Abstract
Clinicians now have five oral antifungal therapeutic agents to choose from when assessing the risk–benefits associated with a particular treatment for onychomycosis (OM): griseofulvin, itraconazole, terbinafine, ketoconazole, and fluconazole. Only the first three are approved by the FDA for this indication. Griseofulvin is fungistatic and inhibits nucleic acid synthesis, arresting cell division at metaphase, and impairing fungal wall synthesis. Due to its low cure rates and high relapse, it is rarely used for treatment of onychomycosis. Itraconazole is a broad spectrum drug and is effective against dermatophytes, candida, and some nondermatophytic molds. Itraconazole works by inhibiting ergosterol synthesis via cytochrome P-450 (CYP450)-dependent demethylation step. This azole antifungal agent is metabolized in the liver by cytochrome P-450 3A4 (CYP3A4), and therefore has the potential to interact with drugs metabolized through this pathway. Terbinafine, an allylamine, is fungicidal and remains at therapeutic levels in keratinized tissues, but with a short plasma half-life of 36 hours. Terbinafine has the advantage in that it does not inhibit CYP3A4 isoenzyme during its metabolism where some 50% of all commonly prescribed drugs are metabolized. The only potentially significant drug interaction with terbinafine is with the cytochrome P-450 2D6 (CYP2D6) isoenzyme. The lack of widely reported or published clinically relevant drug interactions, and extensive experience from a large prospective, surveillance study conducted in “real world” setting with no patient exclusions, suggest that this is not a major issue. The high cure rates of terbinafine against dermatophytes, as shown in many studies since its launch in the 1990s, together with lack of clinically significant drug interactions and well established safety record, indicate the use of continuous oral terbinafine as the top choice for the treatment of onychomycosis in most patients.
Introduction
Onychomycosis is relatively common, with a prevalence of 6.5%–6.8% in the general population in Canada (CitationGupta et al 1997), 8.5% in the general male population in Finland (CitationHeikkila and Stubb 1995), and up to 18.5% in the US (CitationGhannoum et al 2004). Some studies suggest that as much as 48% of the population may be affected by the age of 70 (CitationDrake et al 1998; CitationScher 1999).
Balancing patient safety with therapeutic benefit is a prime directive when treating onychomycosis. There are several oral antifungal agents to choose from when assessing the risk–benefits associated with a particular treatment for onychomycosis; griseofulvin, ketoconazole, fluconazole, itraconazole, and terbinafine, although only three have been approved by the Food and Drug Administration (FDA). Fluconazole, an azole much like itraconazole, can be used, but it is not approved for onychomycosis. Ketoconazole is rarely used due to poor tolerability, low efficacy, and the availability of new antifungal agents. In this review, we compare the mode of action, pharmacokinetics, and potential for drug interactions for various oral antifungal agents. However, the focus is on the mode of action, pharmokinetics, tolerability, and safety of the three FDA approved oral drugs griseofulvin, itraconazole, and terbinafine. An increased understanding of the metabolism of all the oral antifungal agents allows a better appreciation of potential drug–drug interactions, impact on safety, and appropriate choice of therapy. This is particularly relevant as the number of patients on polypharmacy is increasing due to an aging population and increased comorbidities. Moreover, the widespread use of cholesterol-lowering statins and antihypertensive drugs in otherwise healthy individuals may put many patients at risk for drug interactions.
