxaliplatin (IROX), or irinotecan and bolus 5FU plus LV (IFL). Their data showed that FOLFOX4 was superior to the other two regimens in terms of median time to progression, response rates, and median survival. This study was unique by showing an increase in median survival in FOLFOX4 compared to IFL (19.5 months vs 15 months, p = 0.0001). Paresthesias occurred in 18 out of 258 patients in the FOLFOX4 arm around cycles eight to ten (CitationGoldberg et al 2004). Based on these data, the oxaliplatin-containing regimen was approved as first-line therapy in advanced colorectal cancer, making oxaliplatin-induced peripheral neuropathy a growing problem.
Types of neurotoxicity associated with oxaliplatin
Oxaliplatin is a third generation platinate that differs structurally from earlier platinates. The antineoplastic properties of third generation platinum derivatives are based on platinum chelation with the rigid cyclic structure 1,2-diaminocyclohexane (DACH) (CitationGraham et al 2000). The neurotoxicity seen with oxaliplatin can manifest as either of two distinct syndromes: a transient, acute syndrome that can appear during or shortly after infusion, and a dose-limiting, cumulative sensory neuropathy.
Acute, transient neurotoxicity
The acute, transient neurotoxicity observed with oxaliplatin occurs in nearly all patients. This toxicity is rapid in onset, occurring during or within hours of infusion. The symptoms are peculiar in that they are often induced or aggravated by exposure to cold. There may be manifestations of distal sensory and motor toxicity. The sensory component consists of paresthesias and/or dysesthesias in the distal extremities and/or the perioral region. About 1%–2% of patients will report a transient cold-induced pharyngolaryngeal dysesthesia, causing a feeling of difficulty in breathing. These sensory symptoms are less frequently paralleled by motor symptoms including: tetanic spasms, fasiculations, and prolonged muscular contractions. The acute motor toxicity seen with oxaliplatin has been likened to that of neuromyotonias, tetrodotoxin, and ethylene glycol poisoning, which suggests hyperexcitablity of motor neurons as the mechanism (CitationGolleau et al 2001; CitationWilson et al 2002).
Chronic, cumulative sensory neuropathy
The dose-limiting, cumulative sensory neurotoxicity is seen in 10%–15% of patients after cumulative doses of 780–850 mg/m2 (Citationde Gramont et al 2000; CitationGrothey et al 2002). Symptoms consist primarily of noncold-related dysesthesias and paresthesias of the extremities. These symptoms are quite similar to those seen with cisplatin toxicity, although ototoxicity is rare with oxaliplatin. Symptoms generally persist between cycles and increase in intensity with cumulative dose. Impaired sensation, sensory ataxia, and/ or deficit in fine sensory-motor coordination may ultimately occur (CitationGrothey 2003). The symptoms may be severe enough to limit patients from performing their activities of daily living.
Importantly, these symptoms are consistently reversible with the majority of patients recovering from grade 3 neurotoxicity to grade 1 or less within 6–12 months of therapy discontinuation (CitationBrienza et al 1995; CitationGrothey 2003). In a phase III trial done by Citationde Gramont and colleagues (2000), 74% of patients recovered from grade 3. The median time to recovery was 13 weeks (Citationde Gramont et al 2000). This reversibility makes the oncologist role pivotal in using the dosing scheme to manage these side effects.
An atypical presentation of oxaliplatin neurotoxocity is cited in the literature as manifesting as Lhermitte's sign (CitationTaieb et al 2002). Lhermitte's sign, also referred to as the barber shop sign, is described as electric shock type sensation induced by forward flexion of the head. This occurred in patients who had received high cumulative doses of oxaliplatin (> 1000 mg/m2).
Grading of neurotoxicity
The unique set of oxaliplatin-induced neurologic events that patients experience has prompted the development of a specific neurotoxicity scale to score the duration and severity of symptoms. compares the features of this new grading system with the National Cancer Institute neurotoxicity scales.
Table 1 Grading of oxaliplatin-induced neurotoxicity
Pathogenesis
Neurotoxicity has been observed with platinates since the first drug in the class, cisplatin, was introduced to oncologists. The neurotoxicity with cisplatin is cumulative and dose dependent. It is characterized as a distal sensory neuropathy and may be associated with ototoxicity as well. Severely affected patients may experience ataxia. The toxicity with cisplatin is generally not seen until cumulative doses reach the 300 mg/m2 range. Recovery is generally slow and incomplete (CitationScrenci and McKeage 1999). Carboplatin, a second generation platinate, generally shows dose-limiting hematologic toxicities. Although it is less frequent, carboplatin can produce a similar ototoxicity and distal neuropathy to that seen with cisplatin. Symptoms are generally less severe and recovery is better (CitationCavaletti et al 1998; CitationNeijt et al 2000). See .
