Abstract
Opioid receptors are widely expressed in the central and peripheral nervous system as well as in numerous nonneuronal tissues. Both animal models and human clinical data support the involvement of peripheral opioid receptors in analgesia, particularly in inflammation where both opioid receptor expression and efficacy are increased. Immune cells have been shown to contain numerous opioid peptides such as β-endorphin (END), met-enkephalin (ENK), and dynorphin-A (DYN), although the predominant opioid peptide involved in immune-cell mediated antinociception is thought to be END. These opioid-containing immune cells migrate to inflamed tissues during a complex process of recruitment by chemokines, adhesion, and extravasation. In these tissues, opioid peptide is released from the immune cells upon stimulation with corticotrophin-releasing factor (CRF), noradrenaline, and interleukin 1β (IL-1β), and the immune cells return to the local lymph node depleted of peptide. Consistent with this model, systemic immunosuppression may lead to impaired endogenous analgesia as competent immune cells are essential to achieve release of endogenous opioid peptides within inflamed tissue. A further level of complexity is added by the observation that exogenous opioids may impair immune cell function, although there is some evidence to suggest that endogenous opioid peptides do not share this immunosuppressive effect. Improving our understanding of endogenous opioid mechanisms will provide valuable insight towards the development of novel treatments for pain with improved side effect profiles.
Introduction
Pain arising from inflammatory conditions is often treated with agents that inhibit the immune response, such as corticosteroids, or modulators of the inflammatory cascade, such as nonsteroidal antiinflammatory drugs (NSAIDs) (CitationDequeker 1999). These drugs are associated with substantial adverse effects and risks with long-term use, ranging from gastrointestinal (GI) insult to opportunistic infections, and osteoporosis (CitationHirschowitz 1994; CitationDequeker 1999). While opioid receptor agonists have limited or, more likely, poorly understood antiinflammatory action, they remain useful effective analgesic agents in these conditions. However, respiratory depression, sedation, and constipation remain as dose-limiting side effects after systemic administration of this drug class. Tolerance, while not a common occurrence, can develop during long-term use (CitationSessle and Henry 1985; CitationMansour et al 1988; CitationMarinangeli et al 2004; CitationJensen et al 2005).
The immune system is traditionally thought of as a contributor to inflammatory pain. However, a new analgesic role has been described for the immune system in the release of endogenous opioid peptides from immune cells within inflamed tissue (CitationStein et al 1997). The benefit of peripheral immune-mediated opioid analgesia is targeted analgesia at the site of inflammation, and thus avoidance of side effects mediated by activation of opioid receptors in the central nervous system (CNS), or on peripheral organs such as the GI tract. Although immune cell-mediated endogenous opioid analgesia appears to challenge our current understanding of the role of the immune system in inflammation, it is likely that the two approaches to inflammatory pain treatment are not in contrast, but complementary. The role of the immune system in peripheral analgesia is likely to be delicately balanced, depending on whether the action of infiltrating cells is proinflammatory, proalgesic, or both; either predicted by the immune cell, or by the injury, or disease status. In situations where endogenous antinociception exceeds hyperalgesia mediated by immune cells, immuno-suppression or modulation of immune cell trafficking may increase pain (CitationHermanussen et al 2004).
A better understanding of the endogenous pathways and mechanisms involved in peripheral analgesia may not only enable us to target these pathways for novel antinociceptive approaches and avoid systemic side effects of opioid treatment, but it will also allow us to identify patients in whom immunosuppression may have detrimental effects on pain control and minimise the possible algesic effects of immunosuppression in these patients.
Opioid receptor distribution and mechanisms that mediate antinociception
Since their first characterization in 1973, 3 main opioid receptors, referred to as μ (MOR), δ (DOR), and κ (KOR), have been defined (CitationPert and Snyder 1973; CitationSimon et al 1973; CitationTerenius 1973; CitationLaw et al 2000). These opioid receptors can exist as several splice variants, which are differentially expressed in the central and peripheral nervous systems as well as varying nonneuronal tissues, presumably due to different messenger ribonucleic acid (mRNA) processing as they are derived from the same gene (CitationAbbadie et al 2000; CitationLaw et al 2000).
Opioid receptor structure and function
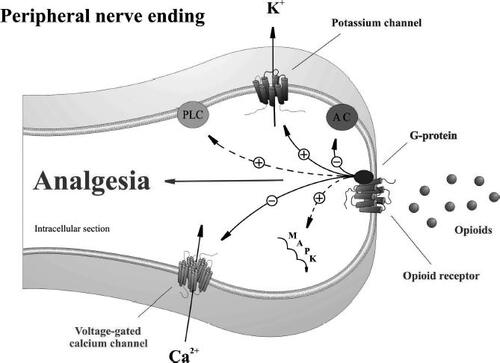
Opioid receptors are prototypical G-protein coupled receptors belonging to the subfamily of rhodopsin receptors and consist of approximately 400 amino acids (CitationLaw et al 2000; CitationPil and Tytgat 2003). On a molecular basis, they possess 7 α-helical transmembrane domains and an extracellular N-terminus with multiple glycosylation sites (CitationLaw et al 2000). MOR, DOR, and KOR are highly homologous to each other at the sequence and post-translational levels, with particularly high conservation in the regions spanning the transmembrane domains and intracellular loops (CitationKnapp et al 1995; CitationAkil et al 1996; CitationLaw et al 2000; CitationPil and Tytgat 2003). Divergence can be found at the N- and C-termini as well as the extracellular loops, accounting for the unique pharmacological properties of these receptors (CitationKnapp et al 1995; CitationAkil et al 1996; CitationLaw et al 2000; CitationPil and Tytgat 2003). Opioid receptors can couple to both pertussis toxin-sensitive and insensitive G-proteins, with coupling characteristics differing slightly between the receptor types. G-protein coupling of opioid receptors to their effectors can be direct or involve intermediate or other indirect effector pathways (CitationConnor and Christie 1999). Activation of inwardly rectifying K+ channels (GIRK+), inhibition of voltage-dependent Ca2+ channels as well as inhibition of adenylyl cyclase are direct G-protein-coupled effects of opioid receptor activation (CitationConnor and Christie 1999; CitationLaw et al 2000). Conversely, opioid receptors can activate phospholipase C (PLC), the mitogen-activated protein kinase (MAPK) cascade, and large conductance Ca2+-activated K+ channels by utilising other intermediary messenger systems (CitationConnor and Christie 1999; CitationLaw et al 2000). Modification of Ca2+ and K+ conductance by opioid receptors can lead to a decrease in neuronal excitability, a decrease in neuronal firing rate, and inhibition of neurotransmitter release (CitationConnor and Christie 1999; CitationLaw et al 2000). Functionally, opioid receptors have been implicated in regulation of pain, reinforcement, reward, neuroendocrine modulation, and alteration in neuro-transmitter release (CitationMansour et al 1995). The consequence of opioid receptor activation appears to be correlated closely with the anatomical location as well as expression levels of the opioid receptor subtypes (CitationMansour et al 1995).
Opioid receptor expression and function in the central nervous system
Opioid receptors are widely and differentially distributed in the CNS. MOR, in particular, is widely distributed throughout the forebrain, midbrain, and hindbrain with greatest expression apparent in the neocortex, caudateputamen, nucleus accumbens, thalamus, hippocampus, amygdala, and nucleus tractus solitarius (CitationMansour et al 1988, Citation1995). This distribution of the MOR is consistent with its suggested role in pain perception as well as sensorimotor integration. Conversely, KOR has been found to be expressed in moderate amounts in many brain areas, with greatest expression in the caudate putamen, nucleus accumbens, hypothalamus, amygdala, and neural lobe of the pituitary (CitationMansour et al 1988, Citation1995). KOR has been implicated to be involved in feeding, pain perception, and neuroendocrine function. DOR is particularly highly expressed in olfactory related neural areas, the neocortex, caudate-putamen, nucleus accumbens, and amygdala while exhibiting limited binding in the thalamus, hypothalamus, and brainstem (CitationMansour et al 1988, Citation1995). This distribution corresponds with the suggested involvement of DOR in motor as well as olfactory and cognitive functioning.
The functional consequences arising from these expression patterns of opioid receptors may affect many other physiological systems, which can have important implications for the clinical use of opioids, particularly in the unwanted side effects of systemically administered opioid receptor agonists. Many side effects arising from systemic administration of opioids are due to activation of central opioid receptors. Administration of MOR agonists has been linked to centrally mediated cardiovascular and renal effects (CitationMansour et al 1988, Citation1995; CitationGutkowska et al 2004; CitationMao and Wang 2005). Of particular relevance to the use of opioids as analgesics are respiratory depression mediated though activation of MOR and DOR in the brainstem, as well as KOR-mediated sedation; both of which can be dose-limiting side effects of systemic treatment with opioids (CitationSessle and Henry 1985; CitationMansour et al 1988).
Opioid receptor expression and function in the spinal cord and peripheral nervous system
Opioid-mediated analgesia is mediated by modulation of ascending and descending pain pathways (CitationMansour et al 1988; CitationVaughan et al 1997; CitationPan et al 2004). Accordingly, MOR, DOR, and KOR expression has been confirmed in dorsal root ganglia, the spinal cord, and trigeminal nucleus of the ascending pain pathway as well as the central Gray area, pontine, gigantocellulare, and intermediate reticular nuclei of the descending pain pathway, with predominantly MOR and KOR expression in the median raphe and raphe magnus (CitationMansour et al 1988). In the periaqueductal gray region and the nucleus locus coeruleus, opioid receptor agonists produce analgesia by disinhibiting descending fibres through inhibition of Gamma-Aminobutyric acid (GABA)-ergic neuronal inputs, which modulates other descending pathways in turn, such as noradrenergic neurons, to produce analgesia (CitationVaughan et al 1997; CitationPan et al 2004).
In addition to spinal and centrally mediated opioid analgesia, opioid receptors expressed on peripheral neurons can also contribute to antinociception. Numerous animal models and human clinical trials in support of this peripheral opioid analgesia have been described and will be discussed in greater detail in the next section. While targeting these peripheral mechanisms may provide analgesic strategies that can avoid some or all of the central side effects of systemic opioid agonists, some peripherally mediated side effects may remain problematic. This is a result of opioid receptor expression on peripheral nervous system neurons innervating peripheral organs such as the skin and GI tract (CitationBagnol et al 1997; CitationFickel et al 1997; CitationBigliardi-Qi et al 2004; CitationHolzer 2004). MOR and KOR have been found to be expressed in the stomach, duodenum, jejunum, ileum as well as proximal and distal colon, where their function is thought to include control of visceral pain, regulation of transit time of luminal contents, and mucosal transport of fluids and electrolytes (CitationBagnol et al 1997; CitationFickel et al 1997; CitationEastwood and Grundy 2000; CitationHolzer 2004; CitationGray et al 2005). Accordingly, the adverse effect of systemic administration of opioid agonists on GI function is thought to arise primarily from modulation of the enteric pathways governing peristalsis by opioid receptor agonists (CitationBagnol et al 1997; CitationFickel et al 1997; CitationHolzer 2004). The effect of opioid receptor agonists on gut motility and secretion has been utilised clinically in the symptomatic management of diarrhea (CitationSun et al 1997). Furthermore, opioid receptors may be involved in the modification of GI inflammation, possibly through the protein kinase C-mediated desensitization of chemokine receptors (CitationPhilippe et al 2003; CitationZhang et al 2003).
