Abstract
Stress urinary incontinence (SUI) is the most common form of urinary incontinence and occurs more frequently in women than in men. Duloxetine is a balanced dual serotonin (5-HT) and norepinephrine (NE) reuptake inhibitor and shows no relevant binding affinity for histaminergic, dopaminergic, cholinergic, and adrenergic receptors. The efficacy of duloxetine in SUI is based on the inhibition of the presynaptic reuptake of 5-HT and NE in Onuf's nucleus of the sacral spinal cord, whereby it may increase the concentration of 5-HT and NE in the synaptic cleft. The effectiveness of duloxetine was studied in a cat model of acetic acid-induced bladder irritation. The results showed that in cats with previous irritated bladder function, there was a dosage-dependent improvement of bladder capacity and periurethral electromyography (EMG) activity. In women with SUI, it is assumed that the clinical efficacy of duloxetine is based on stronger urethral contraction and persistent sphincter tone during the storage phase. In clinical trials in women with SUI, duloxetine has demonstrated efficacy in reducing incontinence episodes and increasing quality of life. Nausea was the most common adverse event and the main cause for discontinuation. In summary, duloxetine appears to be a promising new option for the treatment of SUI.
Introduction
Urinary incontinence is defined by the International Continence Society as an involuntary passage of urine and is commonly found in elderly people. With a rate of approximately 50% prevalence, stress urinary incontinence (SUI) is the most common form of urinary incontinence, and is more frequently found in women than in men (CitationHampel et al 1997) because of the vulnerability of the female sphincter mechanism.
Initial treatment for SUI should include lifestyle intervention and supervised pelvic floor muscle training. The International Consultation on Incontinence (ICI) actually recommends that conservative treatment may be augmented with appropriate drug therapy and mentions dual serotonin (5-HT) and norepinephrine (NE) reuptake inhibitors as examples of therapy for SUI.
Duloxetine is the first medication developed for SUI and has been also developed for the treatment of depression and pain caused by diabetic peripheral neuropathy (). This review focuses on duloxetine in the treatment of SUI. The action of duloxetine is associated with the reuptake inhibition of 5-HT and NE at the presynaptic neuron in Onuf's nucleus (the pudendal nerve motor nucleus) of the sacral spinal cord (CitationMichel and Peters 2004).
Onuf 's nucleus
The Onuf's nucleus is a distinctly confined neuronal group in the ventral horn of the sacral spinal cord, which extends in humans over 2 segments from S1 to S2 or S2 to S3. Onufrowicz originally discovered it as nucleus X (CitationOnufrowicz 1889; Citation1890).
In experimental animal studies conducted with cats, dogs, rabbits, and subhuman primates (retrograde labeling by injection of horseradish peroxidase [HRP]), the Onuf's nucleus was identified as the origin of the innervation of the external sphincter of the urethra and the anus. Generally, the Onuf's nucleus contains 300–500 neurons on each side in animals; however, humans have an average of 625 neurons (CitationDurant et al 1988; CitationDownie et al 1991; CitationHosoya et al 1991; CitationJost 1997).
The corresponding neurons for the bladder sphincter were discovered in the ventromedial (cats) or ventrolateral (rhesus monkey) part, and for the anal sphincter, in the dorsolateral (cats) and dorsomedial (rhesus monkey) part of the nucleus.
Neurotransmitters
The motoneurons in the Onuf's nucleus are densely packed with different neurotransmitters (eg, NE, 5-HT, and dopamine) and receptors. The motoneurons in the Onuf's nucleus, which are involved in bladder–sphincter function, are surrounded by a dense accumulation of norepinephric and serotonergic nerve terminals, which primarily carry 5-hydroxytryptamine2 (5-HT2) and alpha-1 receptors (CitationThor 2003).
Stimulation of these receptors leads to a marked increase in the tonus of the guarding reflex, which prevents voiding by a sudden increase in abdominal pressure initiated by eg, coughing and sneezing. An effect of the neurotransmitters 5-HT and NE, on the motoneuron was only detected in the presence of glutamate. As a result, a glutamate-induced and a 5-HT- and NE-supported activation of the sphincter motoneurons does not appear to impair the bladder– sphincter synergy (CitationThor 2003).
