Abstract
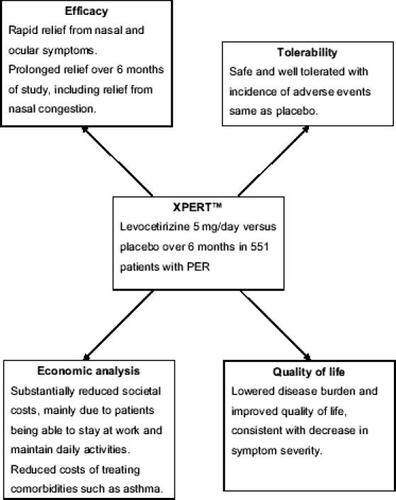
Allergic rhinitis (AR) is a major health problem that can significantly impair quality of life (QoL). The former classification of AR comprises seasonal allergic rhinitis (SAR) and perennial allergic rhinitis (PAR), which do not adequately reflect the clinical course and presentation of AR. The Allergic Rhinitis and its Impact on Asthma (ARIA) classification is based on the duration of symptoms and their severity. Persistent AR (PER) is experienced for periods longer than 4 days/week and for more than 4 consecutive weeks, and may feature mild or moderate-to-severe disease based on the impairment of QoL and symptom severity. Oral antihistamines are a standard treatment option in AR. New second generation antihistamines have a rapid onset of action, are highly effective on AR symptoms, and some were even shown to relieve nasal congestion. Levocetirizine is a potent histamine H1-receptor antagonist with proven efficacy in both SAR and PAR, and it is the best studied therapeutic option in persistent AR. The Xyzal in Persistent Rhinitis Trial (XPERT™) studied 551 patients with PER, showing that levocetirizine (5 mg/day compared with placebo) significantly improved nasal symptoms as early as the first week and for the 6 months of study, with significant improvement in nasal congestion after 6 weeks of treatment. Levocetirizine also improved QoL, was well tolerated, and produced substantial societal and employer cost savings. Thus, levocetirizine is the first tested standard treatment for PER using ARIA classification, and shows prompt short-term and long-term relief of symptoms, improves patients' QoL, and provides economic benefits to employers and the society.
Introduction
Allergic rhinitis (AR) is a major health problem with high and ever-increasing prevalence (CitationBousquet et al 2001). At least one in five adults in Western Europe are estimated to have AR (CitationBauchau and Durham 2004), and its well-known nasal and eye symptoms can be severe enough to have a substantial negative impact on daily activities and sleep, with resulting impairment of quality of life (QoL) similar to that caused by asthma (CitationBousquet, Bullinger et al 1994; CitationBousquet, Knani et al 1994). Despite the debilitating effects of AR, it remains a condition where patients do not seek appropriate treatment, are undertreated, or do not adhere to treatment; all of which lead to high societal costs (CitationMalone et al 1997; CitationSchoenwetter et al 2004). A recent study found a total cost of about €350 per month, mainly due to reduced productivity, for each AR patient who had not received appropriate long-term therapy for their condition (CitationBousquet et al 2005). Therefore, there is a need to radically improve diagnosis, treatment, and management of AR.
Allergic rhinitis and its impact on asthma
The former classification of AR comprised seasonal allergic rhinitis (SAR), which was mainly linked to pollen allergy, and perennial allergic rhinitis (PAR), which was mainly linked to house-dust mites. Many shortcomings of this classification have become apparent over the years. For example, many AR patients are polysensitized to pollen and perennial allergens (CitationBauchau and Durham 2005), and thus cannot be classed as having SAR or PAR. Also, some countries have seasonal pollen, while others have pollen for many months, or even perennially (CitationBousquet et al 2001). Finally, the SAR/PAR classification does not cover severity or duration of the disease, which makes it difficult to decide upon the best treatment option.