Opioid receptor expression and function in nonneuronal tissues
Opioid receptor protein and mRNA have also been described in several nonneuronal tissues such as vascular and cardiac epithelia as well as keratinocytes, although the significance of opioid receptor expression in these nonnociceptive tissues is less clear (CitationBidlack 2000; CitationCadet et al 2000; CitationMousa et al 2001; CitationBigliardi-Qi et al 2004). MOR, DOR, and KOR are also expressed on various immune cells including lymphocytes and macrophages. Activation of the receptors have been reported to modulate immune function including the macrophage oxidative burst and cytokine production (CitationBidlack 2000). Evidence for the expression of all 3 types of opioid receptors on immune cells stems from functional evidence suggesting that MOR, DOR, and KOR agonists can modulate immune cell function, and includes immunohistochemical and binding studies which verify the expression of MOR, DOR, and KOR on numerous immune cells (CitationBidlack 2000). In addition, mRNA encoding for opioid receptors has been obtained from immune cells and supports the identification of opioid receptors expressed on immune cells as identical to neuronal receptors (CitationBidlack 2000).
In summary, opioid receptor protein and mRNA have been described in both central and peripheral nervous systems as well as in several nonneuronal tissues. Activation of these differentially distributed receptors by systemic treatment with opioid receptor agonists not only causes analgesia, but can be directly correlated to clinically observed adverse effects such as respiratory depression, sedation, and constipation (CitationSessle and Henry 1985; CitationMansour et al 1988, Citation1995; CitationFickel et al 1997; CitationVaughan et al 1997; CitationGutkowska et al 2004; CitationHolzer 2004; CitationPan et al 2004; CitationMao and Wang 2005).
Peripheral opioid receptors and their contribution to analgesia
Including centrally mediated effects, the potential that opioid receptors of the peripheral nervous system have for mediating effective analgesia is well documented. Common strategies to isolate the activation of peripheral opioid receptors include the administration of local, systemically inactive doses of opioids, and the use of hydrophilic opioids that do not readily penetrate the blood-brain barrier.
Animal models
In animal models of inflammatory pain, peripheral MOR, DOR, and KOR participate in the suppression of pain. In the inflammatory phase of the formalin test in rats, novel peripherally restricted μ-opioid agonists, administered subcutaneously, produced dose-dependent antinociception (CitationFurst et al 2005). This effect was reversed by the peripherally restricted antagonist naloxone methiodide, confirming the activation of peripheral MOR (CitationFurst et al 2005). The DOR agonist SNC80 produced dose-dependent antinociception in PGE2-induced hyperalgesia of the rat paw following subcutaneous administration (CitationPacheco and Duarte 2005). This effect was not observed after equivalent administration at a remote site, indicating that anti-nociception was delivered by peripheral DOR (CitationPacheco and Duarte 2005). KOR-selective opioid agonists, when administered subcutaneously to the paw, evoked potent dose-dependent increases in pain thresholds after Freund's complete adjuvant (FCA)-induced chronic inflammation, an effect antagonized by naloxone methiodide (CitationBinder et al 2001). In an animal model of inflammatory bowel disease, peripherally restricted κ-opioid agonists produced dose-dependent, peripherally antagonized reductions in visceromotor responses and afferent nerve activity, providing further evidence that peripheral KOR effects antinociception (CitationSengupta et al 1999). In a single study using FCA-induced inflammation of the rat paw, locally acting MOR-, DOR-, and KOR-selective agonists delivered antinociception that was dose-dependent, stereospecific, and reversible by receptor specific antagonists (CitationStein et al 1989). In addition to inflammatory pain, peripheral opioid receptors reportedly attenuate nociceptive responses in rats following thermal injury (CitationNozaki-Taguchi and Yaksh 1999), and in animal models of uterine cervical distension (CitationSandner-Kiesling et al 2002), neuropathic pain (CitationPertovaara and Wei 2001; CitationTruong et al 2003; CitationObara et al 2004), and nociceptive pain (CitationFurst et al 2005). Moreover, a technique involving viral-driven transfer and overproduction of proenkephalin A, a precursor for the endogenous opioid peptide enkephalin in trigeminal ganglion neurons, resulted in long lasting attenuation of allodynia in a rat model of neuropathic pain (CitationMeunier et al 2005). Immunohistochemistry indicated that transgene-derived opioids were transported predominantly to the peripheral ends of primary sensory neurons. The abolition of transgene-derived antinociception by naloxone methiodide confirms that peripheral opioid receptors were responsible for the attenuation of allodynia in this model (CitationMeunier et al 2005).
Human clinical studies
Peripheral opioid receptor-mediated analgesia has also been demonstrated in controlled clinical studies. In experimental human pain models, administration of the peripherally restricted opioid agonists morphine-6-glucoronide (M6G) reduced hyperalgesia induced by freeze lesions and excessive muscle contraction, which was verified to be via peripheral action due to the absence of characteristic CNS effects (CitationTegeder et al 2003). Patients with chronic pancreatitis reported reductions in abdominal pain following intravenous infusion with a peripherally restricted κ-opioid agonist (CitationEisenach et al 2003). Similarly, the administration of locally acting opioids during knee and dental surgery significantly reduces postoperative pain and recovery time (CitationDionne et al 2001; CitationLikar et al 2001; CitationKalso et al 2002). Topical application of opioids for the treatment of painful skin ulcers and oral mucositis has also achieved effective relief of pain (CitationTwillman et al 1999; CitationCerchietti et al 2002). Separate bioavailability studies indicate that therapeutic doses of opioids applied topically to skin ulcers are not readily absorbed into the blood and are therefore likely to act peripherally (CitationRibeiro et al 2004). Some clinical studies assessing the efficacy of locally acting opioids failed to show an improvement in pain intensity scores, but reported more subtle modifications such as increases in time to first analgesic request and a reduction in total analgesic requirement (CitationLikar et al 2004; CitationZajaczkowska et al 2004).
Despite overwhelming evidence that peripheral opioid receptors mediate effective analgesia, there is limited direct evidence describing opioid mechanisms at peripheral nerve endings. This is because elucidation of opioid receptor mechanisms has relied heavily upon techniques that utilize cultured neurons and other cellular expression systems. However, experiments in animal models using ion channel blockers indicate that opioid receptors on peripheral sensory nerve endings mediate analgesia partly through local activation of adenosine triphosphate (ATP)-sensitive K+ channels (CitationPacheco and Duarte 2005), and inhibition of L-type Ca2+ channels () (CitationShimizu et al 2004).
Figure 1 Proposed model for opioid receptor-mediated analgesia at peripheral terminals of primary sensory neurons. Activation of opioid receptors by endogenous or exogenous opioid agonists promotes G-protein coupling. Opioid receptor-coupled G-proteins directly activate inwardly rectifying K+ channels (GIRK+), inhibit voltage-dependent Ca2+ channels, and inhibit adenylyl cyclase (AC). Opioid receptors indirectly activate phospholipase C (PLC), the mitogen-activated protein kinase (MAPK) cascade, and large conductance Ca2+-activated K+ channels by utilizing other intermediary messenger systems.
Inflammation promotes opioid receptor mechanisms in the periphery
An important determination from both animal and human studies is that peripherally acting exogenous opioids produce enhanced analgesia in the presence of inflammation. For example, in the rat paw model, locally acting opioid agonists significantly increase nociceptive thresholds to mechanical stimuli in inflamed tissue, but not in noninflamed tissue (CitationStein et al 1989; CitationBinder et al 2001). In human studies, reports from dental surgery patients show that chronically inflamed tissue exhibits improved analgesic responses compared with acutely inflamed (CitationDionne et al 2001) and noninflamed tissue (CitationLikar et al 2001). These findings indicate that inflammation promotes opioid receptor mechanisms in the periphery.
Opioid receptor expression
In primary afferent neurons, opioid receptors are manufactured in the dorsal root ganglion (DRG) and are transported both centrally and peripherally by axonal transport (CitationHassan et al 1993). In the rat paw model, inflammation induces a significant increase in MOR in the DRG (CitationJi et al 1995; CitationZhang et al 1998; CitationBallet et al 2003; CitationZollner et al 2003; CitationShaqura et al 2004) and in peripheral nerves innervating the inflamed tissue (CitationHassan et al 1993; CitationMousa et al 2001, Citation2002). In the spinal cord, there are reports of either no change (CitationBallet et al 2003; CitationShaqura et al 2004), or increase in MOR expression (CitationJi et al 1995; CitationMousa et al 2002); a discrepancy likely due to differences in disease state and the sensitivity of analytical techniques. Consistent with increased receptor expression, MOR mRNA levels are increased in both the DRG (CitationPuehler et al 2004) and in the spinal cord (CitationMaekawa et al 1996), which supports the thesis that inflammation increases MOR synthesis. With respect to DOR and KOR, the effect of inflammation on receptor expression is less clear in the rat paw model. In separate studies with similar disease states, DOR expression is reported to decrease in DRG (CitationJi et al 1995; CitationZhang et al 1998), increase in peripheral tissues (CitationHassan et al 1993), and both increase and decrease in the spinal cord (CitationJi et al 1995; CitationCahill, Morinville, et al 2003). DOR mRNA levels reportedly remain unchanged in the DRG (CitationPuehler et al 2004) and in the spinal cord (CitationMaekawa et al 1996). In independent studies, KOR expression has been found to both decrease and remain static in DRG (CitationJi et al 1995; CitationZhang et al 1998), and remain constant in the spinal cord (CitationJi et al 1995). A significant increase in KOR mRNA has been observed in the spinal cord of animals with peripheral inflammation (CitationMaekawa et al 1996). Adding further complexity; changes in KOR and DOR expression have also been reported in nonneuronal cells. More specifically, the expression of KOR and DOR have recently been shown to be decreased in the fibroblast-like synoviocytes of patients with rheumatoid arthritis and osteoarthritis, particularly where inflammation was present (CitationShen et al 2005). Upregulation of the KOR occurred through exposure to the specific KOR agonist U69593 (CitationShen et al 2005), and while these changes occurred in cells isolated from patients and established in vitro, these observations could suggest that intra-articular or peripheral opioids may be useful in the management of these inflammatory conditions and could have an impact on pain states in these patients. Despite these apparently conflicting outcomes, DOR- and KOR-selective opioid agonists deliver potent antinociception in the inflamed rat paw model, suggesting the involvement of both DOR and KOR receptors in pain control during inflammation (CitationStein et al 1989; CitationBinder et al 2001; CitationCahill, Morinville, et al 2003). In a separate inflammatory model, the inflamed mice intestine, MOR mRNA, and protein is upregulated in nerves innervating the gut (CitationPol et al 2001). Furthermore, intestinal inflammation significantly increases DOR mRNA in peripheral nerves and in the spinal cord, and increases DOR and KOR receptor protein in peripheral nerves (CitationPol et al 2003).