Pharmacokinetics
Mode of action
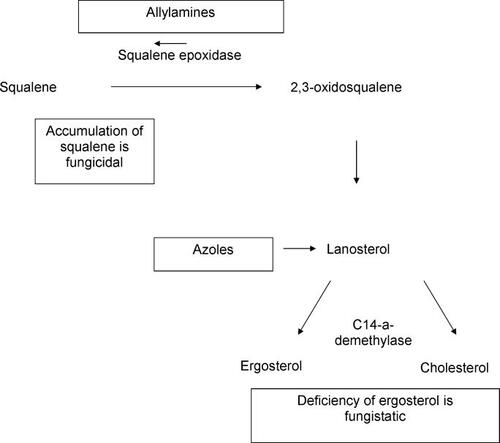
Griseofulvin acts by disrupting the fungal mitotic spindle, inhibiting cell wall synthesis, whereas azoles act to block ergosterol synthesis, required for assembly of the fungal cell wall, by inhibiting C14α-demethylase, a member of the cytochrome P-450 (CYP450) family. Terbinafine works much like azoles, with the exception that it blocks ergosterol synthesis further upstream by inhibiting squalene epoxidase. This results in cells becoming deficient in ergosterol and causes accumulation of toxic squalene, which, in turn, results in fungal death. This activity makes terbinafine a fungicidal drug compared with azoles which are fungistatic. This step does not involve CYP450 enzymes, therefore drug interactions are not typically an issue ().
Absorption
Griseofulvin is poorly absorbed, unless micronized, or coated with polyethylene glycol, or given with fatty meals (CitationLin et al 1982). Its absorption decreases with repeated administration, possibly due to damage to the mucosal wall by unabsorbed griseofulvin (CitationDebruyne and Coquerel 2001). This agent has therefore largely been superceded by compounds with better pharmacokinetics. The bio-availability of the most effective azole antifungal, itraconazole, is increased by coadministration of food, and decreased in the presence of agents that reduce gastric acidity, eg, antacids, H2 blocker antihistamines, proton pump inhibitors, and the anti-HIV agent, oral didanosine. The efficacy of itraconazole may therefore be compromised by drug coadministration. The bioavailability of terbinafine is good, with 70%–80% of the ingested dose being absorbed, and maximal plasma concentrations reached within 8 hours (CitationDebruyne and Coquerel 2001). In addition, the absorption of terbinafine is unaffected by coadministration of food or agents that decrease gastric pH ().
Table 1 Characteristics of oral antifungal agents
Concentrations in the nails and plasma
Steady-state plasma levels of terbinafine are reached after 10–14 days of treatment (CitationDe Doncker 1997), and itraconazole within 3 weeks (CitationLeyden 1998). Both terbinafine and itraconazole can be measured in the nail by 7 days after the start of treatment, indicating that the route of entry into the nail plate is via the nail bed and the matrix (CitationDe Doncker 1997), rather than solely by incorporation into keratin precursor cells, as seen with griseofulvin (CitationDebruyne and Coquerel 2001). Terbinafine reaches a steady state in the nail after 1 week of treatment, whereas itraconazole may require 3–12 weeks; these levels are then sustained in the nail plate for several months.
The older generation of antifungal drugs (eg, griseofulvin) had to be used continuously until an entirely new nail plate was grown out, which could take up to a year. Griseofulvin has low affinity for keratins and drug levels decline rapidly with plasma levels (CitationMeinhof 1993). Also, the drug persists for only a short duration, for approximately two weeks after treatment is discontinued. On the other hand, both itraconazole and terbinafine are keratinophilic and lipophilic, allowing them to be used for only a short period of time. Terbinafine can persist in the nails between 24 and 156 days (CitationDebruyne and Coquerel 2001); this allows terbinafine to be used effectively with relatively short courses of treatment. Plasma levels of terbinafine and itraconazole fall rapidly after the end of treatment, and the shorter treatment requirements with both drugs further minimize the likelihood of systemic side effects (CitationDebruyne and Coquerel 2001).
Drug interactions
Potential drug interactions that could reduce efficacy or drug toxicity must be taken into account when assessing the benefits and risks of the different oral antifungal agents. The most common hepatic enzyme involved in drug metabolism is cytochrome 3A4 (CYP3A4), which is required for the clearance of many different therapeutic agents.