Pharmacological properties of duloxetine
Duloxetine is a balanced 5-HT and NE reuptake inhibitor. There is a weak inhibition of dopamine and no significant affinity for histaminergic, dopaminergic, cholinergic, and adrenergic receptors () (CitationWong et al 1993).
Table 1 Affinity of duloxetine for neuronal receptors
The efficacy of duloxetine in SUI is based on the inhibition of the presynaptic reuptake of 5-HT and NE in the Onuf's nucleus of the sacral spinal cord, whereby it may lead to an increased concentration of 5-HT and NE in the synaptic cleft (CitationMichel and Peters 2004).
The effects of duloxetine on the lower urinary tract were investigated in animal experiments. Duloxetine showed a significant increase of bladder capacity and sphincter– electromyography (EMG) activity. Cats, whose bladders were initially pretreated with acetic acid, showed a dose-dependent improvement of the bladder capacity (5-fold) and periurethral EMG activity (8-fold) of the striated sphincter musculator only during the storage phase of the micturition cycle (CitationKarl and Katofiase 1995).
In women with SUI, it is assumed that an increased urethral contraction and a sustained sphincter tonus during the filling phase may explain the clinical efficacy of duloxetine treatment. The pharmacokinetics of duloxetine have been examined in healthy adults and in patients with SUI or mixed urinary incontinence () (CitationIshigooka et al 1997; CitationSharma et al 2000; CitationLantz et al 2003; CitationSkinner et al 2004).
Table 2 Properties of duloxetine
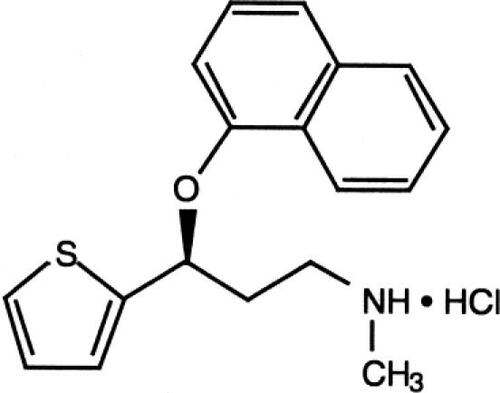
Duloxetine hydrochloride (C18 H19 NOS.HCL; MW333, 88) is a (+)-(S)-N-Methyl-3-(1-naphthyloxy-2-thienyl)-propylamine Hydrochloride and is approximately 96% bound to human plasma protein.
Duloxetine is extensively metabolized by oxidative enzymes (cytochrome P1A2 [CYP1A2] and cytochrome P2D6 [CYP2D6]), followed by a conjugation. A large intersubject variability (due to gender, age, smoking status, and CYP2D6 metabolizer status) has been proven.
The average elimination half-life value after oral intake of duloxetine is 12 hours. The recommended dose of duloxetine for the treatment of SUI is 40 mg twice a day regardless of mealtime. If a woman still experiences troublesome events after 4 weeks, the dosage can be reduced to 20 mg twice a day (CitationEli Lilly Nederland 2004).
Contraindications
Contraindications are pregnancy and lactation. Duloxetine should not be used in combination with nonselective, irreversible monoamine oxidase inhibitors. Duloxetine should not be used in combination with CYP1A2 inhibitors like fluvoxamine, ciprofloxacin, enoxacine, since the combination results in elevated plasma concentration of duloxetine. Further contraindications are liver disease resulting in hepatic impairment and hypersensitivity to the active substance or to any of the excipients (CitationEli Lilly Nederland 2004).
Clinical results
The therapeutic efficacy of doses of 40 mg twice a day has been demonstrated in 4 double-blind placebo-controlled studies. Of the 1913 women between 22 and 83 years of age who participated in these studies, 958 patients received duloxetine and 955 received a placebo.