Table 2 Comparison of neurotoxicity profiles of cisplatin and oxaliplatin
The acute sensory neuropathy seen with oxaliplatin is described in the literature as a “channelopathy”. This is based on the similarities that oxaliplatin-induced neuropathy shares with hereditary myotonias and certain toxin exposures. After an action potential has been elicited, the fast depolarizing current is dependent upon sodium influx through voltage-gated ion channels. The action potential is terminated through sodium channel inactivation coupled with repolarization by an efflux of potassium ions. Multiple different sodium channels are expressed in sensory neurons and each contains different electrical properties. Disorders of these nerve ion channels are characterized by an increase or decrease in the excitability of a neuron.
A study by CitationWu and colleagues (2001) identified a mutation in a voltage-gated sodium channel in a family with cold-aggravated myotonia. This mutation led to functional defects of fast inactivation of the sodium channels presumably provoking myotonic spells (CitationWu et al 2001). It has been shown in rat models that oxaliplatin alters voltage-gated sodium channels. The effects of oxaliplatin were studied on rat nerve preparations by CitationAldersberger et al (2000), who exposed rat nerves to oxaliplatin. They observed an increase in amplitude and duration of compound action potentials and prolongation of the refractory period of peripheral nerves. They also noticed that the potassium current was not altered with the addition of oxaliplatin after sodium channels were blocked using tetrodotoxin. This was an important finding, which suggested that the alterations in the action potential were related to sodium and not potassium channels. The authors implicate that oxalate, a known calcium chelator, may play a role in the sodium channel interaction by chelating calcium; however, they found no difference in the antagonistic effect of oxaliplatin on sodium channels at low calcium concentrations (Aldersberger et al 2000).
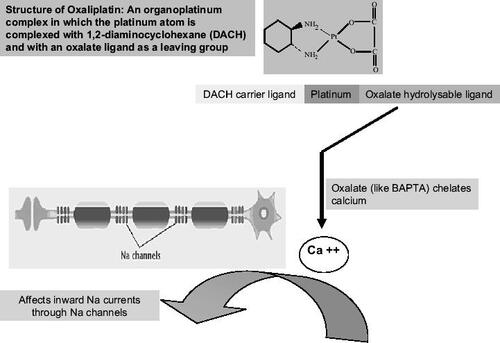
The theory that an oxalate affects the sodium channels has been entertained by other researchers as well. Oxalate is released intracellularly from oxaliplatin by bicarbonate ions. Oxalate and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), another calcium chelator, have produced effects on inward sodium currents in invertebrate models similar to those seen with oxaliplatin (CitationGrolleau et al 2001). See .
Figure 1 Postulated mechanism underlying the pathogenesis of neurotoxicity caused by oxaliplatin. Na+ channelopathy: the theory that an oxalate affects the sodium channels has been entertained by other researchers as well. Oxalate is released intracellularly from oxaliplatin by bicarbonate ions. Oxalate and BAPTA, another calcium chelator, have produced effects on inward sodium currents in invertebrate models similar to those seen with oxaliplatin.
Unlike the acute transient neuropathy, the cumulative toxicity of oxaliplatin appears to be related to direct toxicity to the nerve. Morphological changes have been evident in dorsal root ganglia in rats treated with cumulative doses of oxaliplatin intraperitoneally. The treated rats had evidence of nuclear, nucloeolar, and somatic size reduction on microscopic examination of the dorsal root ganglia (CitationCavaletti et al 2001). These studies have set the framework for various treatments for oxaliplatin-induced neurotoxicity.
Management of oxaliplatin-induced neurotoxicity
Prior to oxaliplatin administration, a brief, standardized neurologic examination should include the testing of exteroceptive sensation at hands and feet (fine touch, cotton, pain, pinprick, and deep pressure pain), and testing of proprioceptive sensation (position of the limbs, Romberg's test, perception of passive movements in fingers and toes, and possibly assessment of vibratory sensation by a tuning fork or digital device at ankles and wrist). Testing of osteotendinous reflexes may confirm the presence of sensory impairment. Patients complaining about difficulties in fine movements may be asked to perform some tasks such as buttoning their shirt, lacing their shoes, picking up coins, or writing a few sentences. Patients who complain about cold related symptoms could develop “cramps” or “spasms” if asked to chill a hand in cold water to assess the presence of muscular contractions, and this should be avoided.
Methods to treat or prevent oxaliplatin-induced neurotoxicity center around two main concepts: dosing strategies and neuromodulatory agents, but educating the patient and care giver is of utmost importance.