Griseofulvin
Griseofulvin is not indicated for those with porphyria and hepatocellular failure. Also, patients on warfarin-type anticoagulants may need an adjustment of their anticoagulant dose (CitationDeveloux 2001). These may cause contraceptive failure especially of low dose pills. The major drug interactions noted are with phenobarbital, anticoagulants, and oral contraceptives.
Azole antifungal agents
These are metabolized in the liver by CYP3A4, and thus have the potential to interact with a long list of clinically important agents (). In particular, concurrent administration of azole antifungal agents and the following therapeutics are contraindicated: the antidysrhymic and antimalarial agent, quinidine; benzodiapines that undergo oxidative metabolism (including alprazolam, chlordiazepoxide, clonazepam, diazepam, estazolam, flurazepam, halazepam, quazepam, and triazolam); dofetilide; the antipsychotic, pimozide; and the statins, lovastatin, simvastatin, and atovastatin (CitationKatz 1999; CitationShapiro and Shear 2002). Peripheral edema resulting from coadministration of itraconazole and calcium channel blocker, nifedipine, has also been reported (CitationTailor et al 1996).
Table 2 Drug–drug interactions observed with azole antifungal drugs and CYP3A4 metabolizing agents
Other commonly used agents that are metabolized by CYP3A4 are warfarin, cisapride, and cyclosporine. Monitoring of serum levels, and dosage adjustment if indicated, should therefore be carried out during treatment with itraconazole (CitationShapiro and Shear 2002). Inhibition of CYP3A4 by itraconazole may increase warfarin's anticoagulant activity when administered together (CitationDel Rosso 2004). Similarly, some oral antidiabetic agents, including reaglinide and pioglitzone, are metabolized by CYP3A4, and concurrent administration may cause hypoglycemia (CitationDel Rosso 2004). Any agent that induces CYP3A4, such as rifampicin and phenytoin, may increase the metabolism and therefore reduce the efficacy of itraconazole (CitationShear et al 2000). Itraconazole levels should therefore be monitored in the event of coadministration with these agents.
Azole antifungal agents have also been implicated in several hormone–drug interactions (CitationVenkatakrishnan et al 2000). Patients with HIV infection undergoing treatment with highly active retroviral therapy are also likely to be receiving protease inhibitors such as ritonavir, saquinavir, or amprenavir that are potent inhibitors of CYP3A4. The effects of ketoconazole on amprenavir and saquinavir have been documented, and in the event of concurrent treatment with azole antifungal agents and protease inhibitors, dose reduction of the latter is required.
Terbinafine (allylamine)
In contrast with azole antifungal agents, the potential of terbinafine for drug interaction is generally considered low. Side effects associated with CYP3A4 are not observed. While terbinafine is metabolized extensively in the liver, this occurs via the action of various P-450 enzymes (eg, CYP2C9, CYP1A2, CYP3A4, CYP2C8, and CYP2C19) (CitationVickers et al 1999). In addition, metabolism of terbinafine requires less than 5% of the total liver CYP450 capacity (CitationVickers et al 1999). Clinically significant drug interactions are limited to cimetidine and rifampicin, which decrease and increase the rate of terbinafine plasma clearance, respectively. The rate of clearance of terbinafine is reduced by one-third in the presence of cimetidine, and doubles in the presence of rifampicin (CitationShear et al 2000). Terbinafine inhibits the cytochrome family member, cytochrome P-450 2D6 (CYP2D6) (CitationAbdel-Rahman et al 1999), and caution may be indicated when administering CYP2D6 substrates, such as nortriptyline, desipramine, perphenazine, metoprolol, encainide, and propafenone (CitationShear et al 2000; CitationDebruyne and Coquerel 2001). Concentrations of warfarin may be altered when coadministered with terbinafine (CitationShear et al 2000).
Terbinafine has a terminal half-life of 16–22 hours (CitationDebruyne and Coquerel 2001). This is prolonged in patients with liver or kidney impairment, and patients with a creatinine clearance less than 50 mL/min or serum creatinine level of more than 300 μmol/L should receive half the normal dose. Terbinafine is primarily excreted (> 70%) in the urine (see ) (CitationBalfour and Faulds 1992).