The Allergic Rhinitis and its Impact on Asthma (ARIA) classification system was introduced in 2001 and is based on duration and severity of symptoms and their impact on QoL () (CitationBousquet et al 2001). The duration of AR is split into intermittent and persistent patterns. Intermittent AR is defined by symptoms that occur for 4 or less days per week or for not more than 4 consecutive weeks, whereas persistent AR (PER) lasts for more than 4 days per week and for more than 4 consecutive weeks. Symptoms of intermittent AR or PER may be mild or moderate-to-severe based on its impact on QoL and symptom severity. Mild rhinitis characteristically does not affect daily, leisure, or sport activities, normal work or school attendance, or disturb sleep, and causes no bothersome symptoms. Moderate-to-severe rhinitis is characterized by impairment of at least one of these parameters. Compared with patients with intermittent AR, PER patients had more severe symptoms, a higher rate of self awareness, previous diagnosis of AR, differed in their use of medication, and had a clearly distinct allergen sensitization pattern (CitationBauchau and Durham 2005).
Figure 1 Allergic Rhinitis and its Impact on Asthma (ARIA). Dark lines show that moderate-to-severe symptoms occur most often in persistent allergic rhinitis (PER) with milder symptoms in intermittent allergic rhinitis. Adapted from CitationBousquet et al (2001).
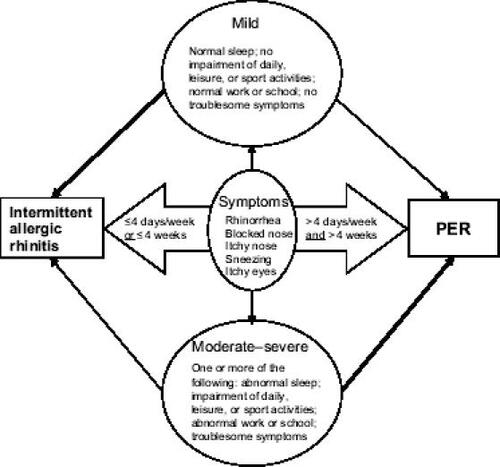
The ARIA classification system is distinct from the classic one. Patients classified as having SAR do not necessarily have intermittent AR, and PAR-classified patients do not always have PER (CitationBauchau and Durham 2005). In one study, nearly half of so-called SAR patients had PER, and nearly half of PAR patients had only intermittent AR (CitationDemoly et al 2003; CitationBauchau and Durham 2005). Another feature of ARIA is that it acknowledges the relationship of AR with asthma and other comorbidities such as rhinosinusitis and conjunctivitis, and recommends a combined treatment strategy of upper and lower airways for optimum management (CitationBousquet et al 2001).
PER management
PER is managed on different levels and includes allergen avoidance, immunotherapy, pharmaceutical treatment, and patient education (CitationBousquet et al 2001). Allergen avoidance measures should always be indicated when possible and appear to be effective, although the level of scientific evidence is low (CitationBousquet et al 2001). Allergen avoidance can be difficult because patients are often sensitised to many allergens, and it may impact negatively on daily activities, family life, and QoL (CitationMartin 1999; CitationMorris 2004). Examples of avoidance measures include removal of carpets, regular vacuuming, regular washing of bedclothes, use of dust mite-resistant covers, removal of pets, and staying inside during high pollen loads (CitationBousquet et al 2001; CitationHorak et al 2004; CitationPeat et al 2004; Citationvan den Bemt et al 2004; CitationSimpson and Custovic 2005).
Allergen immunotherapy may be suitable in PER patients who have severe or long-term symptoms predominantly induced by dominating allergens. ARIA recommends allergen immunotherapy in carefully selected patients with rhinitis (especially when complicated by asthma) and specific sensitivities, and in patients in whom pharmacotherapy produces incomplete symptom control or undesirable adverse events (CitationBousquet et al 2001; CitationDurham et al 1999). It should be initiated early in the disease process to reduce the risk of adverse events and to prevent the further development of severe disease as it may alter the course of disease and prevent asthma. It can be given subcutaneously and is effective; however, it is time consuming, costly, and carries a low risk of anaphylaxis (CitationBousquet et al 2001). Sublingual administration has a lower risk of anaphylaxis and is less time-consuming than subcutaneous administration, but it may have lower efficacy and requires good compliance by the patient (CitationBousquet et al 2001; CitationWilson et al 2005).