Education
Education of the patients and care givers, including both physician and nursing staff, about symptoms resulting from oxaliplatin-induced neurotoxicity is paramount. It is equally essential that they be familiar with measures to be taken to manage these events. Patients must be instructed to avoid exposure to cold objects, environment, and liquids. Reassurance that the acute symptoms of neurotoxicity are transient is very important. The profession of the patient, such as a meat handler, should be considered before offering such an agent. Additionally, patients should be routinely questioned on the events or presence of subjective symptoms. Such questions must be focused on the nature (paresthesias, dysesthesias, hyperesthesias, pain, numbness, muscle contractions, and weakness), location (extremities, perioral area), relationship to cold (if any), time course (onset in relation to oxaliplatin infusion, duration, transient versus persistent), and severity (presence of functional impairment).
In case of reported “difficulty breathing” or “laryngo-spasm”, it is important to distinguish between cold-related symptoms (pharyngolaryngeal dysesthesia, muscular contractions) and noncold-related symptoms (muscular contractions). In the latter case, if a cutaneous rash is present, a differential diagnosis of acute hypersensitivity associated with oxaliplatin is mandatory. Checking O2 saturation may be useful in ruling out the presence of an allergic reaction associated with Quincke's edema. Furthermore, this would substantially help reassure the patient.
Dose modification and change in schedule
The recent clinical trials with oxaliplatin 85 mg/m2 have applied an algorithm for oxaliplatin dose reductions in patients presenting with signs/symptoms of neurotoxicity. The main decisional criteria for dose reduction were transience versus persistence of symptoms, relationship of clinical symptoms to cold, and presence of pain, functional impairment, and/or sensory abnormalities at neurologic examination. The leading principle of the algorithm was to allow for only one dose reduction and to stop treatment until improvement or recovery (see drug information approved by FDA and supplied with injection).
The presence of transient paresthesias associated with pain or functional impairment led to dose lowering from 85 to 75 mg/m2 when neurologic examination was normal in adjuvant setting and 65 mg/m2 in the metastatic setting. If the neurologic examination was abnormal, the cycle of therapy was omitted with resumption of therapy at the scheduled next cycle. If paresthesias with pain or functional impairment persisted between cycles, therapy was interrupted until improvement was noted, at which time therapy was resumed at the lower therapeutic dose (see drug insert supplied with drug). In trials that scheduled oxaliplatin 130 mg/m2 every 3 weeks, the therapeutic dose was reduced to 100 mg/m2, with all other parameters remaining the same (Citationde Gramont et al 2004).
Prolonging the duration of infusion from 2 to 6 hours can usually prevent recurrence of pharyngolaryngeal dysesthesia by decreasing the Cmax by an estimated 32%. In such cases, no dose reduction is not required then.
OPTIMOX (STOP and GO) concept
Observations of reversibility of oxaliplatin-induced neurotoxicity led Citationde Gramont and colleagues (2000) to develop a dosing scheme termed the STOP and GO (OPTIMOX) strategy with the goal of increasing the cumulative oxaliplatin dose that can be given. This strategy uses a 5-FU-LV infusion (without bolus) over 46 hours plus oxaliplatin 130 mg/m2 every 2 weeks for 6 cycles until a cumulative dose of 780 mg/m2 has been achieved. After this, oxaliplatin is held and treatment with 5FU-LV is continued in the following fashion: day 1, LV 200 mg/m2 (over 2 hours); bolus 5-FU 400 mg/m2, infusion of 5-FU 2.4–3.0 mg/m2 over 46 hours every 2 weeks. The oxaliplatin is reintroduced after a 6-month hiatus. A phase III study including 608 patients was presented at the American Society of Clinical Oncology Meeting in May 2003, which compared OPTIMOX concept with FOLFOX4. The OPTIMOX arm showed significantly lower rates of Grade 3 neurotoxicity (13% vs 19%; p = 0.0017) without compromising response rates (RR) or progression free survival (PFS) (RR 63.1% vs 59.8%; PFS 9.2 vs 8.9 months) (Citationde Gramont et al 2004). This appears to be a promising approach to prolong the administration of platinate therapy without compromising efficacy.
In the MOSAIC study evaluating FOLFOX in the adjuvant treatment of colon cancer, the main side effect of FOLFOX4 was also the anticipated sensory neuropathy (CitationAndre et al 2004). The overall incidence of grade 3 neurotoxicity was 12.4% and 18% among patients who received the entire planned 1020 mg/m2 dose of oxaliplatin. However, the neurotoxicity proved reversible in the vast majority of patients so that at 12 and 18 months after discontinuation of therapy only 1.1% and 0.5% of patients, respectively, had residual grade 3 neurotoxicity (CitationAndre et al 2004).