Table 3 Randomized trials with terbinafine 250 mg daily
Liver enzyme elevations
Despite transient asymptomatic liver enzyme changes seen in clinical trials, terbinafine is not listed in the British National Formulary as a potential inducer of liver enzymes. While some rare cases of hepatic failure have been reported among millions of adults treated for OM (Citationvan 't Wout et al 1994; CitationBoldewijn et al 1996; CitationMallat et al 1997; CitationShiloah et al 1997; CitationVivas et al 1997; CitationGupta et al 1998; CitationAnania and Rabin 2002), many of these patients were elderly and/or had preexisting liver diseases; therefore the causal relationship in many such cases has not been unequivocally determined.
The risk of acute liver injury among 69830 patients treated with oral antifungal agents was determined in a cohort study in which patients with prior liver disease were excluded (CitationGarcia Rodriguez et al 1999). The incidence rates of acute liver injury were found to be 134.1 per 100 000 person-months; (95% confidence interval [CI]: 36.8, 488.0) for ketoconazole, 10.4 (95% CI: 2.9, 38.1) for itraconazole, and 2.5 (95% CI: 0.4, 13.9) for terbinafine. Ketoconazole was associated with the highest relative risk with 228.0 (95% CI: 33.9, 933.0), when compared with the risk among non-users, followed by itraconazole (relative risk [RR] 17.7; 95% CI: 2.6, 72.6) and terbinafine (RR 4.2; 95% CI: 0.2, 24.9). This cohort study confirms the finding that most case reports of liver injury after administration of oral antifungal agents occur with ketoconazole and itraconazole, and argues against using these agents as initial treatment for uncomplicated fungal infections. While the Rodriguez study (CitationGarcia Rodriguez et al 1999) highlights low incidence of liver injury for terbinafine, the higher rates of hepatotoxicity seen with azole antifungals has adversely affected the perception of terbinafine-induced liver enzyme elevation. The incidence of terbinafine-related hepatobiliary dysfunction in the same studies are even lower at 1 in 45 000–120 000 patients (CitationHay 1993). To put this finding further into context, the low risk of hepatic injury observed with terbinafine may be comparable to that seen with paracetamol, a medication widely used for pain relief, and perceived as safe by the general population (CitationFriis and Andreasen 1992; CitationSkorepova 2004).
The risk of hepatotoxicity with terbinafine should not be exaggerated, but should be taken into account, together with any other relevant factors. Patients with chronic or active liver diseases should not be treated with terbinafine, and baseline (pretreatment) liver transaminase testing is recommended. While some physicians continue to monitor liver enzymes during the course of terbinafine treatment, this is no longer recommended by the revised current labeling. After many years of experience with terbinafine, the FDA subsequently removed the LFT monitoring recommendation from the terbinafine label (CitationMedWatch 2001). This is in line with early safety data reported for 1508 patients with toenail onychomycosis, with a mean age of 50 years, and extensive intractable disease, averaging over 11 years in duration (CitationPollak and Billstein 1997). The incidence of hepatic or biliary disorders was 2.8%, of which the most common was abnormal liver function tests (2.4%). A recent study of 504 patients, in which patients with baseline abnormal liver enzymes were excluded, showed no clinically significant alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevation in plasma levels when tested 6 weeks into the treatment (250 mg/day) (CitationPollak et al 2004). For griseofulvin, there is a clear dosage-dependent association with hepatic toxicity, particularly in patients with prior liver damage (CitationSkorepova 2004).
Tolerability of oral antifungal (adverse drug events)
Griseofulvin
Availability of newer antifungal agents terbinafine and itraconazole suggest that griseofulvin is no longer the treatment of choice for dermatophyte onychomycosis. Side-effects include nausea and rashes in 8%–15% of patients. In adults, it is contraindicated in pregnancy and the manufacturers caution against men fathering children for 6 months after therapy.