The mechanisms that affect opioid receptor upregulation during inflammation are not yet well understood, however, a number of recent studies provide insight into these mechanisms. The inflammatory mediator interleukin-6 (IL-6) strongly induces MOR, but not DOR transcription and translation in the human neuroblastoma cell line SHSY5Y (CitationBorner et al 2004). IL-6 evoked increases in MOR expression are mediated by the transcription factors signal transducers and activators of transcription 1 (STAT1) and transcription 3 (STAT3) (CitationBorner et al 2004). In vitro results with IL-6 are supported by findings that IL-6 knock out mice have reduced MOR levels in the grey matter of the midbrain compared with wild type individuals and exhibit reduced analgesic responses to morphine (CitationBianchi et al 1999). Interleukin-4 stimulates MOR transcription in cultured immune cells and cultured neurons via transcription 6 (STAT6) activation of the MOR gene promoter (CitationKraus et al 2001). Furthermore, the proinflammatory cytokine tumor necrosis factor (TNF) induces MOR gene transcription, via the transcription factor nuclear factor κB (NFκB) in immune cells (CitationKraus et al 2003) and neurons (CitationBorner et al 2002). Nerve growth factor (NGF) is produced by peripheral tissues, particularly during inflammation (CitationMcMahon 1996), and is implicated in opioid receptor regulation. Transgenic mice that overexpress NGF show an increase in MOR and KOR expression and a decrease in DOR expression in DRG compared with wild type mice, and exhibit increased nociceptive thresholds (CitationZwick et al 2003). Further evidence that NGF influences opioid receptor function is provided by findings that intrathecal NGF restored opioid effectiveness in a rat model of neuropathic pain (CitationCahill, Dray, et al 2003). In addition to the action of inflammatory cytokines and growth factors, the increased conduction of nociceptive signals that occurs during inflammation promotes opioid receptor transcription in sensory neurons, particularly during the early stages of inflammation (CitationPuehler et al 2004). This was evidenced by blocking neuronal conduction with local anesthetic, which prevented the upregulation of MOR mRNA in the first 2 hours following induction of inflammation in the rat hind paw (CitationPuehler et al 2004).
Opioid receptor efficacy
The ability of opioid agonists to stimulate radiolabelled Guanosine 5′-Triphosphate (GTP) ([35S]GTP-γ-S) binding, provides a measure of Gi/Go-protein coupling efficiency and the functional status of the opioid receptor (CitationTraynor and Nahorski 1995). In DRG, but not spinal cord tissue, (D-Ala, N-Me-Phe, Gly-ol)-Enkephalin (DAMGO) stimulated (35S)GTP-γ-S binding is increased in animals with inflammation (CitationBallet et al 2003; CitationZollner et al 2003; CitationShaqura et al 2004). These results may simply reflect the increase in opioid receptor density that occurs in DRG during inflammation, but nevertheless represent an improvement in the efficacy of the μ-opioid agonist DAMGO to stimulate opioid receptor G-protein coupling and may explain the increased antinociceptive effects of exogenous opioids in inflammatory conditions (CitationZollner et al 2003). Moreover, in cultured sensory neurons, the expression of MOR mRNA correlates directly with opioid-evoked inhibition of voltage-gated Ca2+ channels (CitationSilbert et al 2003), which also suggests that the increase in opioid receptor expression evident during inflammation translates into a functional enhancement of opioid efficacy. Inflammation also alters the subcellular distribution of opioid receptors, mobilizing receptors towards the cell membrane (CitationCahill, Morinville, et al 2003), and disrupts the perineurial barrier surrounding sensory nerve fibres, providing opioid peptides better access to cell membrane receptors (CitationAntonijevic et al 1995). Some inflammatory chemokines have been reported to desensitize opioid receptors on peripheral sensory neurons in vitro and to suppress opioid antinociception to the noninflamed periaqueductal gray region of the rat brain when administered before opioid treatment (CitationSzabo et al 2002; CitationZhang et al 2004). These findings suggest that elements of the inflammatory process may, in isolation, impair opioid efficacy. However, opioids can exert a reciprocal desensitization of chemokine receptors (CitationZhang et al 2003), which is evidence of the complexity of the interaction between chemokine and opioid receptors; a relationship that is not fully understood. Furthermore, in the inflamed rat paw, inflammatory chemokines recruit opioid-containing polymorphonuclear cells. The blockade of these chemokines reduces peripheral opioid-mediated antinociception, which is evidence that chemokines promote opioid mechanisms (CitationBrack, Rittner, et al 2004b). Tissue acidosis is another characteristic of inflammation that may affect opioid efficacy. Interstitial pH as low as 5.6 has been observed in various inflamed tissues (CitationEdlow and Sheldon 1971; CitationJacobus et al 1977; CitationWard and Steigbigel 1978; CitationSimmen et al 1994; CitationAndersson et al 1999). In NG108-15 cell membranes, acidic pretreatment increases opioid receptor-mediated inhibition of adenylyl cyclase by impairing GTPase activity (CitationSelley et al 1993). Further evidence that acidic inflammatory pH improves opioid efficacy is provided by studies using DRG neurons, which show that morphine inhibition of Ca2+ transients is enhanced at low extracellular pH (CitationKapitzke et al 2005).
Taken together, the changes that occur during inflammation contribute to enhanced opioid receptor expression and efficacy, particularly for MOR.
Immune cells contain opioid peptides
Endogenous opioid peptides
In mammals, pro-opiomelanocortin (POMC), proenkephalin (PENK) and pro-dynorphin (PDYN) are the precursor proteins from which the main endogenous opioid peptides β-endorphin (END), met-enkephalin (ENK), and dynorphin-A (DYN), respectively, are derived (CitationNakanishi et al 1979; CitationKakidani et al 1982; CitationNoda et al 1982). Dynorphin A and B, which are derived from PDYN, have relatively high affinity for KOR, but also some affinity for MOR and DOR. ENK and leu-enkephalin are endogenous ligands derived from PENK that display preferential binding to DOR, lower affinity for MOR, and negligible binding to KOR (CitationPrzewlocki and Przewlocka 2001). END, along with several nonopioid peptides, is derived from the precursor protein POMC and exhibits equipotent MOR and DOR binding while having lower affinity for KOR (CitationAkil et al 1981; CitationPrzewlocki and Przewlocka 2001). Endomorphin-1 and endomorphin-2 are MOR-selective endogenous peptides of so far unknown origin that have been found in distinct regions of rat brain as well as primary neurons and the spinal cord (CitationMartin-Schild et al 1997; CitationZadina et al 1997; CitationSchulz et al 1998)
Opioid peptides in nonneuronal cells
The role of END, ENK, and DYN has been described in detail for both central and peripheral antinociceptive pathways (CitationPrzewlocki and Przewlocka 2001). Interestingly, nonneuronal tissues have also been described to contain opioid peptides that may contribute to peripheral antinociception.(CitationCabot et al 1997, Citation2001; CitationSchafer et al 1994; CitationFinegold et al 1999; CitationLin et al 2002; CitationMousa et al 2004). Opioid peptides have been shown to be expressed in keratinocytes (CitationKhodorova et al 2003), and it has been suggested that these cells may be an important source of endogenous opioid receptor agonists. In particular, activation of both the endothelin-B and cannabinoid CB2 receptors have been shown to elicit analgesia in vivo through release of END from keratinocytes (CitationKhodorova et al 2003; CitationIbrahim et al 2005) This occurred in models where pain was induced either through injection of endothelin-1, or a noxious thermal stimulus, and may highlight that local release of opioid peptides can achieve anatomically specific analgesia (CitationKhodorova et al 2003; CitationIbrahim et al 2005).
Immune cells are of particular interest with respect to nonneuronal cell types involved in endogenous analgesic mechanisms. Expression of opioid peptides by immune cells have been extensively studied and have been shown to express both full-length and truncated mRNA encoding for the precursor proteins POMC, PENK, and PDYN. Moreover, the respective opioid peptides END, ENK, and DYN have been described in many immune cells of rodent and human origin (CitationLolait et al 1986; CitationSmith et al 1986; CitationZurawski et al 1986; CitationOates et al 1988; CitationBuzzetti et al 1989; CitationRosen et al 1989; CitationStein, Hassan, et al 1990; CitationHassan et al 1992; CitationPrzewlocki et al 1992; Citationvan Woudenberg et al 1992; CitationSchafer et al 1994; CitationLinner et al 1996; CitationCabot et al 1997, Citation2001; CitationLyons and Blalock 1997; CitationKamphuis et al 1998; CitationBlalock 1999; CitationBinder et al 2004; CitationMousa et al 2004). Originally, the role of these opioid peptides was thought to consist of the regulation of local immune function through the neuroendocrine axis (CitationLolait et al 1986; CitationOates et al 1988; CitationRosen et al 1989; CitationManfredi et al 1995; CitationKamphuis et al 1998; CitationBlalock 1999). This was supported by the observation that immune cells express opioid receptors (CitationCarr et al 1989). In addition, functional evidence verified that opioids can modulate immune function, including mitogen-induced proliferation, antibody production, and natural killer (NK) activity (CitationManfredi et al 1995). An additional role for these peptides emerged with the observation that opioid peptides released locally from immune cells can act on opioid receptors expressed on peripheral neurons to exert an antihyperalgesic effect in inflamed tissue (CitationStein, Hassan, et al 1990; CitationSchafer et al 1994, Citation1996; CitationCabot et al 1997, Citation2001; CitationCabot 2001; CitationMousa et al 2001, Citation2004; CitationRittner et al 2001; CitationRittner and Stein 2005).