The goal of the studies was to examine the frequency of incontinence episodes (IEF) obtained from diary notes and the analysis of quality-of-life questionnaires (Incontinence Quality of Life [I-QOL] and Patient Global Impression of Improvement [PGI-I]) specially designed for urinary incontinence. In all four studies, women treated with duloxetine demonstrated a 50%–58% median decrease in IEF compared to 27%–40% seen with placebo (CitationNorton et al 2002; CitationDmochowski et al 2003; CitationMillard et al 2004; Citationvan Kerrebroeck et al 2004).
Phase II study
The double-blind, randomized, placebo-controlled dose-finding study of 553 women (aged between 18–65) with SUI (CitationNorton et al 2002) took place over a period of 12 weeks. A total of 415 patients received duloxetine: 138 patients received 20 mg/day; 137 patients received 40 mg/day; 140 patients received 80 mg/day. 138 patients received a placebo (CitationNorton et al 2002).
The study results showed a significant and clinically relevant reduction in urinary incontinence episodes with duloxetine. The last-visit analysis showed a significant but not dose-dependent reduction in IEF. The pooled diary data showed a dose-dependent pattern. While the I-QOL data “paralleled” the dose-dependent response of the pooled IEF, only the change at 80 mg/day were statistically significant. Thus, 80 mg/day was then used for the subsequent Phase III studies.
Clinical Phase III studies
Overall, 3 double-blind, randomized, placebo-controlled Phase III studies have been conducted worldwide. In all studies, the female patients were treated over a period of 12 weeks with 40 mg duloxetine twice daily compared with placebo. Included in the study were female patients predominantly with symptoms of SUI and who experienced 7 or more incontinence episodes a week (CitationDmochowski et al 2003; CitationMillard et al 2004; Citationvan Kerrebroeck et al 2004). The bladder capacity should have been ≥ 400 ml, and the cough and stress-pad-test should have been positive. Female patients who had predominantly symptoms of urge incontinence were excluded from these studies. Female patients who conducted stable pelvic floor muscle training (PFMT) and patients who had prior incontinence surgery were not excluded.
The efficacy was evaluated based on the reduction of the IEF, which was collected with patient diaries and with the aid of the I-QOL and PGI-I questionnaires that evaluated quality of life. The I-QOL questionnaire collects data in 3 subcategories: avoidance and limiting behavior, psychosocial impact, and social embarrassment. The PGI-I questionnaire allows a self-evaluation based on improvement of the clinical picture by the patient ( and ). Overall, the data of the Phase III studies were comparable with those of the Phase II study.
Table 3 Duloxetine in the treatment of SUI: Results of 3 Phase III efficacy studies, duloxetine 40 mg twice daily
Table 4 Duloxetine in the treatment of SUI: patients with IEF ≥ 14 per week
The 3 Phase III double-blind studies, conducted over a period of 12 weeks, continued with an open phase during which the efficacy was evaluated in 3-month intervals using the PGI-I questionnaire. After 12 months of treatment, 82% of patients viewed their condition as improved.
Study conducted in women with severe SUI
One double-blind placebo-controlled study, in which patients dose escalated from duloxetine 40 mg bid to 60 mg bid, was conducted in women (n = 109 women, age from 33–75 years, with predominant symptoms of SUI), who were suffering from severe SUI (more than 14 incontinence episodes per week) and were awaiting surgery. The results showed a significant reduction in the frequency of incontinence episodes (–60% vs –27%, p < 0.001), a significant improvement in the I-QOL score (10.6% vs 2.4%, p = 0.003) and a reduction in pads (–34.5% vs. –4.8%, p = 0.008) in the duloxetine group compared with placebo (CitationCardozo et al 2004). Twenty percent (10/49) of patients treated with duloxetine were no longer interested in surgery following the 8-week treatment phase compared to no (0/45) patients in the placebo group (p = 0.001).