In another study conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP C-07) and reported at American Society of Clinical Oncology (ASCO) (2005) (CitationWolmark et al 2005), in which 2407 patients were randomized to either Roswell Park schedule 5FU-LV (500 mg/m2 of both given weekly for 6 weeks, followed by 2 weeks' rest for 3 cycles) versus the same 5FU-LV regimen and oxaliplatin (FLOX) (oxaliplatin at 85 mg/m2 every two weeks, but only on weeks 1, 3, and 5 of the 8-week cycle; cumulative dose 765 mg/m2). Seventy-three percent of patients received the planned oxaliplatin treatment. The regimen was tolerable as grade 3 and 4 toxicities were similar in the two arms (Grade 3/4–50%/10% FLOX vs 41%/9% 5FU-LV). Only 8% of patients experienced grade 3 neurotoxicity and this decreased to 0.5% of patients after 12 months (CitationWolmark et al 2005).
On the other hand, there are data suggesting that oxaliplatin dose intensification significantly improves RR and PFS in pretreated metastatic disease without increasing severe toxicity. CitationMaindrault-Goebel et al (2000) retrospectively analyzed data from three phase II studies using different FOLFOX regimens (FOLFOX2, 3, and 6). Data on 126/161 patients was included. FOLFOX2 included oxaliplatin 100 mg/m2; FOLFOX3, 85 mg/m2; and FOLFOX6, 100 mg/m2. Forty-seven patients received low dose intensity (LDI) oxaliplatin (LDI: ≤ 85 mg/m2/2 weeks) and 79 patients high dose intensity (HDI) oxaliplatin (HDI: > 85 mg/m2/2 weeks). It was found that the objective responses occurred in 31 (39%) HDI patients and 9 (19%) LDI patients (p = 0.03). Median PFS was 28 weeks, with 52% of HDI patients progression free at 6 months, and 26 weeks with 36% of LDI patients progression free at 6 months (p = 0.02). Increased oxaliplatin dose intensity was not associated with increased neurotoxicity or other toxicities. FOLFOX are among the most effective regimens for treating LV-5-FU-resistant metastatic colorectal cancer. This study showed that oxaliplatin dose intensification significantly improves RR and PFS in pretreated metastatic disease without increasing severe toxicity (CitationMaindrault-Goebel et al 2000).
Neuromodulatory agents
Ca/Mg infusion
Divalent cations have the ability to modify voltage-gated sodium channels (CitationGremlin et al 2002). It is hypothesized that the acute neurotoxicity of oxaliplatin is related to the ability of oxalate to chelate calcium. Increases in extracellular calcium have been shown to increase the probability of sodium channel closure decreasing the hyperexcitability of peripheral neurons seen in oxaliplatin-induced neuropathy (CitationArmstrong and Cota 1999). Magnesium supplementation has been previously studied in preventing cisplatin-induced hypomagnesemia (CitationLajer and Daugaard 1999).
This promising treatment is based on a retrospective study by CitationGamelin and colleagues (2004) of 161 patients treated with varying regimens of oxaliplatin and 5FU-LV. Patients had received one of three various oxaliplatin regimens (85 mg/m2/2 weeks; 100 mg/m2/2 weeks; or 130 mg/m2/3 weeks). Ninety-six patients received 1 g each of calcium gluconate and magnesium sulfate intravenously over 15 minutes just before the oxaliplatin infusion. This dose was repeated after completion of the infusion. The percentage of patients with grade 3 distal paresthesias was significantly lower in the Ca/Mg group (7% vs 26%, p = 0.001). The acute symptoms of distal and perioral paresthesias were much less frequent. No patients in the Ca/Mg group experienced pseudolaryngospasm. Furthermore, the Ca/Mg group recovered more rapidly from neuropathy especially in the patients receiving 85 mg/m2 of oxaliplatin (< 2 months). The Ca/Mg infusions had no bearing on treatment efficacy (CitationGamelin et al 2004). This is a simple strategy to help ameliorate the symptoms of acute oxaliplatin-induced neuropathy, but further investigation is warranted to determine if this treatment is effective in preventing chronic, cumulative neurotoxicity. It must be borne in mind that this strategy is based on a single retrospective analysis of a nonrandomized study. Prospective, randomized studies (such as the CONCEPT study) are underway to validate the benefit of these minerals in ameliorating the neurotoxicity of oxaliplatin.
We recently reported on a case of a patient in which oral calcium supplements not only was successful in treating his neurotoxicity, but also the patient was able to receive a cumulative dose of 2500 mg/m2 (990 mg/m2 with oral calcium only) (CitationSaif 2004).