Immune cells express opioid peptides and their precursor proteins
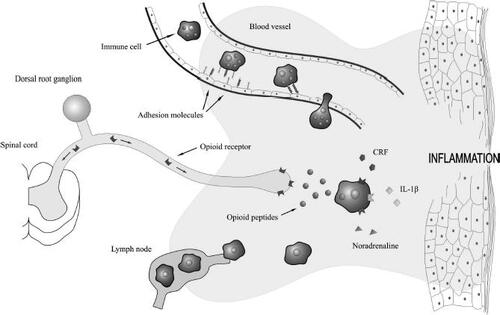
The mechanism proposed for this immune-derived peripheral antinociception involves recruitment of immune cells to local inflamed areas, where they release endogenous opioid peptides upon stimulation with proinflammatory mediators such as interleukin 1β (IL-1β) and corticotrophin-releasing factor (CRF) as well as sympathetic mediators such as noradrenaline (CitationStein, Hassan, et al 1990; CitationSchafer et al 1994, Citation1996; CitationCabot et al 1997, Citation2001; CitationCabot 2001; CitationMousa et al 2001, Citation2004; CitationRittner et al 2001; CitationBinder et al 2004; CitationRittner and Stein 2005). Numerous leukocytes, including lymphocytes, macrophages, monocytes, and human peripheral blood mononuclear cells, have been reported to contain these opioid peptides (CitationLolait et al 1986; CitationSmith et al 1986; CitationZurawski et al 1986; CitationOates et al 1988; CitationBuzzetti et al 1989; CitationRosen et al 1989; CitationStein, Hassan, et al 1990; CitationHassan et al 1992; CitationPrzewlocki et al 1992; Citationvan Woudenberg et al 1992; CitationSchafer et al 1994; CitationLinner et al 1996; CitationCabot et al 1997, Citation2001; CitationLyons and Blalock 1997; CitationKamphuis et al 1998; CitationBlalock 1999; CitationBinder et al 2004; CitationMousa et al 2004). Generally, both precursor protein as well as opioid peptide expression appear to be increased in immune cells from inflamed tissue or mitogen-stimulated cells compared with quiescent leukocytes (CitationSmith et al 1986; CitationZurawski et al 1986; CitationBuzzetti et al 1989; CitationRosen et al 1989; CitationHassan et al 1992; Citationvan Woudenberg et al 1992; CitationCabot et al 1997, Citation2001; CitationLyons and Blalock 1997; CitationMousa et al 2004). Regulation of POMC in lymphocytes has been reported to be dynamic with both mRNA and opioid peptide levels increased in mitogen-stimulated cells, while POMC expression in macrophages was of a more constitutive nature (CitationLyons and Blalock 1997). In addition, the predominant opioid-containing cell type appears to be dynamically regulated depending on the progression of the inflammatory cascade (CitationRittner et al 2001). Increased complexity is additionally suggested by an observation that immune cells from inflamed lymph nodes contain relatively less opioid peptides compared with cells from noninflamed lymphatic tissue, while at the same time opioid peptide content in local inflamed tissue is increased (CitationCabot et al 1997). This observation is consistent with a model of peripheral opioid antinociception whereby immune cells migrate to inflamed tissue in a site directed manner, release these opioid peptides, and then return to lymphatic tissues depleted of peptide () (CitationCabot et al 1997).
Figure 2 Endogenous opioid peptides are released by immune cells to reduce inflammatory pain. Adhesion molecules expressed on immune cells and inflamed endothelium coordinate the migration of circulation immune cells into inflamed tissue. The proinflammatory mediators corticotropin-releasing factor (CRF) and interleukin-1β (IL-1β), as well as the sympathetic neurotransmitter, noradrenaline, stimulate immune cells to secrete their opioid peptides. These peptides activate opioid receptors located on the peripheral ends of sensory neurons and effectively reduce inflammatory pain. Immune cells, devoid of their opioid contents, then continue their passage to neighbouring lymph nodes.
Opioid peptides involved in peripheral antinociception
END and ENK have been proposed to be the most important opioid peptides involved in immune-derived opioid antinociception. Antibodies to both END and ENK could reverse the antihyperalgesic effect observed after release of these peptides from immune cells was stimulated using CRF or IL-1β (CitationStein, Hassan, et al 1990; CitationCabot et al 2001). Although DYN and PDYN levels were generally reported to be low, this opioid peptide is likely to be of some significance in mediating peripheral antinociception as antiDYN has been reported to inhibit IL-1β-induced analgesia (CitationPrzewlocki et al 1992; CitationCabot et al 2001). In addition, expression of both the opioid peptide and its precursor were observed to be increased significantly in immune cells during inflammation, although absolute levels were low (CitationCabot et al 2001). The exact role of DYN in peripheral antinociception remains to be elucidated.
All three opioid peptides have been proposed to be contained within vesicular structures similar to those observed in neurons. The release of these endogenous opioid receptor ligands is Ca2+ dependent and can be evoked by stimulation with high K+ (CitationManfredi et al 1995; CitationCabot et al 1997, Citation2001). Ultrastructural studies have confirmed the presence of END in secretory granules in macrophages, monocytes, granulocytes, and lymphocytes within immune cells from inflamed tissue (CitationMousa et al 2004). These granules were grouped in small vesicular structures within the cytoplasm, while larger vesicles were observed to possess extended processes ready for exocytosis (CitationMousa et al 2004).
Numerous papers have reported that immune cells contain opioid peptides and increasing functional evidence is being accumulated to suggest that these endogenous opioid receptor ligands can be released in local inflamed tissues by leukocytes to alleviate inflammatory hyperalgesia (CitationLolait et al 1986; CitationCabot et al 1997; CitationKamphuis et al 1998; CitationBinder et al 2004; CitationMousa et al 2004). In early inflammation, the degree of antinociception evoked through these locally released opioid peptides relies heavily upon the number of opioid receptors expressed in these tissues rather than the number of opioid peptide-containing immune cells recruited to the inflamed site (CitationBrack, Rittner, et al 2004c). Analgesia, mediated through the release of endogenous opioid peptides, may become increasingly important as the number of opioid receptors expressed increases with the progression of inflammation (CitationZollner et al 2003; CitationBrack, Rittner, et al 2004c).
Inflammation and the migration of circulating leukocytes
Leukocyte migration and adhesion molecules
For opioid-containing immune cells to elicit antinociception by releasing their endogenous opioid peptide load in local inflamed tissue, these cells must first migrate to these tissues.
“Homing” of immune cells to inflamed sites is a complex process and involves numerous inflammatory mediators, chemoattractants, and adhesion molecules. The extra-vasation of leukocytes into inflamed tissue is enabled by the interaction of adhesion molecules expressed on leukocytes and vascular endothelium. These adhesion molecules comprise the carbohydrate binding selectins, immunoglobulins (Ig) such as ICAM and PECAM, and the integrin family of α and β subunit heterodimers (CitationWahl et al 1996). The upregulation of adhesion molecules and the engagement of leukocytes in the extravasation process are promoted by various inflammatory mediators and chemoattractants such as interleukin-1 (IL-1), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), thrombin, histamine, RANTES (Regulated upon Activation Normal T cell Expressed and Secreted), monocyte chemotactic peptide (MCP), and macrophage inflammatory peptides (MIP) (CitationSpringer 1994; CitationWahl et al 1996; CitationVillarreal et al 2001). Passing leukocytes are initially tethered by the action of selectins. P- and E-selectins are expressed on vascular endothelium and L-selectins are constitutively expressed on leucocytes (CitationBrown 1997). Once loosely tethered by selectins, leukocytes roll along the inflamed endothelium, which promotes the upregulation of leukocyte integrins and further adhesion (CitationSpringer 1994; CitationButcher and Picker 1996). Leukocyte integrins bind to the immunoglobulins expressed on endothelium; a firm interaction that results in the arrest of circulating leukocytes (CitationSpringer 1994; CitationButcher and Picker 1996). Leukocyte arrest is followed by diapedesis of the leukocyte, through relaxed endothelial junctions and into the inflamed tissue. These processes enable a targeted and leukocyte subset selective migration at the site of inflammation (CitationSpringer 1994; CitationButcher and Picker 1996).
Presentation of opioid-containing immune cells in inflamed tissue
In the rat paw model, the induction of inflammation with FCA results in a significant increase in opioid-expressing leukocytes in the inflamed tissue (CitationCabot et al 1997; CitationRittner et al 2001). Assessment with fluorescence-activated cell staining indicates that approximately 20% of immune cells that infiltrate inflamed tissue contain endogenous opioid peptides (CitationRittner et al 2001; CitationMachelska et al 2002). At the early stages of inflammation, opioid-expressing immune cells are predominantly from a neutrophil lineage, representing 66% of opioid-containing cells. At later stages, monocytes and macrophages are prominent, representing 73% of opioid-expressing leukocytes (CitationRittner et al 2001; CitationSchmitt et al 2003). Other studies have shown that activated lymphocytes are the prevalent opioid-containing immune cell type in the later stages of inflammation (CitationCabot et al 1997; CitationMousa et al 2001). This discrepancy is likely due to the use of different immunoreactive staining procedures. Opioid-expressing cells roughly reflect general immune cell type representation at different stages of inflammation (CitationRittner et al 2001).
Immune-derived opioids reduce inflammatory pain
Stress-induced analgesia during inflammation in peripheral tissue
Stressful stimuli trigger potent analgesia mediated by endogenous opioid systems in the CNS (CitationYamada and Nabeshima 1995). The response to stress is characterized by stimulation of the hypothalamic–pituitary–adrenocortical axis resulting in the sequential secretion of CRF and adrenocorticotrophic hormone (ACTH); activation of the sympathetic nervous system, which releases noradrenaline from the adrenal medulla and sympathetic nerve endings; and the activation of endogenous opioid systems in the CNS (CitationYamada and Nabeshima 1995). In rats with inflammation of the hind paw however, stress-induced analgesia is mediated predominantly by endogenous opioids that bind to peripheral opioid receptors to effectively reduce inflammatory pain (CitationStein, Gramsch, et al 1990).
A common technique for evoking stress-induced analgesia is cold water swim stress (CWS). In the rat paw model, CWS induces sudden antinociception that manifests as significant increases in pain thresholds in the inflamed paw (CitationStein, Gramsch, et al 1990). This analgesia is most potent immediately following the insult and subsides within 10 to 15 minutes (CitationStein, Gramsch, et al 1990). The antinociceptive effect of CWS is dose-dependently reversible by opioid receptor antagonists, which suggests that antinociception is opioid receptor-mediated. At the early stages of inflammation (6 hours after induction with FCA), CWS-evoked antinociception is completely blocked by systemically acting doses of naloxone and partially blocked by peripherally selective antagonists, which demonstrates a central and peripheral component (CitationMachelska et al 2003). However, as inflammation becomes chronic (described in this study as 4 days post FCA), peripherally selective antagonists completely abolish CWS-evoked anti-nociception, indicating that analgesia is mediated exclusively by opioid receptors on peripheral sensory nerves innervating the inflamed paw (CitationMachelska et al 2003). The use of antibodies against specific endogenous opioid peptides and selective opioid receptor antagonists reveal that at the early stages of inflammation, the peripheral component of CWS-evoked analgesia is mediated by END, ENK, and DYN, which activate MOR, DOR, and KOR (CitationMachelska et al 2003). At later stages of inflammation (as observed at 4 days post FCA), antinociception is delivered wholly by END, activating peripheral MOR and DOR (CitationStein, Gramsch, et al 1990; CitationMachelska et al 2003).