Pharmacotherapeutic options include oral anti-histamines, and topical antihistamines that can be administered in the nose. According to ARIA, antihistamines are recommended for all types of intermittent rhinitis and mild persistent rhinitis; in severe persistent rhinitis, intranasal steroids are the first-line treatment due to their higher efficacy in treating nasal obstruction, and they may be complemented by antihistamines as add-on treatment if required (CitationBousquet et al 2003; CitationSimons 2004).
Compared with older antihistamines, newer anti-histamines have rapid onset of action (minutes to hours), are easy to use, and are highly effective on symptoms such as rhinorrhea, sneezing, and nasal and ocular pruritus. They are mostly free of anticholinergic adverse effects and relatively safe compared with first generation antihistamines, even in population groups such as the elderly (CitationHansen et al 2005). Furthermore, the newer agents are comparatively nonsedating, thus providing further benefit over older antihistamines with sedative properties that are no longer recommended in adults or in children (CitationKay 2000).
It was shown in PER that local inflammation is closely linked to nasal obstruction and airflow (CitationCiprandi et al 2005a). So the antiinflammatory activities exerted by some modern antihistamines may explain the efficacy on nasal obstruction and decongestant effects found in some antihistamines like levocetirizine (CitationCiprandi et al 2005a). In addition, antihistamines may reduce symptoms of AR and asthma in patients with comorbid diseases (CitationSimons 2002, Citation2004; CitationBachert and Demarteau 2005). The antiinflammatory effect of newer antihistamines is gaining significance in allergic conditions where inflammation might occur and lead to asthma (CitationSimons 2004). For example, in the Early Treatment of the Atopic Child (ETACTM) study, the antihistamine cetirizine has been shown to prevent the atopic march from atopic dermatitis to asthma in patients sensitized to grass pollen or house-dust mite (CitationWarner 2001). This interesting result is being investigated in the Early Prevention of Asthma in Atopic Children (EPAAC™) study with levocetirizine (CitationFumagalli et al 2004).
Nasal steroids are highly effective in reducing inflammation and nasal symptoms in AR. They are particularly useful in patients suffering from prominent nasal obstruction. Compared with antihistamines, however, they have a slower onset of action (days), and especially beclomethasone at high doses, may have a less favourable safety profile for chronic use in children. Oral steroids are reserved for very severe cases only, due to serious adverse events from chronic use. Some patients with concomitant asthma may need treatment with both inhaled and oral steroids, so adding a nasal steroid for concomitant rhinitis may not be the primary choice.
Other treatments include chromones (less effective than antihistamines or nasal steroids, but valuable in children and pregnant women in view of their excellent safety profile), nasal decongestants (symptomatic relief for nasal obstruction, only for short-term use), and oral decongestants (may lead to tachycardia, insomnia, anxiety, and may aggravate prostate problems and glaucoma). Leukotriene-receptor antagonists may play a role in treating AR patients with asthma (CitationBousquet et al 2001); however, the consensus of two reviews is that they are no more effective in AR than nonsedating antihistamines and are less effective than nasal steroids (CitationNathan 2003; CitationWilson et al 2004).
The H1-antihistamine levocetirizine
Levocetirizine is a potent histamine H1-receptor antagonist, and is the active enantiomer of the racemate, cetirizine (CitationTillement et al 2003). Studies in healthy volunteers showed that it very effectively blocks the histamine wheal and flare response in human skin, with its potency exceeding those of other tested substances like desloratadine, fexofenadine, ebastine, and cetirizine (CitationClough et al 2001; CitationDevalia et al 2001; CitationGrant et al 2002). In addition to its antihistaminic effect, levocetirizine also demonstrated antiinflammatory effects in cell culture studies (CitationThomson et al 2002; CitationGiustizieri et al 2004) and in vivo (CitationMichel et al 2001; CitationCiprandi et al 2004).
