Abstract
Acute bacterial sinusitis (ABS), acute exacerbations of chronic bronchitis (AECB), and community-acquired pneumonia (CAP) are common conditions and constitute a substantial socioeconomic burden. The ketolides are a new class of antibacterials with a targeted spectrum of antibacterial activity. In vitro, telithromycin is active against common bacterial pathogens that cause upper and lower respiratory tract infections, including some isolates that are resistant to other antibiotic classes. In 2004, telithromycin was the first ketolide antibiotic approved for clinical use by the US Food and Drug Administration for the treatment of adult outpatients with ABS, AECB, and mild-to-moderate CAP. This review discusses the use of telithromycin in the treatment of these infections, providing an overview of its antibacterial activity, pharmacokinetic and pharmacodynamic properties, clinical efficacy, and tolerability–safety, and concludes that telithromycin is an appropriate option for the treatment of community-acquired ABS, AECB, and mild-to-moderate CAP.
Introduction
Acute bacterial sinusitis (ABS), acute exacerbations of chronic bronchitis (AECB), and community-acquired pneumonia (CAP) are common conditions and constitute a substantial socioeconomic burden. Lower respiratory tract infections (RTIs), such as CAP and AECB, represent a particular public health concern owing to the morbidity and mortality associated with these infections (CitationNiederman et al 1998, Citation1999; CitationBartlett et al 2000).
Bacterial pathogens associated with RTIs include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, and atypical–intracellular organisms such as Mycoplasma pneumoniae,Chlamydophila (Chlamydia) pneumoniae, and Legionella pneumophila. Antibacterial treatment of outpatient community-acquired RTIs is usually empiric, since the causative pathogen is rarely identified before initiation of antibacterial therapy. However, the development and spread of antibacterial resistance among respiratory pathogens, in particular S. pneumoniae, now represents a key challenge in the management of RTIs (CitationFile 2004). Although the clinical impact of bacterial resistance is still under debate, there have been a number of recent cases of treatment failure associated with macrolide use (CitationRzeszutek et al 2004; CitationKlugman and Lonks 2005).
The ketolides are a new class of antibacterials with a targeted spectrum of antibacterial activity. In vitro, telithromycin is active against common bacterial pathogens that cause upper and lower RTIs, including some isolates resistant to other antibiotic classes. In 2004, telithromycin was the first ketolide antibiotic approved for clinical use by the US Food and Drug Administration for the treatment of adult outpatients with ABS, AECB, and mild-to-moderate CAP.
This review discusses the use of telithromycin in the treatment of these infections, providing an overview of its antibacterial activity, pharmacokinetic and pharmaco-dynamic properties, clinical efficacy, and tolerability– safety.
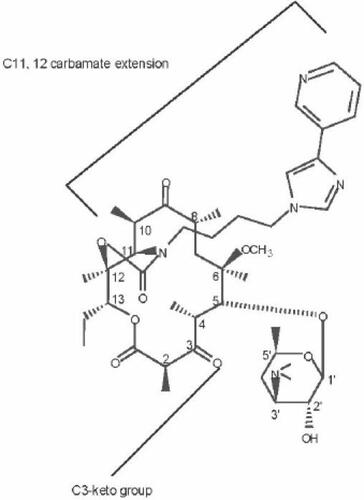
Chemistry of telithromycin
The ketolide class of antibacterials, of which telithromycin is the first to be approved for clinical use, are semisynthetic derivatives of the 14-membered macrolide, erythromycin. Telithromycin retains the macrolactone ring structure and the D-desosamine sugar attached at position 5 (). The defining characteristic of the ketolides is the removal of the neutral sugar, Lcladinose, from the 3 position of the erythronolide ring and the subsequent oxidation of the 3hydroxyl to a 3-keto functional group (). Removal of the Lcladinose moiety improves the acid stability of telithromycin. Furthermore, compounds with the 3-keto group do not trigger the expression of resistance to MLSB (macrolide–lincosamide–streptograminB) antibacterials in strains with inducible erm determinants (CitationBonnefoy et al 1997). This allows telithromycin to remain active against bacterial strains in which MLSB resistance would be induced (CitationBonnefoy et al 1997).
The 11, 12-carbamate present in telithromycin () enhances the activity of ketolides (CitationDouthwaite and Champney 2001) and, together with the 6-O-alkyl group, prevents 6–9 or 9–12 cyclization of the compound, which would result in the formation of unstable hemiketal products (commonly formed by erythromycin). Telithromycin also contains heterocyclic aromatic rings spaced from the lactone ring structure via short alkyl or allyl linkages. These structures impart improved ribosomal binding and thereby increase the activity of the compounds against both macrolide-susceptible and macrolide-resistant strains (CitationMankin et al 2000).
Mechanism of action
Telithromycin inhibits bacterial protein synthesis by interacting close to the peptidyl transferase site of the 50S ribosomal subunit (CitationChampney and Tober 2001); the main sites of macrolide and ketolide interaction are within domains II and V of the 23S ribosomal RNA (CitationHansen et al 1999; CitationXiong et al 1999). Ketolides bind to the ribosome in a 1:1 ratio (CitationHansen et al 1999), indicating that domains II and V of the 23S rRNA lie in close proximity within the tertiary structure of the rRNA and form a single drug-binding pocket. This structure has been confirmed by crystallo-graphic models of the 50S subunit (CitationBan et al 2000; CitationSchlunzen et al 2001).
Chemical footprinting experiments have located the main site of ketolide and macrolide interaction at nucleotides A2058 and A2059 in domain V (CitationHansen et al 1999; CitationXiong et al 1999). Although both macrolides and ketolides protect these bases from chemical modification, the ketolides display a higher affinity than macrolides for forming interactions with the ribosomes (CitationBertho et al 1998; CitationCapobianco et al 2000). This increased affinity has been shown to be caused by the additional interaction at A752 in domain II and is mediated by the 11, 12-carbamate side chain and alkyl or allyl linkages (CitationHansen et al 1999). Base substitutions at position A752 reduce the binding of ketolides, supporting the notion that the adenine base at A752 is an important secondary contact site for the carbamate ketolides (CitationNovotny et al 2001). This additional contact presumably enables the ketolides to retain activity against bacteria that have base modifications in domain V (CitationCapobianco et al 2000; CitationDouthwaite et al 2000; CitationBemerMelchior et al 2000).
Mechanism of resistance
The main factor behind the development of the ketolides was the need for new antibacterials to overcome the problem of macrolide resistance among the common respiratory tract pathogens, particularly S. pneumoniae. The most common macrolide resistance mechanisms in Gram-positive bacteria involve drug efflux and target modification (CitationLeclercq and Courvalin 1991; CitationSutcliffe et al 1996). Efflux resistance in S. pneumoniae and Streptococcus pyogenes is encoded by the mef(A) gene. Target modification involves methylation of the key A2058 nucleotide in the MLSB antibiotic binding site on the ribosome by methylases encoded by erm(B) in S. pneumoniae, and erm(B) and erm(TR) in S. pyogenes (CitationBonnefoy et al 1997; CitationRoberts et al 1999). The L-cladinose moiety present in macrolides contributes to the strong induction of erm(B) resistance by these agents. Other less common macrolide resistance mechanisms include mutations in the ribosomal proteins or 23S rRNA (CitationTaitKamradt et al 2000).