Endogenous opioids are released from leukocytes
CWS-evoked analgesia in the rat paw depends on the local action of CRF and noradrenaline, evidenced by reversal following local injection of CRF receptor antagonists and adrenergic receptor antagonists respectively (CitationSchafer et al 1996; CitationBinder et al 2004). CRF, noradrenaline, and the proinflammatory cytokine, IL-1β, evoke the release of opioid peptides from leukocytes. Immune cell suspensions extracted from rats with inflamed hind paws exhibit receptor-specific and concentration-dependent opioid release when exposed to CRF, noradrenaline, and IL-1β (CitationSchafer et al 1996; CitationCabot et al 1997, Citation2001; CitationBinder et al 2004; CitationMousa et al 2004). CRF induces the release of END, ENK, and DYN (CitationCabot et al 2001); noradrenaline releases END (CitationBinder et al 2004; CitationMousa et al 2004); and IL-1β releases END and DYN (CitationCabot et al 2001). An upregulation of receptors for these mediators on immune cells participating in an inflammatory response indicating that inflammation consolidates opioid release mechanisms (CitationZoukos et al 1994; CitationMousa et al 1996). Separate administration of CRF, noradrenaline, and IL-1β to the inflamed rat paw evokes potent dose-dependent and pharmacologically reversible analgesia (CitationBinder et al 2004; CitationSchafer et al 1994). This antinociceptive effect is the result of local action at the inflamed site as equivalent doses administered systemically are ineffective (CitationSchafer et al 1994; CitationBinder et al 2004). Antibodies against opioid peptides and opioid receptor antagonists abolish the analgesic effects of CRF, noradrenaline, and IL-1β, confirming that the observed analgesia is mediated by endogenous opioids that activate opioid receptors () (CitationSchafer et al 1994; CitationBinder et al 2004). Further evidence supporting immune cells as the source of opioid peptides is provided by findings that the administration of CRF, noradrenaline, and IL-1β into noninflamed paws does not evoke an antinociceptive effect (CitationSchafer et al 1994; CitationBinder et al 2004). The degree of analgesia induced by exposure to CWS increases proportionally with the presentation of opioid-containing immune cells in the inflamed hind paw (CitationRittner et al 2001; CitationMachelska et al 2003).
Impaired immune cell function leads to impaired endogenous analgesia
Numerous studies have reported that impaired immune cell function is associated with diminished opioid analgesia, which is consistent with the model where immune cells release opioid peptides in inflamed tissue to elicit antinociception (CitationStein, Hassan, et al 1990; CitationPrzewlocki et al 1992; CitationSchafer et al 1994; CitationMachelska et al 1998, Citation2003; CitationMousa et al 2000; CitationBrack et al 2004, Citation2004a, Citation2004b; CitationHermanussen et al 2004). This effect has been described for immunomodulatory agents that affect immune cell trafficking as well as numerous treatments that compromise the functional integrity of leukocytes (CitationStein, Hassan, et al 1990; CitationPrzewlocki et al 1992; CitationSchafer et al 1994; CitationMachelska et al 1998, Citation2003; CitationMousa et al 2000; CitationBrack et al 2004, Citation2004a, Citation2004b; CitationHermanussen et al 2004).
Immune cell trafficking
Fucoidin, which binds to selectins and blocks leukocyte “rolling”, has been reported to impair analgesia induced by CWS and CRF (CitationMachelska et al 1998). This impaired antinociception was paralleled by a decrease in END content as well as a decrease in the number of END-containing immune cells in inflamed tissue (CitationMachelska et al 1998). Similarly, intracellular adhesion molecule-1 (ICAM-1) has been found to be upregulated in inflamed tissue and appears to contribute to the recruitment of opioid-containing cells to these local microenvironments as antiICAM-1 decreased CWS- and CRF-induced analgesia (CitationMachelska et al 2002). Other adhesion molecules, including P-selectin, platelet-endothelial adhesion molecule-1 (PECAM-1), and L-selectin, are also upregulated in inflammation, and these increased expression levels correlate with increased END levels (CitationMousa et al 2000). However, while the exact role of these adhesion molecules in endogenous opioid analgesia remains to be elucidated, results indicate that PECAM-1 is not required for endogenous opioid analgesia (CitationMachelska et al 2004). The same study confirmed functional integrity of selectins and integrins as essential for immune-derived antinociception (CitationMachelska et al 2004).
In contrast to adhesion molecule-mediated leukocyte trafficking, chemokine-mediated recruitment of polymorphonuclear cells may target opioid-containing leukocytes more specifically, as immune cells containing opioid peptides have been reported to be mostly chemokine receptor (CXCR2)-positive (CitationBrack, Rittner, et al 2004b). Accordingly, a blockade of the keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) decreased the number of opioid-containing leukocytes in inflamed tissue and abolished CRF-induced antinociception in the inflamed rat paw (CitationBrack, Rittner, et al 2004b).
Effect of immunosuppressants on immune-derived antinociception
Further evidence for the importance of the functional integrity of opioid-containing immune cells in endogenous analgesia stems from the observation that the immunosuppressant Cyclosporine A (CsA) can dose-dependently block CWS-, IL-1- and CRF-induced peripheral analgesia (CitationStein, Hassan, et al 1990; CitationSchafer et al 1994; CitationHermanussen et al 2004). The reduced paw pressure threshold that has been observed in inflamed paws of CsA-treated animals was mirrored by a decrease in the number of infiltrating lymphocytes and could be reversed by administration of activated lymphocytes (CitationHermanussen et al 2004). Nonspecific immunosuppression induced by whole body irradiation also decreased stress-induced antinociception in inflamed paws, while more specific depletion of monocytes/ macrophages by liposomal clodronate similarly decreased stress-induced endogenous opioid antinociception without affecting exogenous opioid analgesia (CitationPrzewlocki et al 1992; CitationBrack et al 2004).
Clinical evidence for association between impaired immune function and increased pain
Reduced END content in immune cells has clinically been reported in numerous painful conditions, including arthritis, Crohn's disease, fibromyalgia, migraine, and tension headache (CitationWiedermann et al 1992, Citation1994; CitationPanerai et al 2002). There is little evidence to suggest that opioid content in circulating immune cells significantly contributes to analgesia. A reduction in opioid content of these cells may compromise the local delivery of opioid peptides to inflamed tissues by migrating immune cells and thus impair endogenous analgesic mechanisms in these patients. In line with this observation, pain associated with disease or inflammation may be increased in immunocompromised patients (CitationMerine et al 1987). A positive correlation between immunosuppression and increased pain has been reported, and in AIDS patients in particular, a decreased CD4+ count appears to be a risk factor for developing painful pathologies such as polyneuropathy (CitationLebovits et al 1994; CitationWyatt and Fishman 1994; CitationLefkowitz 1996; CitationHewitt et al 1997; CitationSchifitto et al 2002). These observations highlight the importance of the immune system and release of endogenous opioid peptides for antinociception.
Immunosuppressive effects of exogenous opioids
Exogenous opioids such as morphine remain the most effective treatment for acute and chronic pain. There is evidence suggesting that opioids have an immunosuppressive action as well as clinically apparent side effects, such as respiratory depression, constipation, tolerance, and dependence (CitationRisdahl et al 1998). The implications of opioid-evoked immunosuppression are particularly relevant during the postoperative period when pain and susceptibility to infection are high; for sufferers of chronic pain and drug users who administer opioids for extended periods; and for patients with immunosuppressive disease such as AIDS, transplant patients, and the elderly, who are predisposed to opportunistic infections.
Evidence for opioid-evoked immunosuppression
Much of the data that suggests opioids have immunosuppressive effects come from epidemiological studies with drug users. These individuals are reported to have markedly increased incidence of skin and soft tissue infections, pulmonary infections, endocarditis, skeletal infections, malaria, and viral hepatitis, although it is accepted that interpretation of these findings is confounded by compromised lifestyle practices associated with this population (CitationHussey and Katz 1950; CitationScheidegger and Zimmerli 1989; CitationRisdahl et al 1998). In healthy nonaddicted individuals, morphine treatment for obstetric procedures resulted in the reactivation of herpes simplex virus (CitationCrone et al 1988), supporting an immunosuppressive action. Animal models of opioid administration and infection show increased mortality rates in opioid treated animals with experimentally-induced sepsis and Toxoplasma gondii infection, reversible by opioid receptor antagonists (CitationChao et al 1990; CitationRoy et al 1999). Morphine-dependent swine, challenged with herpes virus, exhibited enhanced pneumonia secondary to herpes virus infection, although these animals also exhibited a paradoxical decrease in clinical signs associated with neurological disease (CitationRisdahl et al 1993). In mice inoculated with Streptococcus pneumonia, chronic morphine treatment delayed neutrophil recruitment, increased bacterial burden, and increased mortality (CitationWang et al 2005). Various commonly measured immune parameters are significantly suppressed following opioid exposure in drug users, healthy human subjects, and animal models. These include suppressed NK cell activity (CitationCarr et al 1993; CitationYeager et al 1995; CitationSacerdote et al 1997), macrophage phagocytic function (CitationRojavin et al 1993), cytokine production (CitationBhargava et al 1994; CitationSacerdote et al 1997), lymphocyte proliferation (CitationBhargava et al 1994; CitationSacerdote et al 1997), and spleen and thymus weight and cellularities (CitationFuchs and Pruett 1993; CitationBhargava et al 1994). In experiments with opioid receptor knock out mice, mutant mice did not exhibit the abnormal immune parameters that were observed in wild type mice following chronic morphine treatment, indicating that opioid receptors mediate morphine-induced immunosuppression (CitationGaveriaux-Ruff et al 1998). These findings collectively indicate that exposure to exogenous opioids impairs resistance to infection and suppresses immune function.
Centrally mediated opioid-evoked immunosuppression
There is evidence that opioid-evoked immunosuppression is mediated by opioid receptors in the CNS. Systemic administration of morphine significantly reduced lymphocyte proliferation ex vivo but the peripherally restricted N-methylmorphine did not, suggesting that a central mechanism mediates immunosuppression (CitationHernandez et al 1993). Furthermore, the central administration of both morphine and N-methylmorphine effectively inhibited lymphocyte proliferation. Several studies have identified the periaqueductal gray (PAG) matter of the mesencephalon as a site of morphine-induced opioid receptor-mediated immunosuppression. Injection of morphine into the PAG is reported to suppress ex vivo macrophage nitric oxide (NO) production, NK cell activity, lymphocyte proliferation, and leukocyte IL-2 production (CitationGomez-Flores et al 1999; CitationGomez-Flores and Weber 1999). In rats with FCA-induced inflammation of the hind paw, concomitant intrathecal morphine administration selectively attenuated the presentation of END-positive leukocytes in the inflamed paw without affecting total immune cell numbers (CitationSchmitt et al 2003). This result led to the hypothesis that arresting pain transmission in the CNS signals a reduced need for immune-derived opioid peptides in the inflamed tissue (CitationSchmitt et al 2003), and provides further evidence for centrally mediated opioid immuno-modulation.
Opioids activate receptors on immune cells and suppress immune activity
In addition to centrally-mediated immunosuppression, the expression of opioid receptors on immune cells suggest that opioids may modulate immune function by direct action on these cells (CitationBidlack 2000). This is supported by findings that in vitro exposure to morphine resulted in concentration-dependent and antagonist-reversible inhibition of macrophage phagocytic function (CitationRojavin et al 1993. Opioids also directly suppress macrophage production of proinflammatory cytokines IL-1, IL-6, TNF-α (CitationAlicea et al 1996), and impair monocyte chemotaxis in response to cytokines MIP-1α, MCP-1, and RANTES, through opioid receptor-mediated heterologous desensitization of chemokine receptors (CitationGrimm et al 1998). Morphine directly upregulates immune cell Fas receptors that trigger apoptosis of the cell when activated by the ligand FasL (CitationYin et al 1999). In this way, morphine administered in vivo apparently primes leukocytes for elimination by apoptosis and suppresses the immune response (CitationYin et al 1999).