Terbinafine
Safety data from four large-scale post-marketing surveys investigating safety of terbinafine in actual clinical practice in an uncontrolled setting have been pooled and reported (CitationHall et al 1997; CitationO'Sullivan 1999). The incidence of adverse events was 10.5%; the majority involved the gastrointestinal system (4.9%) or skin (2.3%); these tended to be mild, transient, and reversible. Terbinafine was considered a “possible” or “probable” cause of only 11 (0.04%) serious adverse events. No drug–drug interactions were reported, even in patients taking oral antidiabetic agents (astemizole, terfenadine, or cimetidine), nor were any previously unrecognized risks identified.
In a post-marketing survey of terbinafine conducted in 1996, involving 10 000 patients, transient taste disturbance was reported by 0.06% of patients (CitationO'Sullivan et al 1996). All patients with taste loss fully recovered the sensation, at an average of 6 weeks (range 2–186 weeks) (CitationO'Sullivan et al 1996). As per terbinafine's prescribing information, rare incidence of cutaneous abnormalities (eg, Stevens-Johnson syndrome or toxic epidermal necrolysis; CitationCarstens et al 1994; CitationTodd et al 1995) have been reported. Over a period of approximately 15 years, only two instances of longer-term taste disturbance have been reported or reversible taste loss or changes (CitationBeutler et al 1993; CitationDuxbury et al 2000).
The good safety profile has been reported in elderly and diabetic patients in early studies (CitationNedelman et al 1997; CitationPollak and Billstein 1997; CitationSmith et al 2000; CitationElewski and Smith 2001). Good safety results have also been obtained in other special patient populations (CitationCribier and Bakshi 2004; CitationGupta et al 2005).
In a study by CitationPollak and Billstein (1997), comparable numbers of patients received 12, 18, and 24 weeks of 250 mg/day terbinafine. No serious adverse events considered to be related to the study drug were reported, either in the intent to treat population as a whole, in those over 60 years (n = 416), or in those with diabetes (n = 77). The most prevalent adverse event (12.3% of patients) was skin-related, most commonly a skin rash or nail disorder. Gastrointestinal complaints (nausea, diarrhea, dyspepsia, and abdominal pain) were recorded in 11.5% of patients, with 3.9% considered to have a relationship with the study drug. In a group of 30 patients aged over 60 years who received 12 weeks of treatment, no serious adverse events occurred (CitationSmith et al 2000). Use of concomitant medications was widespread, with 93.3% of patients receiving at least one prescription or over-the-counter medication during terbinafine treatment, and 27% receiving medications with known interactions with azole antifungals. Although 16 patients used medications metabolized by CYP2D6, no drug interactions occurred between these agents and terbinafine, and no clinical consequences were seen (CitationSmith et al 2000).
HIV patients are also at increased risk for drug interactions, due to compromised liver function and low white cell count (CitationElewski and Smith 2001). No drug interactions have been observed during terbinafine treatment of fungal infection (onychomycosis, tinea pedis, tinea cruris, tinea corporis) in 57 patients with HIV infection (CitationNandwani et al 1996; CitationHerranz et al 1997; CitationRich et al 2001; CitationSmith et al 2001). Although patient numbers are small, there was no evidence of neutropenia, and no detrimental effect on liver function even in patients with serological evidence of viral hepatitis infection. In a subanalysis of 77 patients with diabetes included in an open-label study, the safety profile did not differ from that observed in the general population (CitationPollak and Billstein 1997). Similarly, no drug interactions were reported in a post-marketing survey in which 3.2% of the 25 884 patients were diabetic (CitationHall et al 1997). In addition, control of glucose levels remained unaltered during 12 weeks of terbinafine treatment in 89 patients with diabetes, and no drug interactions or hypoglycemic episodes were seen (CitationCribier and Bakshi 2004; CitationGupta et al 2005).