Levocetirizine 5 mg once daily was established as the most effective and well tolerated dose, producing significant improvements in AR symptoms compared with placebo in a study of 470 patients with SAR (CitationLeynadier et al 2001). A further placebo comparison in 294 patients with PAR showed it produced rapid and significant improvements in AR symptoms within the first week of treatment, which were maintained throughout a 6-week treatment period (CitationPotter 2003). Interestingly, nasal congestion was also significantly reduced in the levocetirizine group compared with placebo over the treatment period with significance within the first week (p < 0.002), and a relative improvement of 83% over the 6 weeks of treatment (p < 0.001).
Similar results were found in a placebo-controlled study of 177 children with SAR where levocetirizine (5 mg/day) significantly improved ocular and nasal symptoms of AR within the first week of treatment, with maintained activity over 6 weeks (Citationde Blic et al 2005). Relief of nasal congestion was observed in the levocetirizine group throughout the study, reaching a maximum difference to placebo during week 3 (p < 0.05) with a relative improvement over placebo of 78%. This difference was maintained until week 5 (p < 0.05), but during the last week, the difference became smaller and nonsignificant due to a strong placebo effect. Concerning QoL measured by PRQLQ (Paediatric Rhinoconjunctivitis Quality of Life Questionnaire), children treated with levocetirizine presented larger improvements in overall and domain scores after 2 weeks, 4 weeks, and 6 weeks of treatment than children under placebo. Levocetirizine was well tolerated in all of the studies, thus complementing the reported lack of cognitive and psycho-motor side effects with this drug (CitationGandon and Allain 2002).
The efficacy of levocetirizine was tested in various comparative clinical trials. In placebo-controlled studies in allergen challenge chambers (CitationDay et al 2004; CitationStübner et al 2004; CitationHorak et al 2005), it provided better protection from and relief from symptoms than single-dose desloratadine 5 mg (CitationDay et al 2004; CitationDeruaz et al 2004), or fexofenadine 120 mg (CitationHorak 2005), and it was superior to loratadine 10 mg in improving symptoms in SAR (CitationStübner et al 2004). These comparative trial results were corroborated in two clinical trials in SAR and PAR where levocetirizine showed better symptom control, increase in nasal airflow, and antiinflammatory activity than desloratadine (CitationCiprandi et al 2004, Citation2005b).
CitationCiebiada et al (2005) compared levocetirizine 10 mg monotherapy with montelukast 10 mg monotherapy, combined therapy, and placebo over 6 weeks in patients with PAR. Levocetirizine alone was more effective on nasal symptoms and inflammatory markers than montelukast alone; but the combination treatment offered an even better symptom control. This combined receptor blockade may be an interesting therapeutic option. This was also shown by a recent study in SAR patients where levocetirizine 5 mg alone, montelukast 10 mg alone, combined therapy and placebo were compared over 2 weeks (CitationLombardo et al 2005). Both monotherapies were effective on symptoms, but the combined treatment was superior on symptoms and QoL.
Levocetirizine in PER
The above studies were performed in a variety of patients with AR classified by the classic system in SAR and PAR. It is difficult to guess how this evidence can be translated into the new indication PER because PER patients are different from SAR or PAR patients. That is why it is of clinical relevance to study rhinitis medication in patients with PER. As of today, few studies have been performed with antihistamines or nasal steroids in PER, although the ARIA classification has been adopted by the industry and academia. Most of these studies were done with levocetirizine. There have been even fewer studies performed in intermittent rhinitis, and there is no evidence yet for levocetirizine in this indication.
A pilot study in 40 patients with PER showed that levocetirizine 5 mg/day for 4 weeks improved nasal symptoms, including nasal obstruction, and increased total nasal airflow (CitationCiprandi et al 2005a). In addition, levocetirizine showed decongestant effects by decreasing the reversibility of nasal airflow after application of a potent alpha-adrenostimulator.