Opioid stimulation of immune cell function
In contrast to findings that opioids exert an immunosuppressive action, there is evidence that some opioids, particularly endogenous opioid peptides, stimulate aspects of immune function. In vitro experiments demonstrate that endogenous opioids such as END and ENK enhance human NK cell activity (CitationMathews et al 1983); increase IL-2 production in the lymphoid cell line EL-4 (CitationBessler et al 1990); increase leukocyte surface expression of compliment and immunoglobulin receptors (CitationMenzebach et al 2003); increase leukocyte oxidative burst activity (CitationMenzebach et al 2003); and augment macrophage tumoricidal activity (CitationHagi et al 1994). In vivo administration of ENK reportedly improves the survival rate of mice infected with herpes simplex virus and inhibits tumor growth (CitationFaith et al 1987). The immunostimulatory properties of endogenous opioids suggest that their release by immune cells in inflamed tissue not only controls pain but also stimulates immune function.
Clinical implications for pain control
Systemic administration of opioids is clinically associated with numerous side effects. While immunomodulatory effects of opioids can have clinical implications as described above, most dose-limiting adverse events, such as respiratory depression, sedation, and tolerance are mediated through activation of central opioid receptors (CitationKieffer and Gaveriaux-Ruff 2002). A logical consequence is the attempt to target peripheral opioid receptors more specifically. Numerous studies have demonstrated that peripheral opioids can provide effective analgesia (CitationStein et al 1989; CitationLikar et al 2001; CitationTruong et al 2003; CitationFurst et al 2005; CitationPacheco and Duarte 2005). The immunosuppressive effect of opioid receptor agonists may contribute to peripheral opioid analgesia as it has been speculated that a local anti-inflammatory effect contributed to prolonged analgesia produced by intra-articular morphine (CitationStein et al 1999).
Strategies to target peripheral opioid receptors specifically include the development and use of peripherally active opioid receptor agonists, such as loperamide, asimadoline, and frakefamide, which can usually penetrate the blood brain barrier poorly, or local administration of centrally active opioids as well as treatments aimed at reducing degradation of locally released peptides (CitationBarber et al 1994; CitationDeHaven-Hudkins et al 1999; CitationChen et al 2001; CitationZajaczkowska et al 2004; CitationModalen et al 2005). Peripherally selective opioids have met with some clinical success, although most work has been carried out with peripherally selective KOR agonists such as fedotozine (CitationDelvaux 2001). Despite promising pilot studies, both centrally mediated adverse events as well as peripheral side effects arising from systemic administration of opioid agonists, including constipation, could not be completely excluded (CitationDelvaux 2001; CitationEisenach et al 2003; CitationRiviere 2004). Thus, research aimed at the development of opioid receptor agonists that do not cross the blood brain barrier and are peripherally selective, but can be administered orally to achieve peripheral opioid analgesia remains ongoing. Targeted delivery of opioids locally to the site of pain could avoid the problems of peripherally selective opioid receptor agonists which retain the potential to cause side effects mediated through opioid receptor activation in the periphery. Side effects include nausea and vomiting arising from stimulation of opioid receptors in the chemo-receptor trigger zone, and constipation from opioid modulation of GI function. Accordingly, while local administration of centrally active opioid receptor agonists can be effective, limiting factors are problems arising from the required routes of administration, such as intra-articular injection (CitationKizilkaya et al 2004).
Historically, immunosuppression has been used as an analgesic strategy in numerous inflammatory conditions such as rheumatoid arthritis (CitationLipsky 1983). In light of recent evidence suggesting that endogenous opioid peptides are released locally from immune cells to elicit antihyperalgesia (CitationStein, Hassan, et al 1990), current clinical practices are likely to be challenged as our understanding of the complex interaction between the immune system and pain control increases. The role of the immune system in analgesia is likely to be delicately balanced depending on whether infiltrating cells are proinflammatory and proalgesic or their major role is an antiinflammatory and analgesic one. In situations where endogenous antinociception exceeds hyperalgesia mediated by immune cells, immuno-suppression of modulation of immune cell trafficking might increase pain. This hypothesis is supported by the observation that intra-articular naloxone lead to an increase in postoperative pain and analgesic consumption (CitationStein et al 1993).
Approaches could target immune cell trafficking to increase immune-mediated endogenous opioid anti-nociception and thus achieve analgesia, which lacks the side effects usually associated with systemic administration of opioid receptor agonists. The immigration of opioid-containing immune cells could be specifically encouraged by utilising chemokines or adhesion molecules. An increase in antinociceptive and decrease in proalgesic cell migration would complement current immunosuppressive treatment strategies. Endogenous opioid analgesia could be further enhanced by increasing infiltrating analgesic cell numbers or by increasing opioid peptide production in these cells. While stimulation of immune cell production using granulocyte colony-stimulating factor (G-CSF) and stem cell factor (SCF) has not met with the expected increase in endogenous analgesia to date, gene therapy targeting endogenous opioid peptide production in peripheral neurons has had some success and may be applied to opioid-peptide containing immune cells in future (CitationBraz et al 2001; CitationBrack, Rittner, et al 2004a; CitationDuplan et al 2004; CitationChuang et al 2005).
A further approach to enhance peripheral opioid analgesia includes inhibition of degradation of endogenous opioid peptides. Using this approach, enhanced anti-nociception was achieved in a rodent model of thermal pain by administration of enkephalin-degrading enzyme inhibitor prodrugs (CitationChen et al 2001). In addition to enhanced antinociception, an additional benefit of this approach to achieve analgesia is the relative lack of immunosuppressive effects of endogenous opioid peptides, which would render these compounds superior to exogenous opioids in certain patient groups. Accordingly, modifications that would enable dosing with these endogenous peptides by eliminating instability and poor bioavailability of the compounds could be the beginning of a new class of analgesic opioid agonists (CitationDhanasekaran et al 2005)
The clinical implications of the complex interactions between the immune system and pain control are especially pertinent to immunocompromised patients. An impairment in immune cell function may lead to decreased endogenous antinociception and thus increased pain (CitationMerine et al 1987). In AIDS patients in particular, undertreatment of pain has been reported to be common (CitationLebovits et al 1994), which gives greater significance to our understanding of how endogenous opioid antinociception might be altered in immunocompromised patient groups. Future studies directed at investigating changes in immune cell trafficking and endogenous opioid analgesia in these patients may provide clinical strategies to more effectively treat pain.
References
- AbbadieCPanYPasternakGDifferential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processingJ Comp Neurol20004192445610723002
- AkilHMengFMansourACloning and characterization of multiple opioid receptorsNIDA Res Monogr1996161127408784848
- AkilHYoungEWatsonSJOpiate binding properties of naturally occurring N- and C-terminus modified beta-endorphinsPeptides19812289926272244
- AliceaCBelkowskiSEisensteinTKInhibition of primary murine macrophage cytokine production in vitro following treatment with the kappa-opioid agonist U50,488HJ Neuroimmunol19966483908598393
- AnderssonSELexmullerKJohanssonATissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the ratJ Rheumatol19992620182410493685
- AntonijevicIMousaSASchaferMPerineurial defect and peripheral opioid analgesia in inflammationJ Neurosci199515165727823127
- BagnolDMansourAAkilHCellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tractNeuroscience199781579919300443
- BalletSConrathMFischerJExpression and G-protein coupling of mu-opioid receptors in the spinal cord and dorsal root ganglia of polyarthritic ratsNeuropeptides2003372111912906839
- BarberABartoszykGDBenderHMA pharmacological profile of the novel, peripherally-selective kappa-opioid receptor agonist, EMD 6175 3Br J Pharmacol19941131317277889287
- BesslerHSzteinMBSerrateSABeta-endorphin modulation of IL-1-induced IL-2 productionImmunopharmacology19901915141689707
- BhargavaHNThomasPTThoratSEffects of morphine tolerance and abstinence on cellular immune functionBrain Res19946421–21108032870
- BianchiMMaggiRPimpinelliFPresence of a reduced opioid response in interleukin-6 knock out miceEur J Neurosci1999111501710215902
- BidlackJMDetection and function of opioid receptors on cells from the immune systemClin Diagn Lab Immunol200077192310973443
- Bigliardi-QiMSumanovskiLTBuchnerSMu-opiate receptor and beta-endorphin expression in nerve endings and keratinocytes in human skinDermatology2004209183915459530
- BinderWMachelskaHMousaSAnalgesic and antiinflammatory effects of two novel kappa–opioid peptidesAnesthesiology20019410344411465595
- BinderWMousaSASitteNSympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissueEur J Neurosci2004209210015245482
- BlalockJEProopiomelanocortin and the immune–neuroendocrine connectionAnn N Y Acad Sci19998851617210816649
- BornerCHolltVKrausJInvolvement of activator protein-1 in transcriptional regulation of the human mu-opioid receptor geneMol Pharmacol200261800511901219
- BornerCKrausJSchroderHTranscriptional regulation of the human mu-opioid receptor gene by interleukin-6Mol Pharmacol20046617192615448191
- BrackALabuzDSchiltzATissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociceptionAnesthesiology20041012041115220792
- BrackARittnerHLMachelskaHControl of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cellsPain2004a11232293815561377
- BrackARittnerHLMachelskaHEndogenous peripheral antinociception in early inflammation is not limited by the number of opioid-containing leukocytes but by opioid receptor expressionPain2004b1081–2677515109509
- BrackARittnerHLMachelskaHMobilization of opioid-containing polymorphonuclear cells by hematopoietic growth factors and influence on inflammatory painAnesthesiology2004c1001495714695736
- BrazJBeaufourCCoutauxATherapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neuronsJ Neurosci2001217881811588161
- BrownENeutrophil adhesion and the therapy of inflammationSemin Hematol199734319269347582
- ButcherECPickerLJLymphocyte homing and homeostasisScience19962726068600538
- BuzzettiRMcLoughlinLLavenderPMExpression of proopiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignanciesJ Clin Invest19898373372536407
- CabotPJImmune-derived opioids and peripheral antinociceptionClin Exp Pharmacol Physiol200128230211236131