Itraconazole
Overall, itraconazole is well tolerated, with adverse effects reported in approximately 3% of patients (CitationScher 1999). The more common adverse effects are headache and gastrointestinal symptoms such as diarrhea, dyspepsia, abdominal pain, constipation, nausea, and flatulence, and dermatologic symptoms such as rash, pruritus, and urticaria. Acute generalized exanthematous pustulosis is associated with both oral itraconazole and terbinafine and has been rarely reported in the literature (CitationPark et al 1997; CitationHall and Tate 2000). In most cases, there are nearly complete resolution of the pustular eruption within a few weeks following cessation of drugs and treatment with topical and systemic corticosteroids (CitationHall and Tate 2000). Liver enzyme elevations, reported in 0.3%–0.5% of patients receiving itraconazole therapy (CitationGupta and Shear 2000). Also, azoles are potent inhibitors of vitanovir, saquinavir, and abacavir and not recommended for this patient population.
Approximately 26% of diabetic patients have onychomycosis, and, compared with nondiabetics, this patient population is at increased risk of secondary complications, including onychocryptosis, bacterial cellulitis, osteomyelitis, gangrene, or foot ulcers (CitationElewski and Smith 2001). Effective treatment that does not interact with oral hypoglycemic or cardiovascular agents, or worsen glycemic control, is therefore of high importance. Azole antifungal agents are not desirable under such a setting.
Conclusions
Both direct and historical comparison of griseofulvin with studies of the newer antifungal agents terbinafine and itraconazole suggest that griseofulvin is no longer the treatment of choice for dermatophyte onychomycosis. While itraconazole has been used for years with varying success, its major drawback is the high potential for drug interactions. This is particularly relevant since it is a potent inhibitor of CYP3A4 and cannot be conveniently used with statins, antidiabetics, or antihypertensives.
The potential for drug–drug interactions is low with oral terbinafine, and this agent provides a viable treatment option for elderly, diabetics, and immunocompromised patients with HIV, who are likely to be receiving concomitant medication. Moreover, due to the increasing use of cholesterol lowering statins and antihypertensive drugs, many otherwise healthy individuals may become at risk for drug interactions. Terbinafine does not interact with these classes of drugs.
References
- Abdel-RahmanSMMarcucciKBogeTPotent inhibition of cytochrome P-450 2D6-mediated dextromethorphan O-demethylation by terbinafineDrug Metab Dispos199927770510383919
- AnaniaFARabinLTerbinafine hepatotoxicity resulting in chronic biliary ductopenia and portal fibrosisAm J Med2002112741212079721
- BalfourJAFauldsDTerbinafine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycosesDrugs199243259841372222
- BeutlerMHartmannKKuhnMTaste disorders and terbinafineBMJ1993307268343667
- BoldewijnOYOttervangerJPMostartCMHepatitis attributed to the use of terbinafineNed Tijdschr Geneeskd1996140669728668241
- BrautigamMNoltingSSchopfRERandomised double blind comparison of terbinafine and itraconazole for treatment of toenail tinea infection. Seventh Lamisil German Onychomycosis Study GroupBMJ1995311919227580551
- CarstensJWendelboePSogaardHToxic epidermal necrolysis and erythema multiforme following therapy with terbinafineActa Derm Venereol19947439127817681
- CribierBJBakshiRTerbinafine in the treatment of onychomycosis: a review of its efficacy in high-risk populations and in patients with nondermatophyte infectionsBr J Dermatol20041504142015030322