Telithromycin is active against bacterial strains expressing mef(A) resistance (CitationSchito et al 2004; CitationJenkins et al 2005), most likely because telithromycin is a poor substrate for the macrolide efflux pump (CitationLeclercq 2000). Telithromycin also retains activity against pneumococcal isolates that constitutively express the erm(B) resistance gene (CitationSchito et al 2004; CitationJenkins et al 2005). Furthermore, the lack of an L-cladinose group ensures that telithromycin does not induce erm expression and is thereby active against strains inducibly resistant to macrolides (CitationMauvais and Bonnefoy 2000).
Selection experiments have shown that exposure of pneumococci to telithromycin is less likely to result in development of resistance than exposure to macrolides (CitationFernandez-Roblas et al 1999; CitationDavies et al 2000; CitationEdlund et al 2000). Typically, exposure to telithromycin resulted in minimal increases in minimum inhibitory concentration (MIC) values, with mutations occurring at lower frequencies than those obtained for macrolides under the same conditions (CitationDavies et al 2000).
In vitro activity
Results from a number of early in vitro studies confirmed that telithromycin possesses potent activity against both common and atypical–intracellular respiratory pathogens (CitationBiedenbach et al 1998; CitationPankuch, Hoellman, et al 1998; CitationRoblin and Hammerschlag 1998; CitationBébéar et al 2000). summarizes the activity of telithromycin and macrolide antibacterials against key respiratory pathogens collected in the USA (2001–2002) from patients with community-acquired RTIs as part of the PROTEKT US (Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin in the United States) longitudinal surveillance study. In this study, telithromycin was more active against S. pneumoniae, including both penicillin-resistant and macrolide-resistant strains, than the macrolides () (CitationBrown and Rybak 2004). Recently published data from PROTEKT US (2002–2003) demonstrate that MIC90 values for some macrolides against S. pneumoniae increased considerably between 2001 and 2002, and 2002 and 2003; the MIC90 for erythromycin changed from 16 μg/mL to 64 μg/mL and the azithromycin MIC90 increased from 32 μg/mL to ≥ 256 μg/mL. Overall, > 99% of isolates were susceptible to telithromycin, and telithromycin susceptibility was unaffected by reduced susceptibility of these isolates to penicillin and/or erythromycin (CitationBrown and Rybak 2004). Telithromycin is also active against Gram-negative respiratory tract bacteria, such as H. influenzae, and is not affected by β−lactamase production (), making it active against ampicillin-resistant strains of these pathogens (CitationBrown and Rybak 2004). Similar results confirming the activity of telithromycin against respiratory pathogens have been demonstrated in the PROTEKT global surveillance study (CitationKohno et al 2003; CitationFarrell and Felmingham 2004; CitationReinert, Felmingham, et al 2004).
Table 1 In vitro activities of telithromycin, erythromycin, and azithromycin against key respiratory pathogens collected in the USA as part of the PROTEKT US study 2001–2002Table Footnotea
Telithromycin also shows in vitro activity against less common respiratory pathogens including methicillin (oxacillin)-susceptible Staphylococcus aureus (MIC90 ≤ 0.25 mg/mL) (CitationBarry et al 1998; CitationBoswell, Andrews, Ashby, et al 1998) and atypical–intracellular respiratory pathogens, including M. pneumoniae and C. pneumoniae (CitationRoblin and Hammerschlag 1998; CitationBébéar et al 2000). In addition, telithromycin has minimal activity against a range of Gram-negative nonrespiratory pathogens and commensal bacteria, including Acinetobacter spp., Enterobacteriaceae spp., Vibrio spp., Campylobacter jejuni, Aeromonas hydrophila, Plesiomonas shigelloides, and Pseudomonas aeruginosa (CitationFelmingham and Farrell 2006).
Pharmacokinetics
Absorption
Following first-pass metabolism of telithromycin by the liver and the gastrointestinal tract, the absolute bioavailability of this agent (which is unaffected by the age of the subject) is ∼57% (CitationPerret et al 2002). Neither the rate nor the extent of absorption of telithromycin is affected by food intake, meaning that the drug can be taken without regard to mealtimes (CitationBhargava, Lenfant, et al 2002). Administration of telithromycin 800 mg once daily for 7 days to healthy volunteers resulted in rapid absorption: the mean peak serum concentration (Cmax) of 2.27 μg/mL was reached within 1 hour of dosing (CitationNamour et al 2001).
Distribution
Telithromycin is subject to moderate serum protein binding; approximately 60%–70% is protein-bound, mainly to albumin and α1-acid glycoprotein. Studies investigating the tissue distribution of telithromycin indicate that the drug penetrates effectively into respiratory tissues and fluids. Dosing of telithromycin 800 mg once daily for 5 days produced high concentrations of the drug in broncho-pulmonary tissue and fluid, including alveolar macrophages and epithelial lining fluid (CitationMuller-Serieys et al 2001, Citation2004; CitationKhair et al 2001). In addition, telithromycin concentrates in other tissues and fluids as shown in . Drug concentrations at these sites exceeded the peak concentration of the drug in plasma and remained above telithromycin MICs for key respiratory pathogens throughout the 24-hour administration period.
Table 2 Tissue distribution of telithromycin 24 hours after administration of final dose
Metabolism and excretion
Overall, approximately 70% of the administered dose of telithromycin is metabolized (33% pre-systemic and 37% systemic), approximately half of which occurs in the liver via the cytochrome P450 3A4 (CYP3A4) pathway. Of the 57% of administered drug that reaches the systemic circulation unchanged, 13% is excreted unchanged in urine, 7% is excreted unchanged in the feces, and 37% is metabolized by the liver (CitationSultan et al 1999; CitationAventis 2003).
Telithromycin has a biphasic half-life, with an overall terminal half-life of ∼10 hours and renal clearance of 12.5 L/hour (CitationNamour et al 2001). No dosage adjustment is required in patients with mild-to-moderate renal impairment or in patients with mild, moderate, or severe hepatic impairment.
Pharmacodynamics
Telithromycin exhibits bactericidal activity against S. pneumoniae, including isolates resistant to erythromycin (CitationPankuch, Visalli, et al 1998). Telithromycin also demonstrates limited bactericidal activity against S. pyogenes, H. influenzae, and M. catarrhalis by 24 hours, depending upon the concentration of antibiotic (typically ≥ 4 times the MIC value is required) and the size of the inoculum (CitationBoswell, Andrews, Wise, et al 1998; CitationPankuch, Hoellman, et al 1998; CitationPankuch, Visalli, et al 1998; CitationOdenholt et al 2001). In vitro pharmacodynamic models that simulate human unbound plasma or epithelial lining fluid concentrations of telithromycin have shown that this agent rapidly eradicates both macrolide-susceptible and macrolide-resistant S. pneumoniae (regardless of resistance phenotype) at concentrations likely to be achieved in vivo (CitationZhanel et al 2004, Citation2005).
An additional consideration when optimizing the dosing schedule of antibiotics is the post-antibiotic effect (PAE), a measure of the agent’s continued antibacterial activity after its removal from the medium. Telithromycin has demonstrated PAE values equal to or greater than those of macrolide comparators (CitationBoswell, Andrew, Ashby, et al 1998; CitationDubois and St-Pierre 2000; CitationOdenholt et al 2001).