Study comparing duloxetine alone with PFMT and in combination
One study with active treatment comparison between duloxetine and PFMT, alone and in combination, was conducted. This placebo-controlled study included 201 women, aged 18–75 years, with predominant symptoms of SUI. Both duloxetine alone and duloxetine in combination with PFMT significantly (p < 0.05) reduced the median IEF by 57%, compared with 35% reduction with PFMT alone (29% reduction with no treatment) (CitationGhoniem et al 2005). The mean total I-QOL score was increased by 13.1 with combination therapy (p < 0.05 vs no treatment) compared with increases of 8.3 for duloxetine alone, 7.8 for PFMT alone, and 4.8 for no treatment. The median decreases in pad use were: for the combination 46%, for duloxetine alone, 35%; for PFMT alone, 25% (all p < 0.05 vs no treatment); and for no treatment, 10%.
Tolerability
Tolerability of duloxetine was evaluated based on the occurrence of adverse events (AE), discontinuations because of AE, vital parameters, electrocardiograms, and laboratory tests. Adverse events were evaluated by the time of occurrence, if they occurred for the first time, or if they worsened in the course of treatment (CitationDmochowski et al 2003; CitationMillard et al 2004; Citationvan Kerrebroeck et al 2004).
Nausea, dry mouth, fatigue, insomnia, constipation, headache, dizziness, somnolence, and diarrhea were reported signficantly more often under duloxetine treatment compared with placebo and occurred at least in 5% of all female patients. Nausea was the most common adverse event and was reported within the first few days after start of treatment (in 64% within 2 days). The intensity of the adverse events was mostly mild to moderate. Fifty-two percent of patients with nausea reported resolution of the symptoms by 1 week and 81% within 1 month. The discontinuation rate due to AE was significantly higher in the duloxetine group compared with the placebo group, where nausea was reported as the most frequent reason for discontinuation.
There were no clinically relevant effects on blood pressure and heart frequency. The analysis of the corrected QT interval showed no arrhythmogenic tendencies under duloxetine (CitationDmochowski et al 2003).
Current status
Duloxetine is approved throughout the European Union for the treatment of moderate to severe SUI in women. For a long time, the rational treatment of SUI was in the domain of surgical procedures. With the availability of duloxetine, there is now a pharmacological treatment that has been shown to be efficacious and tolerable, and as a result broadens the spectrum of treatment for this indication.
Use in depression and pain
Due to its equivalent reuptake inhibition of 5HT2 and NE and demonstrated low binding affinity for neurotransmitter receptors, duloxetine seems to be superior as an anti-depressant drug to reuptake inhibitors for just one monoamine. Duloxetine is therefore also approved for the treatment of depression. In comparative studies, duloxetine was observed to be more effective than both fluoxetine and paroxetine on several measures of depression (CitationGoldstein et al 2002, Citation2004). Duloxetine is different from fluoxetine because of its dual nature of balanced serotonin and noradrenalin reuptake inhibition. Serotonin and norepinephrine are thought to inhibit pain via descending pain pathways (CitationGoldstein et al 2005). In addition, duloxetine is approved by the US Food and Drug Administration for the treatment of diabetic peripheral neuropathic pain. Other antidepressants such as venlafaxine, which also have dual reuptake inhibition, lack the balance in reuptake inhibition (CitationKatofiasc et al 2002).
Conclusion
In the European Union, duloxetine is approved for the treatment of women with moderate to severe forms of SUI (women were investigated with predominant symptoms of SUI in the discussed studies).
Duloxetine, alone and in combination with pelvic floor training, significantly reduced (p < 0.05) in median incontinence episode frequency (57% vs 35% pelvic floor training alone; 29% no treatment). Combined treatment (duloxetine and pelvic floor training) was superior with regard to reduction in pads and improvement of quality of life. Hence, combination with pelvic floor training (with good compliance) may be appropriate. The recommended dose is 40 mg twice daily.
Rational management of SUI has long been the domain of surgical intervention. Duloxetine, for the first time, appears to be a therapeutic approach with well documented efficacy and tolerance, thus widening the spectrum of treatment options for this indication.