Glutathione
Glutathione, an important biological antioxidant, is able to prevent the accumulation of platinum adducts in the dorsal root ganglia in rat model (CitationHolmes et al 1998). A single randomized, double-blind, placebo-controlled trial has been done assessing the efficacy of glutathione in the prevention of oxaliplatin-induced neurotoxicity (CitationCascinu et al 2002). Fifty-two patients were randomized to receive a 1500 mg/m2 glutathione infusion over 15 minutes or normal saline before oxaliplatin infusion. Oxaliplatin was administered on a bimonthly regimen. The median cumulative dose of oxaliplatin did not differ among the two arms. The glutathione group showed significantly less grade 2 or higher neurotoxicity after 8 cycles of chemotherapy (58% vs 10%). The response rates were similar between the glutathione and placebo groups (26.9% vs 23.1%), suggesting that glutathione does not change the efficacy of oxaliplatin. This finding was of significant importance since glutathione has been shown to affect the efficacy of a variety of anti-neoplastic interventions (CitationArrick and Nathan 1984).
Carbamazepine
The theory that oxaliplatin affects voltage-gated sodium channels has led to the use of carbamazepine, a widely used anticonvulsant, to prevent oxaliplatin-induced neuropathy. Carbamazepine decreases high frequency repetitive firing of action potentials by enhancing sodium-channel inactivation (CitationMacdonald and Kelly 1995). In a small German study, 40 patients refractory to 5-FU were treated with oxaliplatin, 5-FU, and folinic acid as second line therapy (CitationEckel et al 2003). Ten patients were additionally treated with carbamazepine maintaining serum levels of 3–6 mg/L. The patients in the carbamazepine group were able to receive significantly higher cumulative doses of oxaliplatin (722 mg/m2 vs 510 mg/m2; p = 0.02). No neuropathy higher than grade 1 occurred in the carbamazepine group compared with 30% in the control group. Larger trials need to be conducted to make conclusions about the prophylactic efficacy of carbamazepine in oxaliplatin-induced neuropathy; however, initial data is promising.
Gabapentin
Gabapentin, another widely used anticonvulsant, appears to affect the release of gamma-aminobutyric acid (GABA). The side effect profile and therapeutic index of gabapentin make it more tolerable and easier to administer than carbamazepine, thus making it more attractive to use for prophylaxis of oxaliplatin-induced neurotoxicity. In a pilot study, 15 patients were treated with oxaliplatin (85 mg/m2/d every 2 weeks) plus 5-FU, and folinic acid as second therapy for advanced colorectal cancer (CitationMariani et al 2000). Gabapentin at a dose of 200 mg/d was started at the onset of neuropathic symptoms. If the patients' symptoms did not resolve in a period of 3 days, then the dose was increased to 300 mg/d. All patients treated with gabapentin had resolution of their symptoms, and no patients had to stop therapy secondary to neurotoxicity. In another study presented at ASCO (2005), 115 patients with chemotherapy-induced peripheral neuropathy (for ≥ 1 month, with average pain rating of ≥ 4/10 or Eastern Cooperative Oncology Group [ECOG] sensory neuropathy ≥ 1/3) were randomized in a double-blind, placebo-controlled trial to either: gabapentin (target dose = 900 mg three times a day [TID]) for 6 weeks then crossover to placebo for 6 weeks (n = 57) or treatment in the reverse order (n = 58) (CitationWong et al 2005). A 2-week washout occurred between crossover treatments. The co-primary endpoints were the average daily pain numerical analogue intensity rating (0 = no pain to 10 = worst pain imaginable) and the ECOG toxicity rating for sensory neuropathy (0 = none to 3 = severe). The results of the study showed that gabapentin did not significantly improve the co-primary endpoints of pain intensity (–0.5 vs –1.0 change from baseline to week 6 for patients on gabapentin and placebo respectively, p = 0.18) or the ECOG toxicity rating for sensory neuropathy (–0.2 vs –0.1 for gabapentin and placebo respectively, p = 0.38). Patients on gabapentin reported significantly more nystagmus (p = 0.009) and dizziness (p = 0.02). Therefore, the study was not able to confirm the benefit of the use of gabapentin in ameliorating peripheral neuropathy.
Amifostine
Based on the studies of amifostine with cisplatin, its effect has been studied with oxaliplatin. Twenty-one patients with peripheral neuropathies (grade ≥ 2) were treated with amifostine 200 mg/m2 subcutaneously (SC) over 3 minutes, twice a week for 6 weeks (CitationPenz et al 2001). Patients were continued on amifostine for as long as improvement was seen. At study entry, 8 patients had grade 3 neurotoxicity and 12 patients had grade 2 neurotoxicity. Among seventeen evaluable patients who completed at least 6 weeks of amifostine therapy, 12 of 17 (71%) patients showed at least minimal (1 grade) improvement in their peripheral neuropathy. One patient reported an increase in peripheral neuropathy from grade 1 to grade 2. Toxicities were manageable with no grade 3 or 4 toxicities observed. Grade 1 or 2 toxicities included: nausea (33%, n = 7); fatigue (9%, n = 2); hypotension (5%, n = 1); and sneezing (5%, n = 1).