In vivo animal models have confirmed that the antibacterial activity of telithromycin is concentration-dependent and that area under the plasma drug concentration–time curve (AUC) divided by the in vitro MIC of the agent against the organism in question (AUC/MIC) is the pharmacodynamic variable most correlated with efficacy (CitationBermudez et al 2000; CitationBonnefoy et al 2001). In adult patients with CAP treated with telithromycin 800 mg once daily for 7–10 days, a pharmacodynamic analysis has explored the relationships between predictor variables (including AUC/MIC) and microbiologic outcome for the three major CAP pathogens (S. pneumoniae, H. influenzae, and S. aureus) (CitationShi, Pfister, et al 2005). Results from this analysis showed that the probability of cure following treatment with telithromycin in adults with CAP was consistently in the region of 90% across the observed range of AUC/MIC values for each pathogen, providing confirmation that the 800 mg once-daily telithromycin dose exhibits near-maximal antimicrobial activity against S. pneumoniae, H. influenzae, and S. aureus in patients with CAP.
Clinical trials
The clinical efficacy and tolerability of telithromycin 800 mg once daily in adults with ABS, AECB, or mild-to-moderate CAP have been evaluated in 16 multicenter trials (summarized in ).
Table 3 Phase III/IV clinical trials of telithromycin for the treatment of patients with acute bacterial sinusitis, acute exacerbations of chronic bronchitis, or community-acquired pneumonia
The trials included analysis of both the intent to treat (ITT) population (mITT = modified intent to treat; any patient enrolled in the study who received at least 1 dose of antibiotic) and the per-protocol (PP) population. In most studies, the primary efficacy endpoint was clinical outcome in the clinically evaluable per-protocol (PPc) population at the test of cure (TOC) visit (Days 17–24). Clinical cure was defined as improvement in signs and symptoms or a return to pre-infection state without the need for additional antimicrobials. The bacteriologic eradication rate at TOC was also recorded for those patients with a causative pathogen isolated at baseline. Satisfactory bacteriologic outcome was defined as the eradication or presumed eradication of the causative pathogen in the bacteriologically evaluable PP or mITT populations (PPb and bmITT populations, respectively).
Acute bacterial sinusitis
Telithromycin has been evaluated for the treatment of ABS in 4 randomized clinical trials, 3 of which were double-blinded comparative trials () (CitationRoos et al 2002; CitationBuchanan et al 2003; CitationLuterman et al 2003; CitationFerguson et al 2004). A diagnosis of ABS was based on radiologic findings (total sinus opacity and/or air–fluid levels and/or mucosal thickening) accompanied by ≥ 1 clinical symptom (purulent nasal discharge; maxillary tenderness–toothache; maxillary pain on percussion; facial pain, pressure or tightness; or nasal congestion) of < 28 days’ duration. Patients with recurrent (> 3 episodes in the previous year) or chronic (symptoms lasting > 28 days) sinusitis were excluded from all 3 studies.
In the first trial by CitationRoos et al (2002), a 5-day course of telithromycin 800 mg once daily was compared with a 10-day course. Both treatment durations were found to result in comparable clinical success rates, with 91.1% cure in the 5-day treatment group (PPc population) vs 91.0% cure in the 10-day treatment group (cure rates in the mITT populations were 82.6% and 87.5% in the 5-day and 10-day groups, respectively). Bacteriologic outcomes were also comparable between treatment groups: a satisfactory bacteriologic outcome was achieved in 92.9% of patients treated for 5 days and in 89.9% of patients who received the 10-day treatment course. These results led the authors to conclude that a 5-day course of telithromycin 800 mg once daily would be effective in the treatment of ABS, and may result in better patient compliance when compared with 10-day treatment courses.
In a second trial (CitationLuterman et al 2003), telithromycin 800 mg for 5 days was compared with telithromycin 800 mg for 10 days and to amoxicillin–clavulanate 500 mg/125 mg 3 times daily for 10 days. Per-protocol clinical cure rates were equivalent for all 3 treatment modalities (75.3% for 5-day telithromycin, 72.9% for 10-day telithromycin, and 74.5% for 10-day amoxicillin–clavulanate). In both the 5-day and 10-day telithromycin treatment groups, a satisfactory bacteriologic outcome was achieved in 6/7 patients, whereas 8/10 comparator-treated patients recorded a satisfactory bacteriologic outcome.
A 5-day course of once-daily telithromycin was also shown to be as clinically effective as a 10-day course of cefuroxime axetil 250 mg twice daily (CitationBuchanan et al 2003). Per-protocol clinical cure rates for telithromycin and cefuroxime axetil were 85.2% and 82.0%, respectively, and 84.0% of telithromycin-treated patients vs 79.6% of comparator-treated patients achieved a satisfactory bacteriologic outcome (). The fourth trial (Study 4017; CitationFerguson et al 2004) demonstrated that a 5-day telithromycin regimen provides equivalent clinical and bacteriologic efficacy to 10 days’ treatment with the fluoroquinolone moxifloxacin (400 mg once daily).
Pooled clinical cure rates from 3 of these studies (Studies 3002, 3005, and 3011) were 80.9% (271/355) for patients treated with telithromycin vs 77.4% (175/226) in the comparator-treated group (amoxicillin–clavulanate and cefuroxime axetil) (CitationRoos et al 2005). Clinical cure rates were also comparable between telithromycin and comparator antibacterials for patients in subgroups of special interest, including those with severe infection (as assessed by the investigator) (81.8% [45/55] vs 77.8% [35/45]), patients with radiologic findings of total sinus opacity (85.7% [84/98] vs 76.7% [33/43]), and those with a symptom duration of ≥ 7 days (81.5% [229/281] vs 78.0% [135/173]). Across the 3 studies, clinical cure rates by pathogen in patients who received 5 days of telithromycin treatment were 90.2% (55/61) for S. pneumoniae, 87.5% (42/48) for H. influenzae, 92.9% (13/14) for M. catarrhalis, and 94.7% (18/19) for S. aureus (CitationRoos et al 2005).
Acute exacerbations of chronic bronchitis
The efficacy of telithromycin 800 mg once daily for 5 days has been evaluated in a total of 480 patients (PPc population) in 3 double-blind comparator-controlled trials (CitationAubier et al 2002; CitationZervos et al 2003; CitationFogarty, de Wet, et al 2005) (). Patients eligible for entry into the studies included those with a documented history of chronic bronchitis (characterized by cough and excessive sputum production for ≥ 2 consecutive years) and a clinical diagnosis of AECB based on the presence of increased cough or dyspnea, increased sputum volume, and increased sputum purulence.
Clinical and bacteriologic efficacy
CitationAubier and colleagues (2002) reported that a 5-day course of telithromycin 800 mg once daily was as effective as a 10-day course of amoxicillin–clavulanate 500 mg/125 mg 3 times daily. Clinical cure rates (PPc population) for telithromycin and amoxicillin–clavulanate were 86.1% and 82.1%, respectively. Among mITT patients, 81.3% of telithromycin-treated patients achieved clinical cure vs 78.1% of comparator-treated patients. Among PPb patients, a satisfactory bacteriologic outcome was recorded for 69.2% of those treated with telithromycin and 70.0% of patients who received amoxicillin–clavulanate. Re-infections at the late post-therapy visit (Days 31–36) were slightly more common for amoxicillin–clavulanate than telithromycin (9 vs 2).