References
- CardozoLDrutzHPBayganiSKPharmacological treatment of women awaiting surgery for stress urinary incontinenceAm Coll Obstet Gynecol200410451119
- DmochowskiRRMiklosJRNortonPADuloxetine versus placebo for the treatment of North American women with stress urinary incontinenceJ Urol200317012596314501737
- DownieJEspeyMGajewskiJAlpha 2-adrenoceptors not imidazole receptors mediate depression of a sacral spinal reflex in the catEur J Pharmacol199119530141678710
- DurantPLucasPYakshTMicturition in the unanesthetized rat: spinal vs. peripheral pharmacology of the adrenergic systemJ Pharmacol Exp Ther1988245426353367300
- Eli Lilly NederlandBVYentreve: summary of product characteristics [online]2004 Accessed 11 Oct 2004 URL: http://www.emea.eu.int/humandocs/Humans/EPAR/yentrve/yentreve.htm
- GhoniemGMVan LeeuwnJSElserDMA randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment, and no active treatment in women with stress urinary incontinenceJ Urol20051731453415821458
- GoldsteinDJLuYDetkeMJDuloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetineJ Clin Psychopharmacol2004243899915232330
- GoldsteinDJLuYDetkeMJDuloxetine vs placebo in patients with painful diabetic neuropathyPain20051161091815927394
- GoldsteinDJMallinckrodtCLuYDuloxetine in the treatment of major depression disorder: a double-blind clinical trialJ Clin Psychiatry2002632253111926722
- HampelCWienholdDBenkenNPrevalence and natural history of female incontinenceEur Urol199732Suppl 23129248806
- HosoyaYOkadoNSuiuraYKohnoKCoincidence of “ladder-like patterns” in distributions of monoaminergic terminals and sympathetic preganglionic neurons in the rat spinal cordExp Brain Res19918622481756794
- IshigookaJNagataETakahashiASimultaneous monitoring of inhibition of serotonin uptake by platelets and plasma concentration following administration of duloxetine, a new antidepressant candidate, to healthy volunteersCurr Ther Res19975867992
- JostWHNeurologie des Beckenbodens1997London, Glasgow, WeinheimChapman & Hall
- KarlKBKatofiascMAEffects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female catJ Pharmacol Exp Ther19952741014247636716
- KatofiascMANissenJAudiaJEComparison of the effects of serotonin selective, norepinephrine selective, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in catsLife Sci20027112273612106588
- LantzRJGillespieTARashTJMetabolism, excretion, and pharmacokinetics of duloxetine in healthy human subjectsDrug Metab Dispos20033111425012920170
- MichelMCPetersSLRole of serotonin and noradrenaline in stress urinary incontinenceBJU Int200494Suppl I233015139860
- MillardRJMooreKRenckenRDuloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trialBJU Int2004933111814764128
- NortonPZinnerNYalcinIBumpRDuloxetine versus placebo in the treatment of stress urinary incontinenceAm J Obstet Gynecol200218740812114886
- OnufrowiczBNotes on the arrangement and function of the cell groups of the sacral region of the spinal cordJ Nerv Men Dis188926498504
- OnufrowiczBOn the arrangement and function of the cell groups of the sacral region of the sacral spinal cord in manArch Neurol Psychopathol18903387411
- SharmaAGoldbergMJCerimeleBJPharmacokinetics and safety of duloxetine, a dual serotonin and norepinephrine reuptake inhibitorJ Clin Pharmacol200040161710664922
- SkinnerMHKuanHYSkerjanecAEffect of age on the pharmacokinetics of duloxetine in womenBr J Clin Pharmacol200457546114678340
- ThorKSerotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: Implication for treating stress urinary incontinenceUrology200362Suppl 4A3914550831
- van KerrebroeckPAbramsPLangeRDuloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinenceB J Obstet Gynaecol200411124957
- WongDBymasterFMayleDLY 2486 8, a new inhibitor of serotonin and norepinephrine uptakeNeuropsychopharmacology1993823338424846