The only major clinical trial to assess any agent as a standard treatment option for PER is the Xyzal in Persistent Rhinitis Trial (XPERT™) (CitationBachert et al 2004), a 6-month, double-blind, placebo-controlled multicenter trial of 551 patients with PER who received levocetirizine 5 mg/day or placebo. The study assessed individual symptoms, QoL (both general and disease-specific), comorbidities, pharmacoeconomics, and safety (CitationBachert et al 2004).
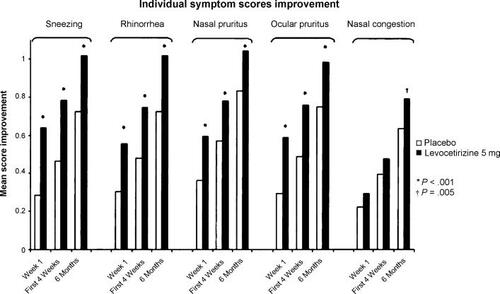
Results from XPERT showed that levocetirizine significantly improved nasal and ocular symptoms compared with placebo as early as 1 week after starting treatment and this improvement was maintained over the 6-month period of study (). Notably, significant improvement in nasal congestion was observed versus placebo after the first month of treatment and maintained over 6 months (p < 0.05 for months 2–6) (CitationVan Cauwenberge et al 2005), which the authors attributed to the long-term antiinflammatory action of levocetirizine (CitationBachert et al 2004). Levocetirizine also improved QoL (assessed by Rhinoconjunctivitis Quality of Life Questionnaire and Short-Form 36), and was well tolerated (CitationBachert et al 2004) (). An economic analysis showed the relatively low cost of long-term levocetirizine therapy compared with the substantial cost of untreated PER and its comorbidities (€350/patient/ month). Levocetirizine treatment produced cost savings to society of more than €150/patient/month, mainly due to patients being able to maintain work ability and daily activities (CitationBousquet et al 2005). In an exploratory analysis of the XPERT study, levocetirizine reduced the percentage of patients with at least one asthma event to 7.4 (from 13.6 for placebo, p = 0.04) and reduced the mean number of asthma medication events from 0.23 per placebo patient to 0.11 (p < 0.001) (CitationBachert and Demarteau 2005).
Figure 2 Change in individual symptom scores among 551 patients with persistent allergic rhinitis who received levocetirizine 5 mg/day or placebo over a period of 6 months. Source: CitationBachert C, Bousquet J, Canonica GW, et al. 2004. Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J Allergy Clin Immunol, 114:838–44. Copyright © 2004 Elsevier, USA. Reproduced with permission from Elsevier.
Conclusion
ARIA defines AR as intermittent AR or PER, based on symptom duration and severity. It complements the classic SAR/PAR classification, and clearly defines PER as different from PAR. Long-term studies in PER will be able to show the effect of drug therapy on allergic symptoms, including nasal congestion. Levocetirizine is currently the best studied treatment in PER, although studies in intermittent rhinitis are still lacking. XPERT showed that over a period of 6 months, levocetirizine was effective against PER symptoms (including nasal congestion), improved QoL, reduced the incidence of comorbidities like asthma, and reduced societal costs. These results show that assessment of drug efficacy and tolerability according to ARIA is clinically relevant, and that significant benefit may be achieved with long-term treatment of PER with levocetirizine.