In the second study, CitationZervos et al (2003) compared telithromycin 800 mg once daily for 5 days with cefuroxime axetil 500 mg twice daily for 10 days. Both treatments provided equivalent clinical efficacy, with PP cure rates of 86.4% in the telithromycin group and 83.1% in the cefuroxime axetil group. Among mITT patients, 78.0% of telithromycin-treated patients and 72.3% of cefuroxime-treated patients were assessed as clinical cures at the TOC visit. When causative pathogens were identified, a satisfactory bacteriologic outcome was achieved in 76.0% of patients in the telithromycin group vs 78.6% in the cefuroxime axetil group. Relapses or re-infection occurred in a small number of patients in both groups at the late post-therapy visit (10 in the telithromycin group and 8 in the cefuroxime axetil group).
The third study of telithromycin in patients with AECB compared the clinical and bacteriologic efficacy of telithromycin 800 mg once daily for 5 days and clarithromycin 500 mg twice daily for 10 days (CitationFogarty, de Wet, et al 2005). Clinical cure (PP population) was achieved in 85.8% and 89.2% of telithromycin and clarithromycin recipients, respectively. Among mITT patients, 83.0% of telithromycin-treated patients and 83.7% of clarithromycin-treated patients were assessed as clinical cures. Eradication (presumed or documented) of the causative pathogen occurred in 81.9% of telithromycin patients vs 82.9% of clarithromycin patients.
Overall, an analysis of pooled data from the 3 studies indicated similar levels of clinical and bacteriologic efficacy for telithromycin and comparator antibacterials (CitationFogarty, Zervos, et al 2005). Per-protocol clinical cure rates at TOC were 86.0% (413/480) and 85.8% (416/485) for telithromycin and comparators, respectively, and a satisfactory bacteriologic outcome was achieved in 77.2% (105/136) of telithromycin-treated patients and 79.1% (106/134) of comparator-treated patients. Clinical cure rates were also comparable between telithromycin and comparator antibacterials for patients in subgroups of special interest, including those aged ≥ 65 years (85.3% [157/184] vs 83.3% [179/215]), patients with significant airway obstruction (77.1% [64/83] vs 81.4% [79/97]), patients with severe AECB (as assessed by the investigator) (84.4% [38/45] vs 75.0% [36/48]), and patients who smoked (86.8% [190/219] vs 85.4% [169/189]) (CitationFogarty, Zervos, et al 2005). Across the 3 studies, clinical cure rates by pathogen in patients who received 5 days of telithromycin treatment were 81.5% (22/27) for S. pneumoniae, 73.3% (44/60) for H. influenzae, and 93.1% (27/29) for M. catarrhalis (CitationFogarty, Zervos, et al 2005).
Health outcomes Healthcare resource utilization (including unscheduled AECB-related outpatient visits, emergency room [ER] visits, hospitalizations, and time lost from work) has been documented in one of the AECB clinical trials (comparing telithromycin with clarithromycin) (CitationFogarty, de Wet, et al 2005). Percentages of patients attending an unscheduled AECB-related ER visit were lower in the telithromycin group compared with the clarithromycin group (0% vs 2.8%, respectively), fewer telithromycin-treated patients were hospitalized owing to AECB (0.4% vs 1.4%, respectively), and fewer employed telithromycin patients reported days lost from work (23% vs 31%, respectively) (CitationFogarty, de Wet, et al 2005). These differences in healthcare resource use were estimated to contribute to a direct cost saving of approximately US$146 per patient.
Community-acquired pneumonia
The efficacy of telithromycin 800 mg for 5 or 7–10 days has been evaluated in a total of 9 trials involving adult or adolescent patients with a radiologically confirmed diagnosis of CAP (CitationHagberg et al 2002; Citationvan Rensburg et al 2002; CitationCarbon, Moola, et al 2003; CitationPullman et al 2003; CitationMathers Dunbar et al 2004; CitationTellier, Niederman, et al 2004; CitationFogarty, Patel, et al 2005; CitationMouton et al 2005) ().
Clinical and bacteriologic efficacy
Administration of telithromycin for 7–10 days in 2 open-label studies (Citationvan Rensburg et al 2002; CitationCarbon, Moola, et al 2003) resulted in clinical cure rates of 92.9% and 93.6% among PPc patients (cure rates in the mITT populations were 79.6% and 85.8%, respectively). The remaining 2 open-label studies in which the duration of telithromycin treatment was 7 days (CitationFogarty, Patel, et al 2005) reported PPc clinical cure rates of 89.6% and 93.0%, with corresponding mITT cure rates of 83.1% and 85.4%.
In 3 of the comparative trials, a 7- to 10-day regimen of telithromycin once daily was shown to be as clinically effective (PPc population) as amoxicillin 1000 mg 3-times daily for 10 days (94.6% vs 90.1%) (CitationHagberg et al 2002), clarithromycin 500 mg twice daily for 10 days (88.3% vs 88.5%) (CitationMathers Dunbar et al 2004), and trovofloxacin 200 mg once daily for 7–10 days (90.0% vs 94.2%) (CitationPullman et al 2003). In the mITT populations, telithromycin clinical success rates were 85.9%, 78.9%, and 82.0% vs 78.5%, 84.6%, and 85.6% for amoxicillin, clarithromycin, and trovofloxacin, respectively. The fourth comparative study demonstrated that telithromycin administered once daily for either 5 or 7 days was as effective as clarithromycin 500 mg twice daily for 10 days with PPc clinical cure rates of 89.3%, 88.8%, and 91.8%, respectively (CitationTellier, Niederman, et al 2004). The corresponding mITT cure rates were 82.4%, 82.2%, and 81.2%.
Across the 8 studies described above, a satisfactory bacteriologic outcome was achieved in 80.0%–92.9% of telithromycin-treated patients at the TOC visit (PP population) (). Among patients who received a comparator antibacterial, a satisfactory bacteriologic outcome was achieved in 83.3%–100% of patients (amoxicillin, 87.5%; clarithromycin, 96.4% and 83.3%; trovafloxacin, 100%). Pooled analyses of data from the 8 CAP studies have been used to investigate the efficacy of telithromycin in subgroups of patients, including those with infections caused by pneumococci resistant to penicillin and/or erythromycin (Citationvan Rensburg et al 2005), patients infected with atypical–intracellular pathogens (CitationDunbar et al 2005), and patients with pneumococcal bacteremia (CitationCarbon, van Resnburg, et al 2003). Among a total of 37 PP patients infected with S. pneumoniae isolates resistant to penicillin and/or erythromycin, 33 (89.2%) achieved clinical cure and eradication of the causative pathogen at TOC (Citationvan Rensburg et al 2005). The clinical success rate was also high for telithromycin-treated patients infected with atypical– intracellular pathogens: C. pneumoniae, 34/36 (94.4%); M. pneumoniae, 36/37 (97.3%); and L. pneumophila, 13/13 (100%) (CitationDunbar et al 2005). Among telithromycin-treated patients with documented pneumococcal bacteremia (n = 82; PP population), clinical cure and bacterial eradication rates were 90.2% (74/82) and 93.9% (77/82), respectively (CitationCarbon, van Rensburg, et al 2003).
Recent data from a randomized, open-label, comparative, international study (Study 4015) suggest that telithromycin may be more effective in treating CAP than a range of commonly prescribed antibiotics, at least in areas where pneumococcal macrolide resistance is high (CitationMouton et al 2005). The study was conducted in 9 countries with documented erythromycin resistance rates ≥ 30% (Greece, Hong Kong, Hungary, South Africa, South Korea, Spain, Taiwan, Thailand, and Tunisia). Adults with CAP received either telithromycin 800 mg once daily for 7–10 days or a comparator oral antibiotic usually prescribed locally and/or recommended by local guidelines (β-lactam, macrolide, or fluoroquinolone). The clinical cure rate in the telithromycin group was significantly higher than that in the comparator group (mITT population): 86.0% vs 78.8%, respectively (p = 0.04). The results of this trial are the first to demonstrate superior clinical efficacy of telithromycin over comparators.