- CabotPJCarterLGaiddonCImmune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in ratsJ Clin Invest199710014289202066
- CabotPJCarterLSchaferMMethionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory painPain2001932071211514079
- CadetPBilfingerTVFimianiCHuman vascular and cardiac endothelia express mu opiate receptor transcriptsEndothelium200071859110912912
- CahillCMDrayACoderreTJIntrathecal nerve growth factor restores opioid effectiveness in an animal model of neuropathic painNeuropharmacology2003455435212907315
- CahillCMMorinvilleAHoffertCUp-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain controlPain200310119920812507715
- CarrDJDe CostaBRKimCHOpioid receptors on cells of the immune system:evidence for delta- and kappa-classesJ Endocrinol198912216182549147
- CarrDJGebhardtBMPaulDAlpha adrenergic and mu-2 opioid receptors are involved in morphine-induced suppression of splenocyte natural killer activityJ Pharmacol Exp Ther19932641179868383741
- CerchiettiLCNaviganteAHBonomiMREffect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinomaCancer2002952230612412178
- ChaoCCSharpBMPomeroyCLethality of morphine in mice infected with Toxoplasma gondiiJ Pharmacol Exp Ther199025260592313590
- ChenHNobleFRoquesBPLong lasting antinociceptive properties of enkephalin degrading enzyme (NEP and APN) inhibitor prodrugsJ Med Chem20014435233011585456
- ChuangYCYangLCChiangPHGene gun particle encoding preproenkephalin cDNA produces analgesia against capsaicin-induced bladder pain in ratsUrology2005658041015833551
- ConnorMChristieMDOpioid receptor signalling mechanismsClin Exp Pharmacol Physiol199926493910405772
- CroneLAConlyJMClarkKMRecurrent herpes simplex virus labialis and the use of epidural morphine in obstetric patientsAnesth Analg198867318233354864
- DeHaven-HudkinsDLBurgosLCCasselJALoperamide (ADL 2–1294), an opioid antihyperalgesic agent with peripheral selectivityJ Pharmacol Exp Ther1999289149450210087042
- DelvauxMPharmacology and clinical experience with fedotozineExpert Opin Investig Drugs200110197110
- DequekerJNSAIDs/corticosteroids–primum non nocereAdv Exp Med Biol19994553192510599363
- DhanasekaranMPalianMMAlvesIGlycopeptides related to beta-endorphin adopt helical amphipathic conformations in the presence of lipid bilayersJ Am Chem Soc200512754354815826181
- DionneRALepinskiAMGordonSMAnalgesic effects of peripherally administered opioids in clinical models of acute and chronic inflammationClin Pharmacol Ther2001701667311452246
- DuplanHLiRYVueCGrafts of immortalized chromaffin cells bio-engineered to improve met-enkephalin release also reduce formalin-evoked c-fos expression in rat spinal cordNeurosci Lett200437011615489007
- EastwoodCGrundyDOpioid-receptor-mediated excitation of rat mesenteric afferent fibres supplying the rat jejunumNeurogastroenterol Motil2000125172211123706
- EdlowDWSheldonWHThe pH of inflammatory exudatesProc Soc Exp Biol Med19711371328325138458
- EisenachJCCarpenterRCurryRAnalgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitisPain20031011–2899512507703
- FaithREMurgoAJClinkscalesCWEnhancement of host resistance to viral and tumor challenge by treatment with methionineenkephalinAnn N Y Acad Sci1987496137453474965
- FickelJBagnolDWatsonSJOpioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brainBrain Res Mol Brain Res1997461–2189191072
- FinegoldAAMannesAJIadarolaMJA paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic painHum Gene Ther1999101251710340556
- FuchsBAPruettSBMorphine induces apoptosis in murine thymocytes in vivo but not in vitro: involvement of both opiate and glucocorticoid receptorsJ Pharmacol Exp Ther1993266417238392561
- FurstSRibaPFriedmannTPeripheral versus central antinociceptive actions of 6-amino acid-substituted derivatives of 14-O-methyloxymorphone in acute and inflammatory pain in the ratJ Pharmacol Exp Ther20053126091815383636
- Gaveriaux-RuffCMatthesHWPelusoJAbolition of morphine-immunosuppression in mice lacking the mu-opioid receptor geneProc Natl Acad Sci U S A1998956326309600964
- Gomez-FloresRSuoJLWeberRJSuppression of splenic macrophage functions following acute morphine action in the rat mesencephalon periaqueductal grayBrain Behav Immun1999132122410469523
- Gomez-FloresRWeberRJInhibition of interleukin-2 production and downregulation of IL-2 and transferrin receptors on rat splenic lymphocytes following PAG morphine administration: a role in natural killer and T cell suppressionJ Interferon Cytokine Res1999196253010433363
- GrayACWhite PJ CouparIMCharacterisation of opioid receptors involved in modulating circular and longitudinal muscle contraction in the rat ileumBr J Pharmacol20051446879415678085
- GrimmMCBen-BaruchATaubDDOpiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitizationJ Exp Med1998188317259670044
- GutkowskaJMukaddam-DaherSJankowskiMThe cardiovascular and renal effects of the potent and highly selective mu opioid agonist [Dmt1]DALDAJ Cardiovasc Pharmacol200444651815550783
- HagiKUnoKInabaKAugmenting effect of opioid peptides on murine macrophage activationJ Neuroimmunol19945017167905488
- HassanAHAbleitnerASteinCInflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissueNeuroscience199355185957688879
- HassanAHPzewlockiRHerzADynorphin, a preferential ligand for kappa-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the ratNeurosci Lett199214018581357608
- HermanussenSDoMCabotPJReduction of beta-endorphin-containing immune cells in inflamed paw tissue corresponds with a reduction in immune-derived antinociception: reversible by donor activated lymphocytesAnesth Analg200498723914980927
- HernandezMCFloresLRBayerBMImmunosuppression by morphine is mediated by central pathwaysJ Pharmacol Exp Ther19932671336418263796
- HewittDJMcDonaldMPortenoyRKPain syndromes and etiologies in ambulatory AIDS patientsPain1997702–3117239150284
- HirschowitzBINonsteroidal antiinflammatory drugs and the gastrointestinal tractGastroenterologist19942207237987619
- HolzerPOpioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humansNeurosci Lett2004361192515135926
- HusseyHHKatzSInfections resulting from narcotic addiction; report of 102 casesAm J Med195091869315432465
- IbrahimMMPorrecaFLaiJCB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioidsProc Natl Acad Sci U S A20051023093815705714
- JacobusWETaylorGJHollisDPPhosphorus nuclear magnetic resonance of perfused working rat heartsNature1977265756816217
- JensenMKThomsenABHojstedJ10-year follow-up of chronic non-malignant pain patients: Opioid use, health related quality of life and health care utilizationEur J Pain2005epub
- JiRRZhangQLawPYExpression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammationJ Neurosci1995158156668613750
- KakidaniHFurutaniYTakahashiHCloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursorNature198229824596123953
- KalsoESmithLMcQuayHJNo pain, no gain: clinical excellence and scientific rigour – lessons learned from IA morphinePain2002982697512127028
- KamphuisSErikssonFKavelaarsARole of endogenous pro-enkephalin A-derived peptides in human T cell proliferation and monocyte IL-6 productionJ Neuroimmunol19988453609600708
- KapitzkeDHermanussenSJenkinsNAcidic pH enhances morphine inhibition of high-K+ induced calcium responses in dorsal root ganglion neurons [abstract]Proceedings of the 11th World Congress on Pain2005
- KhodorovaANavarroBJouavilleLSEndothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injuryNat Med2003910556112847519
- KiefferBLGaveriaux-RuffCExploring the opioid system by gene knockoutProg Neurobiol20026628530612015197
- KizilkayaMYildirimOSDoganNAnalgesic effects of intraarticular sufentanil and sufentanil plus methylprednisolone after arthroscopic knee surgeryAnesth Analg2004981062515041599
- KnappRJMalatynskaECollinsNMolecular biology and pharmacology of cloned opioid receptorsFaseb J19959516257737460
- KrausJBornerCGianniniERegulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding siteJ Biol Chem200127643901811572871
- KrausJBornerCGianniniEThe role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu–opioid receptor geneMol Pharmacol2003648768414500744
- LawPYWongYHLohHHMolecular mechanisms and regulation of opioid receptor signalingAnnu Rev Pharmacol Toxicol20004038943010836142
- LebovitsAHSmithGMaignanMPain in hospitalized patients with AIDS: analgesic and psychotropic medicationsClin J Pain199410156618075469
- LefkowitzMPain management for the AIDS patientJ Fla Med Assoc19968370149019060
- LikarRKoppertWBlatnigHEfficacy of peripheral morphine analgesia in inflamed, non-inflamed and perineural tissue of dental surgery patientsJ Pain Symptom Manage200121330711312048
- LikarRMousaSAPhilippitschGIncreased numbers of opioid-expressing inflammatory cells do not affect intra-articular morphine analgesiaBr J Anaesth2004933758015247115
- LinCRYangLCLeeTHElectroporation-mediated pain-killer gene therapy for mononeuropathic ratsGene Ther2002912475312215892
- LinnerKMQuistHESharpBMExpression and function of proenkephalin A messenger ribonucleic acid in murine fetal thymocytesEndocrinology1996137857638603595
- LipskyPERemission-inducing therapy in rheumatoid arthritisAm J Med1983754B4096356907
- LolaitSJClementsJAMarkwickAJPro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophagesJ Clin Invest198677177692423557
- LyonsPDBlalockJEPro-opiomelanocortin gene expression and protein processing in rat mononuclear leukocytesJ Neuroimmunol1997781–247569307227
- MachelskaHBrackAMousaSASelectins and integrins but not platelet-endothelial cell adhesion molecule-1 regulate opioid inhibition of inflammatory painBr J Pharmacol20041427728015159283
- MachelskaHCabotPJMousaSAPain control in inflammation governed by selectinsNat Med19984142589846582
- MachelskaHMousaSABrackAOpioid control of inflammatory pain regulated by intercellular adhesion molecule-1J Neurosci20022255889612097510
- MachelskaHSchopohlJKMousaSADifferent mechanisms of intrinsic pain inhibition in early and late inflammationJ Neuroimmunol20031411–230912965251
- MaekawaKMinamiMMasudaTExpression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic ratsPain199664365718740615
- ManfrediBClementiESacerdotePAge-related changes in mitogen-induced beta-endorphin release from human peripheral blood mononuclear cellsPeptides1995166997067479305
- MansourAFoxCAAkilHOpioid-receptor mRNA expression in the rat CNS: anatomical and functional implicationsTrends Neurosci1995182297535487
- MansourAKhachaturianHLewisMEAnatomy of CNS opioid receptorsTrends Neurosci198811308142465635
- MaoLWangJQCardiovascular responses to microinjection of nociceptin and endomorphin-1 into the nucleus tractus solitarii in conscious ratsNeuroscience200513210091515857705
- MarinangeliFCiccozziALeonardisMUse of strong opioids in advanced cancer pain:a randomized trialJ Pain Symptom Manage2004274091615120769
- Martin-SchildSZadinaJEGerallAALocalization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cordPeptides199718164199437728
- MathewsPMFroelichCJSibbittWLJrEnhancement of natural cytotoxicity by beta-endorphinJ Immunol19831301658626300232
- McMahonSBNGF as a mediator of inflammatory painPhilos Trans R Soc Lond B Biol Sci1996351431408730782
- MenzebachAHirschJHempelmannGEffects of endogenous and synthetic opioid peptides on neutrophil function in vitroBr J Anaesth2003915465014504158
- MerineDSFishmanEKJonesBRight lower quadrant pain in the immunocompromised patient: CT findings in 10 casesAJR Am J Roentgenol1987149117793500605
- MeunierALatremoliereAMauborgneAAttenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neuronsMol Ther2005116081615771963