References
- BachertCBousquetJCanonicaGWLevocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitisJ Allergy Clin Immunol20041148384415480324
- BachertCDemarteauNLevocetirizine reduces asthma comorbidity in patients with persistent allergic rhinitis: an exploratory analysis of the XPERT™ trialAllergy Clin Immunol Int2005S11001
- BauchauVDurhamSRPrevalence and rate of diagnosis of allergic rhinitis in EuropeEur Respir J2004247586415516669
- BauchauVDurhamSREpidemiological characterization of the intermittent and persistent types of allergic rhinitisAllergy200560350315679721
- BousquetJBullingerMFayolCAssessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 Health Status QuestionnaireJ Allergy Clin Immunol19949418288064070
- BousquetJDemarteauNMullolJCosts associated with persistent allergic rhinitis are reduced by levocetirizineAllergy2005607889415876309
- BousquetJKnaniJDhivertHQuality of life in asthma. I. Internal consistency and validity of the SF-36 questionnaireAm J Respir Crit Care Med199414937158306032
- BousquetJVan CauwenbergePBachertCRequirements for medications commonly used in the treatment of allergic rhinitisAllergy200358192712653792
- BousquetJVan CauwenbergePKhaltaevNAllergic rhinitis and its impact on asthmaJ Allergy Clin Immunol2001108S14733411707753
- CiebiadaMGorska-CiebiadaMDu BuskeLMMontelukast and levocetirizine in the treatment of perennial allergic rhinitis: a randomised, double-blind, placebo-controlled studyAllergy Clin Immunol Int2005S1264
- CiprandiGCirilloIVizzaccaroALevocetirizine improves nasal obstruction and modulates cytokine pattern in patients with seasonal allergic rhinitis: a pilot studyClin Exp Allergy2004349586415196286
- CiprandiGCirilloIVizzaccaroALevocetirizine improves nasal symptoms and airflow in patients with persistent allergic rhinitis: a pilot studyAllerg Immunol (Paris)2005a3725915745374
- CiprandiGCirilloIVizzaccaroADesloratadine and levocetirizine improve nasal symptoms, airflow, and allergic inflammation in patients with perennial allergic rhinitis: A pilot studyInt Immunopharmacol2005bin press
- CiprandiGMarsegliaGLKlersyCRelationships between allergic inflammation and nasal airflow in children with persistent allergic rhinitis due to mite sensitizationAllergy2005609576015932388
- CloughGFBoutsioukiPChurchMKComparison of the effects of levocetirizine and loratadine on histamine-induced wheal, flare, and itch in human skinAllergy200156985811576078
- DayJHBriscoeMPRafeiroEComparative clinical efficacy, onset and duration of action of levocetirizine and desloratadine for symptoms of seasonal allergic rhinitis in subjects evaluated in the Environmental Exposure Unit (EEU)Int J Clin Pract2004581091815055856
- de BlicJWahnUBillardELevocetirizine in children: evidenced efficacy and safety in a 6-week randomized seasonal allergic rhinitis trialPediatr Allergy Immunol2005162677515853959
- DemolyPAllaertFALecasbleMValidation of the classification of ARIA (Allergic Rhinitis and its Impact on Asthma)Allergy200358672512823130
- DeruazCLeimgruberABerneyMLevocetirizine better protects than desloratadine in a nasal provocation with allergenJ Allergy Clin Immunol20041136697615100671
- DevaliaJLDe VosCHanotteFA randomized, double-blind, crossover comparison among cetirizine, levocetirizine, and ucb 28557 on histamine-induced cutaneous responses in healthy adult volunteersAllergy20015650711167352
- DurhamSRWalkerSMVargaEMLong-term clinical efficacy of grass-pollen immunotherapyNew Engl J Med19993414687510441602
- FumagalliFBaiardiniIPasqualiMAntihistamines: do they work? Further well-controlled trials involving larger samples are neededAllergy200459Suppl 7874715245363
- GandonJMAllainHLack of effect of single and repeated doses of levocetirizine, a new antihistamine drug, on cognitive and psychomotor functions in healthy volunteersBr J Clin Pharmacol20025451812100225
- GiustizieriMLAlbanesiCFluhrJH1 histamine receptor mediates inflammatory responses in human keratinocytesJ Allergy Clin Immunol200411411768215536428
- GrantJARiethuisenJMMoulaertBA double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjectsAnn Allergy Asthma Immunol200288190711868924