Health outcomes
CAP-associated healthcare resource utilization data were collected for telithromycin- and comparator-treated patients enrolled in two of the four comparator-controlled clinical trials (CitationNiederman et al 2004a; CitationTellier, Chang, et al 2004). The similar design of these studies and the use of a common comparator agent (clarithromycin) allowed data from the two studies to be pooled to investigate whether there were any differences in overall healthcare resource utilization associated with telithromycin for 5, 7, or 10 days vs clarithromycin for 10 days (CitationNiederman et al 2004b). Although the two treatments showed equivalent clinical efficacy, telithromycin treatment (for 5, 7, or 10 days) was associated with significantly fewer CAP-related hospitalizations (1.2 vs 3.6 per 100 patients) and CAP-related days spent in hospital (8.8 vs 33.8 days per 100 patients). Similar differences were noted among subsets of patients with risk factors for morbidity. Among patients aged ≥ 65 years, there were 0 vs 6.6 hospitalizations and 0 vs 64.5 hospital days per 100 patients receiving telithromycin and clarithromycin, respectively. In patients with a Fine score > II, there were 2.1 hospitalizations per 100 telithromycin-treated patients vs 9.5 hospitalizations per 100 clarithromycin patients (resulting in 15.6 vs 94.6 hospital days per 100 patients, respectively [CitationNiederman et al 2004b]). Overall, CAP-related hospitalization costs were significantly lower for telithromycin recipients, resulting in an estimated cost saving of US$ 302 per patient.
Safety and tolerability
The safety and tolerability of telithromycin have been evaluated in a total of 16 Phase III clinical trials, comprising 14 of the 16 studies presented in (Study 4017 and Study 4015 were not included in the analysis) and 2 studies conducted in patients with tonsillitis–pharyngitis. The safety-evaluable population included all patients who received at least 1 dose of study medication and had at least 1 safety assessment following randomization.
Telithromycin 800 mg once daily for up to 10 days was generally well tolerated and most adverse events (AEs) were of mild to moderate intensity. In the 11 comparator-controlled clinical trials, 1348 patients out of a total of 2702 (49.9%) treated with telithromycin reported at least 1 treatment-emergent AE (TEAE) compared with 48.4% (1035/2139) of comparator-treated patients. TEAEs considered by the investigator to be possibly related to study medication were reported in 31.9% (861/2702) of telithromycin-treated patients and 28.3% (606/2139) of comparator-treated patients participating in the 11 trials. The most frequent of these possibly related TEAEs (occurring in >1% of patients in either treatment group) are listed in . Overall, TEAEs of the gastrointestinal and nervous system were the most commonly reported events possibly related to study medication. Incidences of blurred vision were reported more frequently with telithromycin (30/2702 [1.1%]) than with comparator antibacterials (6/2139 [0.28%]). Fifteen of the 20 cases reported in telithromycin-treated patients were considered by the investigator to be possibly related to study medication. The events were generally mild in intensity and started most frequently within the first 2 days of treatment. All cases resolved without sequelae and no cases were reported as serious AEs. Rates of discontinuation of study medication were similar between telithromycin and comparator agents (4.4% and 4.3%, respectively). Most treatment discontinuations were attributable to gastrointestinal TEAEs, primarily diarrhea (telithromycin 0.9%, comparators 0.6%) and vomiting (telithromycin 0.8%, comparators, 0.5%). Clinically noteworthy elevations in liver enzymes (eg, aspartate aminotransferase and alanine aminotransferase) occurred in similar proportions of telithromycin-treated (1.2% and 1.6%, respectively) and comparator-treated patients (1.3% and 1.7%, respectively). Overall, 1.1% of patients in the controlled Phase III trials reported treatment-emergent visual AEs compared with 0.28% of patients who received comparators (CitationAventis 2005). Visual disturbances included blurred vision, diplopia, or difficulty focusing. Most of these events were mild to moderate, although severe cases have been reported; all visual AEs were reversible upon cessation of drug treatment (CitationAventis 2005).
Table 4 Treatment-emergent adverse events (TEAEs) possibly related to study medication reported by >1% of patients in 11 comparator-controlled trials of telithromycin
Across all 16 trials, the incidence of death was low (telithromycin, 17/4472 [0.4%]; comparators, 9/2139 [0.4%]) and none of the deaths was considered to be related to study medication. Similarly, the frequency of serious TEAEs considered related to study medication was low and comparable across treatment groups in the controlled trials (telithromycin, 0.3% [9/2702]; comparators, 0.3% [6/2139]).
At therapeutic doses, telithromycin was associated with a small mean increase (1.5 milliseconds) in QTc interval. However, QTc outlier values were uncommon and similar in frequency to those seen with clarithromycin and nonmacrolide antibacterials, and no excess in risk for significant QTc prolongation was noted in telithromycin-treated patients, including those in at-risk populations.
Post-marketing surveillance
The results of a post-marketing surveillance study conducted in Germany (the country in which telithromycin was first introduced in 2001) provide the first broad-ranging evidence of the safety and effectiveness of telithromycin in routine practice (CitationLorenz and Roscher 2004). Data are available for a total of 34 929 outpatients with community-acquired RTIs treated with telithromycin 800 mg once daily for a median of 5 days (AECB, ABS, or tonsillitis–pharyngitis) or 7 days (CAP). There was a high level of satisfaction with telithromycin therapy, with 95.6% of physicians and 94.1% of patients reported to be satisfied or very satisfied. The overall rate of clinical cure or improvement with telithromycin was 98.4%. Adverse drug reactions were reported by 1.9% of patients and were predominately gastrointestinal in nature. Serious adverse drug reactions were observed in 32 patients (0.1%).
Rare occurrences of allergic reactions, atrial arrhythmias, syncope (usually associated with vagal syndrome), and exacerbations of myasthenia gravis have been reported in the worldwide post-marketing surveillance for telithromycin (CitationAventis 2005).
Interactions with other drugs
Telithromycin, like the macrolides, is both a substrate and an inhibitor of the CYP3A4 metabolic pathway. A number of specific drug interaction studies have been undertaken in humans to assess the effects of co-administration of telithromycin with CYP3A4 inhibitors, CYP3A4 inducers, and CYP3A4 substrates. In addition, drug interaction studies have been conducted with several other concomitantly prescribed drugs, including paroxetine and metoprolol, theophylline, warfarin, digoxin, class III antiarrhythmic agents, and oral contraceptives. The results of the drug interaction studies are summarized in .
Table 5 Interactions between telithromycin and commonly prescribed drugsTable Footnotea
CYP3A4 inhibitors
The azole antifungals ketoconazole and itraconazole both strongly inhibit hepatic CYP3A4. However, although both ketoconazole and itraconazole moderately increased telithromycin Cmax and AUC (CitationShi, Montay, et al 2005), the extent of these increases is considered unlikely to be clinically relevant. Grapefruit juice (components of which inhibit intestinal, but not hepatic, CYP3A4) had no effect on telithromycin pharmacokinetics during co-administration (CitationShi, Montay, et al 2005).