Other neuromodulatory agents
Other agents including acetyl-L-carnitine (ALCAR), and α-lipoic acid have shown some promise in small trials. CitationMaestri et al (2002) presented at ASCO (2002) a study evaluating the role of ALCAR in the management of neurotoxicity associated with oxaliplatin. They found that ALCAR was effective in treating patients with established chemotherapy-induced peripheral neuropathy (CitationMaestri et al 2002). Twenty patients were followed with neurotoxicity defined by the WHO criteria. The patients had been treated with various agents including platinum compounds, taxanes, and vinca alkaloids. All were treated with ALCAR 1 g infusion over 1–2 hours for at least 10 days. Sixteen of 20 patients showed at least one grade improvement in their peripheral neuropathy.
Similarly α-lipoic acid has shown beneficial effects in patients with established platinum-induced chemotherapy (CitationGedlicka et al 2002). In a study of 15 patients, neurologic symptoms improved (by at least one grade) in seven patients with grade 2 peripheral neuropathy and in one patient who suffered from grade 3 symptoms. The median time to response was 4 weeks (range, 3–12 weeks), and the median duration of treatment with α-lipoic acid was 2 months (range, 1–4 months).
Ginkgo biloba extract
Ginkgo biloba (GB) is a neuroprotective agent used in neurodegenerative diseases. The possible mode of action may include the prevention of uncoupling of oxidative phophorylation and protection of cell membrane through free radical scavenging (CitationMarshall et al 2004). Preclinical data support the anticancer benefit of GB through targeted inhibition of peripheral-type benzodiazepine receptors over-expressed on mitochondrial membranes in many tumors including colon cancer. There are also data suggesting that GB synergizes with chemotherapy in addition to potential prevention of neurotoxicity. Georgetown University conducted a retrospective analysis of 17 patients with colorectal cancer who were treated with either FOLFOX or CAPEOX regimens (CitationMarshall et al 2004). Ginkgo Biloba was added to chemotherapy at the dose of 120 mg orally bid and Ca or Mg was not given. Eleven of 17 patients initiated GB at cycle 1 or 2 of treatment, only reporting grade 1 acute neuropathy limited to less than 6 days, and 9/11 patients lasted only 2–3 days. The other 6 patients initiated GB after cycle 2, and 5/6 patients noticed decreased intensity and duration in neuropathy with the use of GB. Laryngospasm was not observed in any patient on GB. No GB-related side effects have been observed.
Celecoxib
In a recent study, celecoxib 200 mg bid was added to the regimen consisting of continuous infusion 5-FU 200 mg/m2/d for 10 weeks with a 2-week break and oxaliplatin 130 mg/m2 every 3 weeks (CIFOX). Among 263 patients, 73 patients received celecoxib and 179 did not (CitationAgafitei et al 2004). Patients on both regimens had similar characteristics. None of the 73 patients who received celecoxib experienced any grade 3 or 4 neurotoxicity, compared with 10 of the 179 patients who did not receive celecoxib (p = 0.024). The patients who received celecoxib were less likely to experience grade 3 or 4 neuropathy (0% vs 6%) but more likely to experience grade 1 neuropathy (67% vs 48%) than those who did not receive celecoxib.