- HansenJKlimekLHörmannKPharmacological management of allergic rhinitis in the elderly: safety issues with oral antihistaminesDrugs Aging200522Suppl 42899615839718
- HorakFJrMatthewsSIhorstGEffect of mite-impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth-cohort study – 24 months results of the Study of Prevention of Allergy in Children in EuropeClin Exp Allergy2004341220515298561
- HorakFZieglmayerPZieglmayerRLevocetirizine has a longer duration of action on improving total nasal symptoms score than fexofenadine after single administrationBr J Clin Pharmacol200560243115963090
- KayGGThe effects of antihistamines on cognition and performanceJ Allergy Clin Immunol2000105S622710856168
- LombardoGQuattrocchiPLombardoGRLevocetirizine and montelukast in the treatment of seasonal allergic rhinitisEur Respir J200526Suppl 49137s
- LeynadierFMeesKArendtCEfficacy and safety of levocetirizine in seasonal allergic rhinitisActa Otorhinolaryngol Belg2001553051211859651
- MaloneDCLawsonKASmithDHA cost of illness study of allergic rhinitis in the United StatesJ Allergy Clin Immunol1997992279003207
- MartinIRThe feasibility of allergen avoidance: compliance and effect on quality of life of a house dust mite allergen avoidance strategy for families with asthma. University of Otago Public Health Group [online]1999 Accessed 13 June 2005. URL: http://www chmeds ac nz departments/pubhealth/theses13 htm
- MichelLJean-LouisFDubertretLPharmacological study of levocetirizine in lgE-dependent hypersensitivity cutaneous reaction in grass pollen allergic volunteers: Demonstration of mediator release and eosinophil recruitment modulation by levocetirizineAllergy200156Suppl 681501
- MorrisATalking to patients about managing their allergiesPrescriber2004151423
- NathanRAPharmacotherapy for allergic rhinitis: a critical review of leukotriene receptor antagonists compared with other treatmentsAnn Allergy Asthma Immunol2003901829012602664
- PeatJKMihrshahiSKempASThree-year outcomes of dietary fatty acid modification and house dust mite reduction in the Childhood Asthma Prevention StudyJ Allergy Clin Immunol20041148071315480319
- PotterPCLevocetirizine is effective for symptom relief including nasal congestion in adolescent and adult (PAR) sensitized to house dust mitesAllergy200358893912911418
- SchoenwetterWFDupclayLJrAppajosyulaSEconomic impact and quality-of-life burden of allergic rhinitisCurr Med Res Opin2004203051715025839
- SimonsFEH1-antihistamines in childrenClin Allergy Immunol2002174376412113226
- SimonsFEAdvances in H1-antihistaminesN Engl J Med200435122031715548781
- SimpsonACustovicAThe role of allergen avoidance in the secondary prevention of atopic disordersCurr Opin Allergy Clin Immunol20055223715864079
- StübnerPZieglmayerRHorakFA direct comparison of the efficacy of antihistamines in SAR and PAR: randomised, placebo-controlled studies with levocetirizine and loratadine using an environmental exposure unit – the Vienna Challenge Chamber (VCC)Curr Med Res Opin20042089190215200748
- ThomsonLBlaylockMGSextonDWCetirizine and levocetirizine inhibit eotaxin-induced eosinophil transendothelial migration through human dermal or lung microvascular endothelial cellsClin Exp Allergy20023211879212190657
- TillementJPTestaBBreeFCompared pharmacological characteristics in humans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonistsBiochem Pharmacol2003661123614505791
- Van CauwenbergePEfficacy and safety of levocetirizine in persistent allergic rhinitis (PER)Eur Resp J200526Suppl 49136s
- van den BemtLvan KnapenLde VriesMPClinical effectiveness of a mite allergen-impermeable bed-covering system in asthmatic mite-sensitive patientsJ Allergy Clin Immunol20041148586215480327
- WarnerJOA double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months' treatment and 18 months' posttreatment follow-upJ Allergy Clin Immunol20011089293711742270
- WilsonAMO'ByrnePMParameswaranKLeukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysisAm J Med20041163384414984820
- WilsonDRLimaMTDurhamSRSublingual immunotherapy for allergic rhinitis: systematic review and meta-analysisAllergy20056041215575924