Rifampicin and other CYP3A4 inducers
Rifampicin (a potent inducer of hepatic metabolic enzymes, including CYP3A4) has been shown to reduce serum levels of clarithromycin (CitationKolars et al 1992). Similarly, concomitant administration of telithromycin with rifampicin reduced telithromycin exposure by approximately 80% (CitationAventis 2005). Co-administration of the two drugs is therefore not recommended.
CYP3A4 substrates
Administration of telithromycin with the CYP3A4 substrates cisapride and pimozide is contraindicated owing to the potential for prolongation of the cardiac QTc interval if exposure to cisapride or pimozide is increased. Following oral co-administration with the benzodiazepine midazolam, the plasma level of oral midazolam was increased 6-fold (CitationAventis 2005); patients receiving oral midazolam and telithromycin concomitantly should therefore be monitored, and a reduction in the midazolam dose should be considered if necessary. In a study with healthy volunteers, Cmax and AUC of simvastatin increased 5.3-fold and 8.9-fold, respectively, during co-administration with telithromycin (CitationAventis 2005). In a second study in healthy patients in which simvastatin and telithromycin were administered 12 hours apart, there was much lower increase in simvastatin Cmax and AUC (3.4-fold and 4.0-fold, respectively) (CitationAventis 2005). Nevertheless, it is recommended that co-administration of telithromycin with simvastatin, lovastatin, or atorvastatin should be avoided; if telithromycin is prescribed, therapy with any of these statins should be suspended during the course of treatment. Pravastatin and fluvastatin are not extensively metabolized by CYP3A4; therefore, telithromycin is not expected to interact with these agents.
Other drug interactions
In line with previous drug interaction studies involving macrolides, telithromycin has been shown to exert moderate effects on the pharmacokinetics of theophylline. The increased exposure to theophylline when co-administered with telithromycin may increase the incidence of gastrointestinal side-effects, and it is recommended that the two drugs be given 1 hour apart (CitationBhargava, Leroy, et al 2002). Although telithromycin has been observed to increase digoxin levels, no significant changes in electrocardiogram parameters occurred and there were no signs of digoxin toxicity (CitationMontay et al 2002). However, as suggested for macrolides, digoxin side-effects or serum levels should be monitored during concomitant administration with telithromycin. Post-marketing reports suggest that telithromycin could potentiate the effects of oral anticoagulants when the two drugs are co-administered. Consideration should therefore be given to monitoring prothrombin times/International Normalized Ratio (INR) while patients are receiving telithromycin and oral anticoagulants simultaneously (similar interactions between warfarin and macrolides have been reported).
No clinically significant interactions have been observed between telithromycin and antacids containing aluminum or magnesium. In addition, telithromycin does not influence the anti-ovulatory effects of oral contraceptives containing ethinylestradiol or levonorgestrel (CitationScholtz et al 2000).
Conclusions
The ketolide antibacterial telithromycin demonstrates potent activity against key common RTI pathogens, including S. pneumoniae isolates resistant to other antibiotics and atypical–intracellular organisms, and has a low potential to select for or induce resistance. In European countries where telithromycin has been available for clinical use since 2001– 2002, antibiotic surveillance data indicate that telithromycin has retained in vitro activity against the common respiratory pathogens, including penicillin-resistant or macrolide-resistant strains of S. pneumoniae (> 99% susceptible) (CitationReinert, Felmingham, et al 2004; CitationReinert, Rodloff, et al 2004). Clinical data support these in vitro findings, with results of randomized controlled studies showing telithromycin 800 mg once daily to be as effective as current standards of care for the treatment of ABS, AECB, and CAP. Telithromycin displays pharmacokinetic parameters allowing for once-daily dosing; in addition, it can be administered in short-duration therapy for most RTI indications, which may encourage good patient compliance. In clinical trials, treatment with telithromycin was well tolerated, with AEs being typically mild to moderate in intensity, transient in nature, and occurring at rates comparable with those seen with other first-line treatment options. Assessment of additional healthcare resource utilization in patients with CAP and AECB also indicates that telithromycin may be associated with reduced rates of hospitalization compared with standard first-line treatment options, suggesting the potential for significant cost savings with wider use.
In summary, based on clinical experience to date, telithromycin is an appropriate option for the treatment of community-acquired ABS, AECB, and mild to moderate CAP.
References
- AubierMAldonsPMLeakATelithromycin is as effective as amoxicillin/clavulanate in acute exacerbations of chronic bronchitisRespir Med2002968627112418583
- AventisKetek® (telithromycin) briefing document for the FDA anti-infective drug products advisory committee meeting [online]2003 Accessed 27 June 2005 URL: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3919B1_01_Aventis-KETEK.pdf
- Aventis2005 Ketek® (telithromycin) tablets [package insert]. August.
- BanNNissenPHansenJThe complete atomic structure of the large ribosomal subunit at 2.4 A resolutionScience20002899052010937989
- BarryALFuchsPCBrownSDIn vitro activities of the ketolide HMR 3647 against recent gram-positive clinical isolates and Haemophilus influenzaeAntimicrob Agents Chemother1998422138409687424
- BartlettJGDowellSFMandellLAPractice guidelines for the management of community-acquired pneumonia in adultsClin Infect Dis2000313478210987697
- BébéarCMRenaudinHBryskierAComparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmasAntimicrob Agents Chemother2000441980210858366
- Bemer-MelchiorPJuvinMETassinSIn vitro activity of the new ketolide telithromycin compared with those of macrolides against Streptococcus pyogenes: influences of resistance mechanisms and methodological factorsAntimicrob Agents Chemother2000442999300211036012
- BermudezLEInderliedCBWuMActivities of ABT-773, telithromycin and linezolid against Mycobacterium avium (MAC) in vitro and in vivo [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesMarch 16, 2000Seville, Spain 03.16
- BerthoGGharbi-BenarousJDelaforgeMConformational analysis of ketolide, conformations of RU 004 in solution and bound to bacterial ribosomesJ Med Chem1998413373869719590
- BhargavaVLenfantBPerretCLack of effect of food on the bioavailability of a new ketolide antibacterial, telithromycinScand J Infect Dis200234823612578152
- BhargavaVLeroyBShiJEffect of telithromycin on pharmacokinetics of theophylline in healthy volunteers [Abstract]2002Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2002San Diego, CA, USA A-1832
- BiedenbachDJBarrettMSJonesRNComparative antimicrobial activity and kill-curve investigations of novel ketolide antimicrobial agents (HMR 3004 and HMR 3647) tested against Haemophilus influenzae and Moraxella catarrhalis strainsDiagn Microbiol Infect Dis199831349539635909
- BonnefoyAGirardAMAgouridasCKetolides lack inducibility properties of MLS(B) resistance phenotypeJ Antimicrob Chemother19974085909249208