Pharmacogentics
It is postulated that variations in genes potentially involved in the detoxification (glutathione system: glutathione-Stransferase genes P1 [GSTP1], glutathione-S-transferase genes M1 [GSTM1]) or the cytotoxic mechanism (nucleotide excision repair: excision repair cross-complementing rodent repair deficiency, complementation group 2 [ERCC2], x-ray repair cross-complementing group 1 protein [XRCC1]) of oxaliplatin could serve as genetic predictors of susceptibility to oxaliplatin's neurotoxic effects. This concept was tested by extracting germline DNA from whole blood of 299 patients who received FOLFOX4 in the Intergroup study N9741 (CitationGrothey et al 2005). Four genetic variants (GSTP1 I105V, GSTM1 del, ERCC2 K751Q, XRCC1 R399Q) were tested for association with the time to development of grade 2/3 sensory neuropathy (sNT) and sNT as reason for treatment discontinuation. In addition, patients who developed grade 2 (3) neurotoxicity before a cumulative oxaliplatin dose of 600(800) mg/m2 was reached were compared to an equal number of patients that tolerated extremely high cumulative doses of oxaliplatin. The study showed that the patients with GSTP1 I105V C/C polymorphism were more likely to discontinue FOLFOX due to sNT (23.7%) than patients with T/T (9.2%) or C/T (10%) variants (p = 0.039). Patients with GSTP1 I105V C/ C also had lower cumulative dose to onset of grade 3 sNT compared to T/T or C/C pts (p = 0.05). Patients carrying at least one GSTP1 I105V C-allele (C/C or C/T) were more likely to experience rapid onset grade 3 neurotoxicity than T/T patients, who were more likely to be able to tolerate high doses (p = 0.027 for grade 2, p = 0.03 for grade 3). No significant correlation of GSTM1, ERCC2, and XRCC1 polymorphisms with the onset of sNT on FOLFOX was observed. This study offers an early evidence that genetic variations in GSTP1 may serve as predictors of susceptibility to oxaliplatin-mediated sNT. Such findings should be validated in prospective studies so that GSTP1 polymorphisms can be used to identify patients in whom neurotoxicity prevention strategies on oxaliplatin-based chemotherapy are warranted.
Conclusion
Oxaliplatin, a third generation platinum compound, has been shown to be effective as first line therapy in advanced colorectal cancer. Neurotoxicity is the dose-limiting side effect with this drug. This side effect can manifest as two distinct forms: the acute, reversible sensory neuropathy and a chronic, cumulative neuropathy. The acute form occurs with infusion or in the ensuing hours. The chronic toxicity does not become apparent until cycles 8–10 in the commonly used FOLFOX dosing regimen, making it more predictable. The treatment of oxaliplatin-induced neurotoxicity needs to start at the bedside. Nursing staff and oncologists should inform patients to avoid contact with cold objects, food, and beverages. This can prevent paresthesias and more importantly pseudolaryngospasm. Promising strategies exist that include dosing modification and neuromodulatory agents, although larger trials need to be conducted to confirm efficacy.
References
- AdelsbergerHQuasthoffSGrosskreutzJThe chemotherapeutic oxaliplatin alters voltage-gated Na+ channel kinetics on rat sensory neuronsEuro J Pharmacol20004062532
- AgafiteiRDSchneiderSIqbalSEffect of celecoxib on neurotoxicity in patients with metastatic colorectal cancer treated with 5-FU/oxaliplatin (CIFOX) [abstract]J Clin Oncol2004223600
- AndreTBoniCMounedji-BoudiafLOxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancerN Engl J Med2004350234351 Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators15175436
- ArmstrongCMCotaGCalcium block of Na+ channels and its effect on closing rateProc Natl Acad Sci U S A1999964154710097179
- ArrickBANathanCFGlutathione metabolism as a determinant of therapeutic efficacy: a reviewCancer Res1984444224326380705
- BrienzaSVignoudJItzhakiMOxaliplatin: global safety in 682 patients [abstract]Proc Am Soc Clin Oncol199514209
- CascinuSCatalanoVCordellaLNeuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo controlled trialJ Clin Oncol20021634788312177109
- CavalettiGBogliunGZinconeANeuro- and ototoxicity of high dose carboplatin treatment in poor prognosis ovarian cancer patientsAnticancer Res19981837978029854499
- CavalettiGTrediciGPetruccioliMGEffects of different schedules of oxaliplatin treatment on the peripheral nervous system of the ratEur J Cancer20013724576311720843
- ContiJAKemenyNESaltzLBIrinotecan is an active agent in untreated patients with metastatic colorectal cancerJ Clin Oncol199614709158622015
- de GramontACervantesAAndreTOPTIMOX study: FOLFOX7/LV5FU2 compared to FOLFOX4 in patients with advanced colorectal cancerJ Clin Oncol2004223525
- de GramontAFigerASeymourMLeucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancerJ Clin Oncol20001829384710944126