- ModalenAOQuidingHFreyJA novel molecule (frakefamide) with peripheral opioid properties:the effects on resting ventilation compared with morphine and placeboAnesth Analg20051007131715728057
- MousaSAMachelskaHSchaferMCo-expression of beta-endorphin with adhesion molecules in a model of inflammatory painJ Neuroimmunol20001081–21607010900350
- MousaSAMachelskaHSchaferMImmunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory painJ Neuroimmunol20021261–251512020952
- MousaSASchaferMMitchellWMLocal upregulation of corticotropin-releasing hormone and interleukin-1 receptors in rats with painful hindlimb inflammationEur J Pharmacol1996311221318891603
- MousaSAShakibaeiMSitteNSubcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory painEndocrinology200414513314114630714
- MousaSAZhangQSitteNbeta-Endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissueJ Neuroimmunol20011151–271811282156
- NakanishiSInoueAKitaTNucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursorNature19792784237221818
- NodaMFurutaniYTakahashiHCloning and sequence analysis of cDNA for bovine adrenal preproenkephalinNature198229520266276759
- Nozaki-TaguchiNYakshTLCharacterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist – loperamideAnesthesiology199990225349915332
- OatesELAllawayGPArmstrongGRHuman lymphocytes produce pro-opiomelanocortin gene-related transcripts. Effects of lymphotropic virusesJ Biol Chem19882631004142839471
- ObaraIPrzewlockiRPrzewlockaBLocal peripheral effects of mu-opioid receptor agonists in neuropathic pain in ratsNeurosci Lett20043601–285915082185
- PachecoDFDuarteIDDelta-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channelsEur J Pharmacol2005512123815814086
- PanYLiDChenSActivation of mu-opioid receptors excites a population of locus coeruleus-spinal neurons through presynaptic disinhibitionBrain Res20049971677814715151
- PaneraiAEVecchietJPanzeriPPeripheral blood mononuclear cell beta-endorphin concentration is decreased in chronic fatigue syndrome and fibromyalgia but not in depression: preliminary reportClin J Pain200218270312131069
- PertCBSnyderSHOpiate receptor: demonstration in nervous tissueScience19731791011144687585
- PertovaaraAWeiHPeripheral effects of morphine in neuropathic rats: role of sympathetic postganglionic nerve fibersEur J Pharmacol20014291394511698036
- PhilippeDDubuquoyLGrouxHAnti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammationJ Clin Invest200311113293812727924
- PilJTytgatJSerine 329 of the mu-opioid receptor interacts differently with agonistsJ Pharmacol Exp Ther20033049243012604666
- PolOAlamedaFPuigMMInflammation enhances mu-opioid receptor transcription and expression in mice intestineMol Pharmacol200160894911641416
- PolOPalacioJRPuigMMThe expression of delta- and kappa-opioid receptor is enhanced during intestinal inflammation in miceJ Pharmacol Exp Ther20033064556212724348
- PrzewlockiRHassanAHLasonWGene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociceptionNeuroscience1992484915001603330
- PrzewlockiRPrzewlockaBOpioids in chronic painEur J Pharmacol20014291–3799111698029
- PuehlerWZollnerCBrackARapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conductionNeuroscience2004129473915501604
- RibeiroMDJoelSPZeppetellaGThe bioavailability of morphine applied topically to cutaneous ulcersJ Pain Symptom Manage200427434915120772
- RisdahlJMKhannaKVPetersonPKOpiates and infectionJ Neuroimmunol1998831–24189610668
- RisdahlJMPetersonPKChaoCCEffects of morphine dependence on the pathogenesis of swine herpesvirus infectionJ Infect Dis1993167128178388899
- RittnerHLBrackAMachelskaHOpioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory painAnesthesiology200195500811506126
- RittnerHLSteinCInvolvement of cytokines, chemokines and adhesion molecules in opioid analgesiaEur J Pain200591091215737796
- RivierePJPeripheral kappa-opioid agonists for visceral painBr J Pharmacol20041411331415051626
- RojavinMSzaboIBussiereJLMorphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophagesLife Sci19935399710068361330
- RosenHBeharOAbramskyORegulated expression of proenkephalin A in normal lymphocytesJ Immunol1989143370372584715
- RoySCharboneauRGBarkeRAMorphine synergizes with lipopolysaccharide in a chronic endotoxemia modelJ Neuroimmunol1999951–21071410229120
- SacerdotePManfrediBMantegazzaPAntinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity studyBr J Pharmacol1997121834409208156
- Sandner-KieslingAPanHLChenSREffect of kappa opioid agonists on visceral nociception induced by uterine cervical distension in ratsPain2002961–2132211932057
- SchaferMCarterLSteinCInterleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissueProc Natl Acad Sci U S A1994914219237910403
- SchaferMMousaSAZhangQExpression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesiaProc Natl Acad Sci U S A19969360961008650225
- ScheideggerCZimmerliWInfectious complications in drug addicts: seven-year review of 269 hospitalized narcotics abusers in SwitzerlandRev Infect Dis198911486932749105
- SchifittoGMcDermottMPMcArthurJCIncidence of and risk factors for HIV-associated distal sensory polyneuropathyNeurology2002581764812084874
- SchmittTKMousaSABrackAModulation of peripheral endogenous opioid analgesia by central afferent blockadeAnesthesiology20039819520212502997
- SchulzSSchreffMSchmidtHImmunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root gangliaEur J Neurosci199810370089875349
- SelleyDEBreivogelCSChildersSRModification of G protein-coupled functions by low-pH pretreatment of membranes from NG108-15 cells:increase in opioid agonist efficacy by decreased inactivation of G proteinsMol Pharmacol199344731418232223
- SenguptaJNSniderASuXEffects of kappa opioids in the inflamed rat colonPain1999791758510068163
- SessleBJHenryJLEffects of enkephalin and 5-hydroxytryptamine on solitary tract neurones involved in respiration and respiratory reflexesBrain Res1985327221303986500
- ShaquraMAZollnerCMousaSACharacterization of mu opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory painJ Pharmacol Exp Ther2004308712814593084
- ShenHAeschlimannAReischNKappa and delta opioid receptors are expressed but down-regulated in fibroblast-like synoviocytes of patients with rheumatoid arthritis and osteoarthritisArthritis Rheum20055214021015880345
- ShimizuNKishiokaSMaedaTInvolvement of peripheral mechanism in the verapamil-induced potentiation of morphine analgesia in miceJ Pharmacol Sci200495452715297768
- SilbertSCBeachamDWMcCleskeyEWQuantitative single-cell differences in mu-opioid receptor mRNA distinguish myelinated and unmyelinated nociceptorsJ Neurosci2003231344212514199
- SimmenHPBattagliaHGiovanoliPAnalysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgeryInfection19942238697698834
- SimonEJHillerJMEdelmanIStereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenateProc Natl Acad Sci U S A197370194794516196
- SmithEMMorrillACMeyerWJ 3rdCorticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphinsNature198632188123014342
- SpringerTATraffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigmCell199476301147507411
- SteinAYassouridisASzopkoCIntraarticular morphine versus dexamethasone in chronic arthritisPain1999835253210568861
- SteinCGramschCHerzAIntrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphinJ Neurosci199010129282158530
- SteinCHassanAHLehrbergerKLocal analgesic effect of endogenous opioid peptidesLancet199334232148101583
- SteinCHassanAHPrzewlockiROpioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammationProc Natl Acad Sci U S A199087593591974052
- SteinCMillanMJShippenbergTSPeripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptorsJ Pharmacol Exp Ther19892481269752539460
- SteinCSchaferMCabotPJPeripheral opioid analgesiaPain Reviews1997417387
- SunWMReadNWVerlindenMEffects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinenceScand J Gastroenterol1997323489018764
- SzaboIChenXHXinLHeterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of painProc Natl Acad Sci U S A200299102768112130663
- TegederIMeierSBurianMPeripheral opioid analgesia in experimental human pain modelsBrain2003126109210212690049
- TereniusLStereospecific interaction between narcotic analgesics and a synaptic plasm a membrane fraction of rat cerebral cortexActa Pharmacol Toxicol (Copenh)197332317204801733
- TraynorJRNahorskiSRModulation by mu-opioid agonists of guanosine-5'-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cellsMol Pharmacol199547848547723747
- TruongWChengCXuQGMu opioid receptors and analgesia at the site of a peripheral nerve injuryAnn Neurol2003533667512601704
- TwillmanRKLongTDCathersTATreatment of painful skin ulcers with topical opioidsJ Pain Symptom Manage1999172889210203881
- van WoudenbergADHolEMWiegantVMEndorphin-like immunoreactivities in uncultured and cultured human peripheral blood mononuclear cellsLife Sci199250705141371179
- VaughanCWIngramSLConnorMAHow opioids inhibit GABA-mediated neurotransmissionNature199739061149403690
- VillarrealGZagorskiJWahlSMInflammation: Acute [online]2001 In Encyclopedia of life sciences. Nature Publishing Group. Accessed on 8 November 2005. URL: http://els.net
- WahlSMFeldmanGMMcCarthyJBRegulation of leukocyte adhesion and signaling in inflammation and diseaseJ Leukoc Biol199659789968691062
- WangJBarkeRACharboneauRMorphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infectionJ Immunol20051744263415611267
- WardTTSteigbigelRTAcidosis of synovial fluid correlates with synovial fluid leukocytosisAm J Med197864933626220
- WiedermannCJSacerdotePMurEDecreased immunoreactive beta-endorphin in mononuclear leucocytes from patients with rheumatic diseasesClin Exp Immunol199287178821735181
- WiedermannCJSacerdotePPropstADecreased beta-endorphin content in peripheral blood mononuclear leukocytes from patients with Crohn's diseaseBrain Behav Immun1994826197865897
- WyattSHFishmanEKThe acute abdomen in individuals with AIDSRadiol Clin North Am1994321023438084997
- YamadaKNabeshimaTStress-induced behavioral responses and multiple opioid systems in the brainBehav Brain Res199567133457779288
- YeagerMPColacchioTAYuCTMorphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteersAnesthesiology19958350087661350
- YinDMufsonRAWangRFas-mediated cell death promoted by opioidsNature19993972189930695
- ZadinaJEHacklerLGeLJA potent and selective endogenous agonist for the mu-opiate receptorNature19973864995029087409
- ZajaczkowskaRWnekWWordliczekJPeripheral opioid analgesia in laparoscopic cholecystectomyReg Anesth Pain Med200429424915372386
- ZhangNHodgeDRogersTJCa2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptorsJ Biol Chem2003278127293612551901
- ZhangNRogersTJCaterinaMProinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neuronsJ Immunol2004173594915210821
- ZhangQSchafferMEldeREffects of neurotoxins and hindpaw inflammation on opioid receptor immunoreactivities in dorsal root gangliaNeuroscience199885281919607719
- ZollnerCShaquraMABopaiahCPPainful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neuronsMol Pharmacol2003642021012869624
- ZoukosYThomaidesTMathiasCJHigh beta-adrenoceptor density on peripheral blood mononuclear cells in progressive multiple sclerosis: a manifestation of autonomic dysfunction?Acta Neurol Scand19949038277892755
- ZurawskiGBenedikMKambBJActivation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesisScience198623277252938259
- ZwickMMolliverDCLindsayJTransgenic mice possessing increased numbers of nociceptors do not exhibit increased behavioral sensitivity in models of inflammatory and neuropathic painPain200310649150014659533