- BonnefoyAGuittonMDelachaumeCIn vivo efficacy of the new ketolide telithromycin (HMR 3647) in murine infection modelsAntimicrob Agents Chemother20014516889211353612
- BoswellFJAndrewsJMAshbyJPThe in-vitro activity of HMR 3647, a new ketolide antimicrobial agentJ Antimicrob Chemother199842703910052892
- BoswellFJAndrewsJMWiseRPharmacodynamic properties of HMR 3647, a novel ketolide, on respiratory pathogens, enterococci and Bacteroides fragilis demonstrated by studies of time-kill kinetics and postantibiotic effectJ Antimicrob Chemother199841149539533455
- BrownSDRybakMJAntimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001–2002, as part of the PROTEKT US studyJ Antimicrob Chemother200454Suppl 1i71515265831
- BuchananPPStephensTALeroyBA comparison of the efficacy of telithromycin versus cefuroxime axetil in the treatment of acute bacterial maxillary sinusitisAm J Rhinol2003173697714750614
- CapobiancoJOCaoZShortridgeVDStudies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniaeAntimicrob Agents Chemother2000441562710817709
- CarbonCMoolaSVelancsicsITelithromycin 800 mg once daily for seven to ten days is an effective and well-tolerated treatment for community-acquired pneumoniaClin Microbiol Infect2003969170312925111
- CarbonCvan RensburgDFogartyCFive- to 10-day therapy with telithromycin is effective in outpatients with pneumococcal bacteraemia associated with community-acquired pneumonia [Abstract]2003Abstracts of the 13th European Congress of Clinical Microbiology and Infectious DiseasesJune 2003Glasgow, UK P1129
- ChampneyWSToberCLStructure–activity relationships for six ketolide antibioticsCurr Microbiol2001422031011270656
- CiervoCAShiJOutline Pharmacokinetics of telithromycin: application to dosing in the treatment of community-acquired respiratory tract infectionsCurr Med Res Opin20052116415016238904
- DaviesTADewasseBEJacobsMRIn vitro development of resistance to telithromycin (HMR 3647), four macrolides, clindamycin, and pristinamycin in Streptococcus pneumoniaeAntimicrob Agents Chemother2000444141710639373
- DouthwaiteSChampneySStructures of ketolides and macrolides determine their mode of interaction with the ribosomal target siteJ Antimicrob Chemother200148Suppl. T118
- DouthwaiteSMauvaisPChampneyWSStructure–activity relationship of the ketolide telithromycin (HMR 3647) [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesApril 2000Seville, Spain 02.02
- DuboisJSt-PierreCIntracellular activity and post-antibiotic effect of ABT-773 against Legionella [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesApril 2000Seville, Spain 02.33
- DunbarLMCarbonCvan RensburgDEfficacy of telithromycin in community-acquired pneumonia caused by atypical and intracellular pathogensInfect Dis Clin Pract2005131016
- EdlundCAlvanGBarkholtLPharmacokinetics and comparative effects of telithromycin (HMR 3647) and clarithromycin on the oropharyngeal and intestinal microfloraJ Antimicrob Chemother200046741911062193
- FarrellDJFelminghamDActivities of telithromycin against 13,874 Streptococcus pneumoniae isolates collected between 1999 and 2003Antimicrob Agents Chemother2004481882415105150
- FelminghamDFarrellDJIn vitro activity of telithromycin against Gram-negative bacterial pathogensJ Infect2006521788015996744
- FergusonBJGuzzettaRVSpectorSLEfficacy and safety of oral telithromycin once daily for 5 days versus moxifloxacin once daily for 10 days in the treatment of acute bacterial rhinosinusitisOtolaryngol Head Neck Surg20041312071415365537
- Fernandez-RoblasRCalvoREstebanJThe bactericidal activities of HMR 3004, HMR 3647 and erythromycin against gram-positive bacilli and development of resistanceJ Antimicrob Chemother199943285911252337
- FileTMJrStreptococcus pneumoniae and community-acquired pneumonia: a cause for concernAm J Med2004117Suppl 3AS3950
- FogartyCde WetRMandellLFive-day telithromycin once daily is as effective as 10-day clarithromycin twice daily for the treatment of acute exacerbations of chronic bronchitis and is associated with reduced healthcare resource utilizationChest20051281980816236845
- FogartyCZervosMTellierGTelithromycin for the treatment of acute exacerbations of chronic bronchitisInt J Clin Pract2005b5929630515857326
- FogartyCMPatelTCDunbarLMEfficacy and safety of telithromycin 800 mg once daily for 7 days in community-acquired pneumonia: an open-label, multicenter studyBMC Infect Dis2005c54315927060
- GehannoPSultanEPassotVTelithromycin (HMR 3647) achieves high and sustained concentrations in tonsils of patients undergoing tonsillectomyInt J Antimicrob Agents200321441512727077
- HagbergLTorresAvan RensburgDEfficacy and tolerability of once-daily telithromycin compared with high-dose amoxicillin for treatment of community-acquired pneumoniaInfection2002303788612478329
- HansenLHMauvaisPDouthwaiteSThe macrolide–ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNAMol Microbiol1999316233110027978
- JenkinsSGFarrellDJPatelMTrends in anti-bacterial resistance among Streptococcus pneumoniae isolated in the USA, 2000–2003: PROTEKT US years 1–3J Infect2005513556315950288
- KhairOAAndrewsJMHoneybourneDLung concentrations of telithromycin after oral dosingJ Antimicrob Chemother2001478374011389116
- KlugmanKPLonksJRHidden epidemic of macrolide-resistant pneumococciEmerg Infect Dis200511802715963272
- KohnoSHobanDand the PROTEKT Surveillance Study GroupComparative in vitro activity of telithromycin and beta-lactam antimicrobials against bacterial pathogens from community-acquired respiratory tract infections: data from the first year of PROTEKT (1999–2000)J Chemother2003153354112962361
- KolarsJCSchmiedlin-RenPSchuetzJDIdentification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytesJ Clin Invest199290187181430211
- LeclercqRResistance to ketolides: role of ribosomal modification and macrolide efflux [Abstract]2000Abstracts of the 40th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2000Toronto, Canada 1135
- LeclercqRCourvalinPBacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modificationAntimicrob Agents Chemother199135126772 Erratum in: Antimicrob Agents Chemother, 35:2165.1929280
- LorenzJRoscherKEfficacy and safety of telithromycin in 34,929 patients with respiratory tract infections [Abstract]2004Abstracts of the 44th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2004Washington, DC, USA L-914
- LutermanMTellierGLaskoBEfficacy and tolerability of telithromycin for 5 or 10 days vs amoxicillin/clavulanic acid for 10 days in acute maxillary sinusitisEar Nose Throat J2003825768014503094
- MankinAXiongLKhaitovichPInteraction of macrolides and ketolides with the ribosome [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesApril 2000Seville, Spain 01.02
- Mathers DunbarLHassmanJTellierGEfficacy and tolerability of once-daily oral telithromycin compared with clarithromycin for the treatment of community-acquired pneumonia in adultsClin Ther200426486214996517
- MauvaisPBonnefoyALack of in vitro MLSB resistance induction by the ketolide telithromycin (HMR 3647): role of the 3-keto group [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesApril 2000Seville, Spain 02.10
- MiyamotoNMurakamiSYajinKPharmacokinetic study of a new ketolide antimicrobial telithromycin (HMR 3647) in otorhinolaryngology [Abstract]2000Abstracts of the 40th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2000Toronto, Canada 2144
- MontayGShiJLeroyBBhargavaVEffects of telithromycin on the pharmacokinetics of digoxin in healthy men [Abstract]2002Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2002San Diego, CA, USA A-1834