- De BackerMDe VroeyCLesaffreETwelve weeks of continuous oral therapy for toenail onychomycosis caused by dermatophytes: a double-blind comparative trial of terbinafine 250 mg/day versus itraconazole 200 mg/dayJ Am Acad Dermatol199838S57639594939
- De DonckerPPharmacokinetics of oral antifungal agentsDermatol Ther199734657
- DebruyneDCoquerelAPharmacokinetics of antifungal agents in onychomycosesClin Pharmacokinet2001404417211475469
- Del RossoJQOral Antifungals: What you should know about drug interactionsPodiatry Today2004116615
- DevelouxMGriseofulvin can kill intestinal bacteria whose enzymes help steroid absorptionAnn Dermatol Venereol200112813172511908134
- DrakeLAScherRKSmithEBEffect of onychomycosis on quality of lifeJ Am Acad Dermatol19983870249591814
- DrakeLAShearNHArletteJPOral terbinafine in the treatment of toenail onychomycosis: North American multicenter trialJ Am Acad Dermatol19973774059366820
- DuxburyAJOliverRJPembertonMNPersistent impairment of taste associated with terbinafineBr Dent J2000188295610800234
- ElewskiBSmithSThe safety and efficacy of terbinafine in patients with diabetes and patients who are HIV positiveCutis20016823911499331
- EvansEGSigurgeirssonBDouble blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. The LION Study GroupBMJ19993181031510205099
- FriisHAndreasenPBDrug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987J Intern Med199223213381506809
- Garcia RodriguezLADuqueACastellsagueJA cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugsBr J Clin Pharmacol1999488475210594489
- GhannoumMAChaturvediVEspinel-IngroffAIntra- and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytesJ Clin Microbiol2004422977915243047
- GoodfieldMJAndrewLEvansEGShort term treatment of dermatophyte onychomycosis with terbinafineBr Med Journal1992304115141392793
- GuptaAKdel RossoJQLyndeCWHepatitis associated with terbinafine therapy: three case reports and a review of the literatureClin Exp Dermatol1998236479692307
- GuptaAKJainHCLyndeCWPrevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologists' offices in Ontario, Canada–a multicenter survey of 2001 patientsInt J Dermatol19973678379372358
- GuptaAKRyderJETavakkolAThe use of terbinafine in the treatment of onychomycosis in adults and special patient populations: a review of the evidenceJ Drugs Dermatol20054299305
- GuptaAKShearNHA risk-benefit assessment of the newer oral antifungal agents used to treat onychomycosisDrug Safety20002215210647972
- HallAPTateBAcute generalized exanthematous pustulosis associated with oral terbinafineAustralasian J Dermatol200041425
- HallMMonkaCKruppPSafety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patientsArch Dermatol19971331213199382559
- HanekeETauschIBrautigamMShort-duration treatment of fingernail dermatophytosis: a randomized, double-blind study with terbinafine and griseofulvin. LAGOS III Study GroupJ Am Acad Dermatol1995327277822520
- HavuVHeikkilaHKuokkanenKA double-blind, randomized study to compare the efficacy and safety of terbinafine (Lamisil) with fluconazole (Diflucan) in the treatment of onychomycosisBr J Dermatol20001429710210651701
- HayRJRisk/benefit ratio of modern antifungal therapy: focus on hepatic reactionsJ Am Acad Dermatol199329S5048315062
- HeikkilaHStubbSThe prevalence of onychomycosis in FinlandBr J Dermatol19951336997038555019
- HerranzPGarciaJDe LucasRToenail onychomycosis in patients with acquired immune deficiency syndrome: treatment with terbinafineBr J Dermatol1997137577809390334
- HofmannHBrautigamMWeidingerGTreatment of toenail onychomycosis. A randomized, double-blind study with terbinafine and griseofulvin. LAGOS II Study GroupArch Dermatol1995131919227632064