- EckelFSchmelzRAdelsbergerHPrevention of oxaliplatin neuropathy by carbamazepine. A pilot studyDtsch Med Wochenschr2003127788211797144
- GamelinLBoisdron-CelleMDelvaRPrevention of oxaliplatin-related neurotoxicity by calcium magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancerClin Cancer Res20041040556115217938
- GedlickaCScheithauerWSchüllBEffective treatment of oxaliplatin-induced cumulative polyneuropathy with alpha-lipoic acidJ Clin Oncol20022033596112149316
- GemlinEGamelinLBossiLClinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measuresSemin Oncol20025Suppl 152133
- GoldbergRSargentDMortonRA randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancerJ Clin Oncol200422233014665611
- GrahamMLockwoodGGreensladeDClinical pharmokinetics of oxaliplatin: a critical reviewClin Cancer Res2000612051810778943
- GrolleauFGamelinLBoisdron-CelleMA possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channelsJ Neurophysiol2001852293711353042
- GrotheyAOxaliplatin-safety profile: neurotoxicitySemin Oncol20034Suppl 1551314523789
- GrotheyADeschlerBKroeningHPhase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24h 5-FU infusion/FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC)Proc Am Soc Clin Oncol200221129a
- GrotheyAMcLeodHLGreenEMGlutathione S-transferase P1 I105V (GSTP1 I105V) polymorphism is associated with early onset of oxaliplatin-induced neurotoxicity [abstract]J Clin Oncol200523350915908661
- HoffPPazdurRProgress in the development of novel treatments for colorectal cancerOncology200418705815214591
- HolmesJStankoJVarchenkoMComparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in the Wistar rat modelToxicol Sci1998463425110048138
- HsiangYHLihouMGLiuLFArrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecinCancer Res1989495077822548710
- JemalATiwariRCMurrayTCancer statistics, 2004CA Cancer J Clin200454814974761
- LajerHDaugaardGCispaltin and hypomagnesemiaCancer Treat Rev199925475810212589
- Maindrault-GoebelFde GramontALouvetCEvaluation of oxaliplatin dose intensity in bimonthly leucovorin and 48-hour 5-fluorouracil continuous infusion regimens (FOLFOX) in pretreated metastatic colorectal cancer. Oncology Multidisciplinary Research Group (GERCOR)Ann Oncol20001114778311142489
- MacdonaldRLKellyKMAntiepileptic drug mechanisms of actionEpilepsia199536Suppl 2S2128784210
- MaestriAPasquale CerattiADCalandriCAcetyl-L-carnitine (ALCAR) in patients with chemotherapy-induced peripheral sensory neuropathyProc Am Soc Clin Oncol200221247b
- MarianiGGarroneOGranettoCOxaliplatin induced neuropathy: Could gabapentin be the answer?Proc Am Soc Clin Oncol200019609a
- MarshallJZakariAHwangJGinkgo Biloba (GB) extract as a neuroprotective agent in oxaliplatin (Ox)-induced neuropathy [abstract]J Clin Oncol2004223670
- MeyerhardtJAMayerRJSystemic therapy for colorectal cancerN Engl J Med20053524768715689586
- MidgleyRKerrDColorectal cancerLancet199935339199950460
- NeijtJEngelholmSTuxenMExploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancerJ Clin Oncol20001830849210963636
- PenzMKornekGVRadererMSubcutaneous administration of amifostine: a promising therapeutic option in patients with oxaliplatin-related peripheral sensitive neuropathyAnn Oncol200112421211332158
- PetrelliNDouglasHOJrHerreraLThe modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study GroupJ Clin Oncol198971419262674331
- PoonMAO'ConnellMJMoertelCGBiochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinomaJ Clin Oncol198971407182476530
- SaifMWOral calcium ameliorating oxaliplatin-induced peripheral neuropathyJ Appl Res2004457682
- SaltzLCoxJBlankCIrinotecan plus fluoruracil and leucovorin for metastatic colorectal cancerN Engl J Med20003439051411006366
- ScrenciDMcKeageMPlatinum neurotoxicity: clinical profiles, experimental models and neuroprotective approachesJ Inorg Biochem1999771051010626361
- ShimadaYYoshinoMWakuiAPhase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancerJ Clin Oncol199311909138487053
- TaiebSTrillet-LenoirVRambaudLLhermitte sign and urinary retention: atypical presentation of oxaliplatin neurotoxicity in four patientsCancer20029424344012015768
- TournigandCAndreTAchilleEFOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR studyJ Clin Oncol2004222337
- WilsonRLehkyTThomasRRAcute oxaliplatin-induced peripheral nerve hyperexcitabilityJ Clin Oncol20022017677411919233
- WolmarkMWieandHSKueblerJPA phase III trial comparing FULV to FULV + oxaliplatin in stage II or III carcinoma of the colon: results of NSABP Protocol C-07 [abstract]J Clin Oncol2005233500
- WongGYMichalakJCSloanJAA phase III double blinded, placebo controlled, randomized trial of gabapentin in patients with chemotherapy-induced peripheral neuropathy: a North Central Cancer Treatment Group StudyProceedings of ASCO, 2005: May 14-172005Orlando, FL, USA8001
- WuFTakahashiMPegoraroEA new mutation in a family with cold-aggravated myotonia disrupts Na+ channel inactivationNeurology2001568788411294924