- MoutonYThamlikitkulVNiemanRBTelithromycin versus other first-line single-agent antibiotics in the treatment of community-acquired pneumonia: a randomized superiority trialClin Microbiol Infect Dis200511Suppl 2S267
- Muller-SerieysCAndrewsJVacheronFTissue kinetics of telithromycin, the first ketolide antibacterialJ Antimicrob Chemother2004531495714729764
- Muller-SerieysCSolerPCantalloubeCBronchopulmonary disposition of the ketolide telithromycin (HMR 3647)Antimicrob Agents Chemother2001453104811600363
- NamourFWesselsDHPascualMHPharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple dosesAntimicrob Agents Chemother200145170511120961
- NiedermanMSChangJRStewartJComparison of hospitalization rates in patients with community-acquired pneumonia treated with 10 days of telithromycin or clarithromycinCurr Med Res Opin2004a2074956 Erratum in: Curr Med Res Opin 20:1331.15140342
- NiedermanMSChangJRStewartJHospitalization rates among patients with community-acquired pneumonia treated with telithromycin vs clarithromycin: results from two randomized, double-blind, clinical trialsCurr Med Res Opin2004b209698015265241
- NiedermanMSMcCombsJSUngerANThe cost of treating community-acquired pneumoniaClin Ther199820820379737840
- NiedermanMSMcCombsJSUngerANTreatment cost of acute exacerbations of chronic bronchitisClin Ther1999215769110321424
- NovotnyGWAndersenNMPoehlsgaardJTelithromycin interacts directly with the base of A752 in domain II of 23S ribosomal RNA, in contrast to erythromycin and clarithromycin [Abstract]2001Abstracts of the 11th European Congress of Clinical Microbiology and Infectious DiseasesJune 2001Istanbul, Turkey P480
- OdenholtILowdinECarsOPharmacodynamics of telithromycin in vitro against respiratory tract pathogensAntimicrob Agents Chemother20014523911120939
- PankuchGAHoellmanDBLinGActivity of HMR 3647 compared to those of five agents against Haemophilus influenzae and Moraxella catarrhalis by MIC determination and time-kill assayAntimicrob Agents Chemother199842303249797250
- PankuchGAVisalliMAJacobsMRSusceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agentsAntimicrob Agents Chemother199842624309517943
- PerretCLenfantBWeinlingEPharmacokinetics and absolute oral bioavailability of an 800-mg oral dose of telithromycin in healthy young and elderly volunteersChemotherapy2002482172312476037
- Pham GiaHRoederVNamourFThe new ketolide, HMR 3647, achieves high and sustained concentrations in white blood cells in man [Abstract]J Antimicrob Chemother199944Suppl A57P79
- PullmanJChamplinJVroomanPSJrEfficacy and tolerability of once-daily oral therapy with telithromycin compared with trovafloxacin for the treatment of community-acquired pneumonia in adultsInt J Clin Pract2003573778412846341
- ReinertRRFelminghamDthe EU PROTEKT Study GroupSustained antimicrobial activity of telithromycin in Europe post-launch: the PROTEKT study (Years 1–4) [Abstract]2004Abstracts of the 44th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2004Washington, DC, USA C2-813
- ReinertRRRodloffACHalleEAntibacterial resistance of community-acquired respiratory tract pathogens recovered from patients in Germany and activity of the ketolide telithromycin: results from the PROTEKT surveillance study (1999–2000)Chemotherapy2004501435115272227
- RobertsMCSutcliffeJCourvalinPNomenclature for macrolide and macrolide–lincosamide–streptogramin B resistance determinantsAntimicrob Agents Chemother19994328233010582867
- RoblinPMHammerschlagMRIn vitro activity of a new ketolide antibiotic, HMR 3647, against Chlamydia pneumoniaeAntimicrob Agents Chemother1998421515169624507
- RoosKBrunswig-PitschnerCKostricaREfficacy and tolerability of once-daily therapy with telithromycin for 5 or 10 days for the treatment of acute maxillary sinusitisChemotherapy200248100812011543
- RoosKTellierGBazMClinical and bacteriological efficacy of 5-day telithromycin in acute maxillary sinusitis: a pooled analysisJ Infect2005502102015780415
- RzeszutekMWierzbowskiAHobanDJA review of clinical failures associated with macrolide-resistant Streptococcus pneumoniaeInt J Antimicrob Agents2004249510415288306
- SchitoGCMarcheseAElkharratDComparative activity of telithromycin against macrolide-resistant isolates of Streptococcus pneumoniae: results of two years of the PROTEKT surveillance studyJ Chemother200416132215077994
- SchlunzenFZarivachRHarmsJStructural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteriaNature20014138142111677599
- ScholtzHESultanEWesselsDTelithromycin (HMR 3647), a new ketolide antimicrobial, does not affect the reliability of low-dose, triphasic oral contraceptives [Abstract]2000Abstracts of the 5th International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and OxazolidinonesApril 2000Seville, Spain 09.29
- ShiJMontayGHardyPEffects of itraconazole or grapefruit juice on the pharmacokinetics of telithromycinPharmacotherapy200525425115767219
- ShiJPfisterMJenkinsSGPharmacodynamic analysis of the microbiological efficacy of telithromycin in patients with community-acquired pneumoniaClin Pharmacokinet2005443172915762772
- SultanENamourFMauriacCHMR 3647, a new ketolide antimicrobial, is metabolized and excreted mainly in feces in manJ Antimicrob Chemother199944Suppl A54
- SutcliffeJTait-KamradtAWondrackLStreptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux systemAntimicrob Agents Chemother1996401817248843287
- Tait-KamradtADaviesTCronanMMutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passageAntimicrob Agents Chemother20004421182510898684
- TellierGChangJRAscheCVComparison of hospitalization rates in patients with community-acquired pneumonia treated with telithromycin for 5 or 7 days or clarithromycin for 10 daysCurr Med Res Opin2004207394715140341
- TellierGNiedermanMSNusratRClinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumoniaJ Antimicrob Chemother2004545152315269191
- van RensburgDJFogartyCKohnoSEfficacy of telithromycin in community-acquired pneumonia caused by pneumococci with reduced susceptibility to penicillin and/or erythromycinChemotherapy2005511869215980629
- van RensburgDJMatthewsPALeroyBEfficacy and safety of telithromycin in community-acquired pneumoniaCurr Med Res Opin20021839740012487505
- WatanukiYOdagiriSOguraTA new ketolide antibacterial, telithromycin, achieves high and sustained concentrations in sputum in patients [Abstract]2001Abstracts of the 22nd International Congress of ChemotherapyJune 2001Amsterdam, The Netherlands P9.007
- XiongLShahSMauvaisPA ketolide resistance mutation in domain II of 23S rRNA reveals the proximity of hairpin 35 to the peptidyl transferase centreMol Microbiol199931633910027979
- ZervosMJHeyderAMLeroyBOral telithromycin 800 mg once daily for 5 days versus cefuroxime axetil 500 mg twice daily for 10 days in adults with acute exacerbations of chronic bronchitisJ Int Med Res2003311576912870368
- ZhanelGGJohansonCHisanagaTPharmacodynamic activity of telithromycin against macrolide-susceptible and macrolide-resistant. Streptococcus pneumoniae simulating clinically achievable free serum and epithelial lining fluid concentrationsJ Antimicrob Chemother2004541072715531596
- ZhanelGGJohansonCLaingNPharmacodynamic activity of telithromycin at simulated clinically achievable free-drug concentrations in serum and epithelial lining fluid against efflux (mef(E)-producing macrolide-resistant Streptococcus pneumoniae for which telithromycin MICs varyAntimicrob Agents Chemother2005491943815855517