- KatzHIDrug interactions of the newer oral antifungal agentsBr J Dermatol1999141263210730911
- LeydenJPharmacokinetics and pharmacology of terbinafine and itraconazoleJ Am Acad Dermatol199838S42479594936
- LinCLimJDi GioreCComparative bioavailability of a microsize and ultramicrosize griseofulvin formulation in manJ Int Med Res19821027477117684
- MallatAZafraniESMetreauJMTerbinafine-induced prolonged cholestasis with reduction of interlobular bile ductsDig Dis Sci199742148689246051
- MedwatchSummary Of Safety-Related Drug Labeling Changes Approved by FDA Center for Drug Evaluation and Research (CDER)2001Washington, DC, USAFood and Drug Administration
- MeinhofWKinetics and spectrum of activity of oral antifungals; the therapeutic implicationsJ Am Acad Dermatol199329S37418315060
- NandwaniRParnellAYouleMUse of terbinafine in HIV-positive subjects: pilot studies in onychomycosis and oral candidiasisBr J Dermatol19961342248763464
- NedelmanJCramerJARobbinsBThe effect of food on the pharmacokinetics of multiple-dose terbinafine in young and elderly healthy subjectsBiopharm Drug Dispos199718127389099449
- O'SullivanDPTerbinafine: tolerability in general medical practiceBr J Dermatol199914121510730910
- O'SullivanDPNeedhamCABangsAPostmarketing surveillance of oral terbinafine in the UK: report of a large cohort studyBr J Clin Pharmacol199642559658951186
- ParkYMKimJWKimCWAcute generalized exanthematous pustulosis induced by itraconazoleAm Acad Dermatol1997367946
- PollakRBillsteinSASafety of oral terbinafine for toenail onychomycosisJ Am Podiatr Med Assoc199787565709425805
- PollakRAHarklessLBJenningsMBTerbinafine: Reality versus perception concerning liver functionAm Podiatric Med Assoc2004Boston, USA International Annual Meeting, August 2004
- RichPHouptKRLa MarcaASafety and efficacy of short-duration oral terbinafine for the treatment of tinea corporis or tinea cruris in subjects with HIV infection or diabetesCutis200168152211499330
- ScherRKOnychomycosis: therapeutic updateJ Am Acad Dermatol199940S21610367912
- ShapiroLEShearNHDrug interactions: Proteins, pumps, and P-450sJ Am Acad Dermatol20024746784 quiz 485–812271287
- ShearNDrakeLGuptaAKThe implications and management of drug interactions with itraconazole, fluconazole and terbinafineDermatology200020119620311096189
- ShiloahEHorowizMZeclerETerbinafine-induced cholestatic liver injuryHarefuah19971331112 80–19332048
- SkorepovaMRisk of liver damage casued by modern systemic antimycoticsCes-lov Derm2004795961
- SmithEBSteinLFFivensonDPThe safety of terbinafine in patients over the age of 60 years: a multicenter trial in onychomychosis of the feetInt J Dermatol2000398596411123451
- SmithSHouptKRichPShort-duration oral terbinafine for the treatment of tinea pedis in HIV-positive patientsCutis20016830911499332
- TailorSAGuptaAKWalkerSEPeripheral edema due to nifedipine-itraconazole interaction: a case reportArch Dermatol199613235028607648
- ToddPHalpernSMunroDDOral terbinafine and erythema multiformeClin Exp Dermatol19952024787671425
- van 't WoutJWHerrmannWAde VriesRATerbinafine-associated hepatic injuryJ Hepatol199421115177963410
- VenkatakrishnanKvon MoltkeLLGreenblattDJEffects of the antifungal agents on oxidative drug metabolism: clinical relevanceClin Pharmacokinet2000381118010709776
- VickersAESinclairJRZollingerMMultiple cytochrome P-450sb involved in the metabolism of terbinafine suggest a limited potential for drug-drug interactionsDrug Metab Dispos19992710293810460803
- VivasSRodriguezMPalacioMAAcute hepatitis associated with terbinafineGastroenterol Hepatol19972045689445740
- WatsonAMarleyJEllisDTerbinafine in onychomycosis of the toenail: a novel treatment protocolJ Am Acad Dermatol19953377597593777