Abstract
Community-acquired pneumonia (CAP) is the cause of substantial morbidity, mortality, and resource utilization worldwide. When choosing an antimicrobial, effective treatment depends on proper patient evaluation and the identification of numerous risk factors, such as recent antibiotic exposure or the presence of comorbidity. Patients without any risk factor should be treated effectively with a narrow spectrum β-lactam agent, like amoxicillin, or a macrolide. If a risk factor is present, agents with a broader spectrum of activity should be selected for the empirical therapy. The newer-generation quinolones are suitable agents with their excellent in vitro activity and pharmacodynamic–pharmacokinetic properties. They are not only active against susceptible CAP pathogens, but also against the resistant strains. Among the quinolones, gemifloxacin has the best in vitro activity. Its improved bioavailability, pharmacokinetic–pharmacodynamic properties, and safety profile make this agent an excellent option for the treatment of CAP.
Introduction
Community-acquired pneumonia (CAP) remains a frequent and important clinical entity. Each year, there are 2 to 3 million cases of CAP in the US, resulting in approximately 10 million physician visits (CitationBartlett et al 2000). Despite substantial progress in therapeutic options, CAP remains a significant cause of morbidity and death, and there continue to be major controversies concerning the antimicrobial management of this infection (CitationPaganin et al 2004). The mixed etiology and the changing susceptibility of pathogens causing CAP, in particular that of Streptococcus pneumoniae, has created a challenge, in some circumstances, to clinicians as to which therapeutic approaches may be the most appropriate in terms of optimal patient outcome (CitationOncu et al 2005). Initial antimicrobial therapy is normally given empirically, before the bacterial cause of the infection can be determined in the laboratory, and in many cases treatment is empirical throughout due to the lack of reliable microbiological data. An understanding of the possible pathogens and resistance patterns is helpful in guiding antibiotic choice, and a detailed knowledge of the local susceptibility of the potential pathogens would ensure a more appropriate selection of the antimicrobial agent to be used (CitationAppelbaum et al 2004). This review focuses on the treatment options of CAP with special emphasis on gemifloxacin.
Etiology of CAP
Although CAP may be caused by many possible pathogens, a limited number of common pathogens are responsible for most cases (CitationLim et al 2001). In fact, no etiologic agent is found in as many as 50% of cases, even when extensive diagnostic testing is performed (CitationNiederman et al 2001). In those cases in which an etiologic agent is identified, S. pneumoniae accounts for the majority of bacterial pneumonia (CitationJokinen et al 2001). Relative to other pathogens, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, and respiratory viruses are also common.
Staphylococcus aureus and Enterobacteriaceae pathogens are found in a selected group of patients such as those who have had influenza, have previously taken antimicrobial drugs, or have comorbidities (CitationArancibia et al 2002). lists the most common pathogens associated with CAP based on the collective results of recent studies and based on the severity of illness as judged by the site of care (outpatient versus inpatient) (CitationFile 2003; CitationFile and Niederman 2004).
Table 1 Microbiological etiology in CAP by site of care
Of the respiratory pathogens, penicillin-resistant S. pneumoniae (PRSP) has attracted the greatest interest. PRSP is a widespread problem, with rates of resistance ranging from 5% to 80% in various parts of the world (CitationForward 1999; CitationOncu et al 2005). Risk factors for infection with PRSP strains include young age, day-care center attendance, prior administration of antimicrobial agents, and severe underlying diseases (CitationClavo-Sanchez et al 1997). As the use of non-penicillin antimicrobials has increased, so has the development of resistance to these agents among S. pneumoniae. Worldwide rate of macrolide resistance has risen dramatically in recent years. The prevalence of resistance is highly variable between countries, ranging from <3% to >70% (CitationCizman et al 1999; CitationOster et al 1999). Emergence of S. pneumoniae with reduced susceptibility to quinolones has also been reported in England and the US (CitationBrueggemann et al 2002; CitationJohnson et al 2003). Fortunately, the incidence of quinolone resistance is currently low worldwide (<1%) (CitationGarcia-Rodriguez and Munoz Bellido 2000).
Outpatient vs inpatient treatment
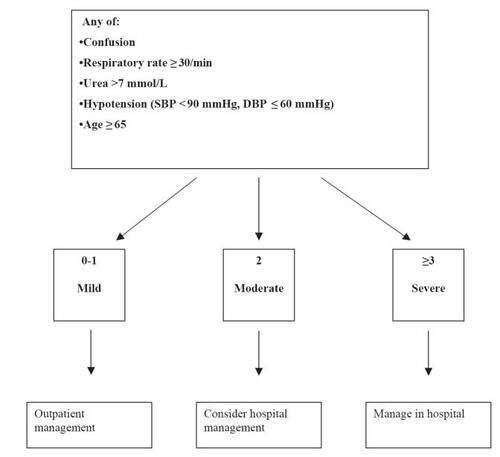
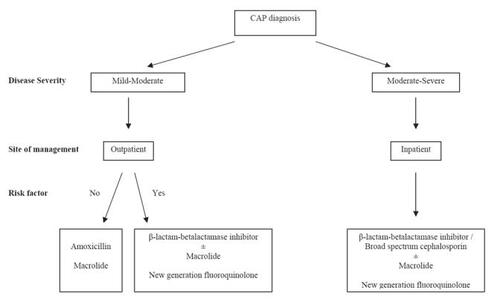
A clinical prediction rule has been tested based upon the likelihood of mortality from CAP (CitationFine et al 1997; CitationLim et al 2003). It is especially useful to predict the severity of CAP, to identify patients who can be safely treated as outpatients () (CitationLim et al 2003). Prediction of the severity of CAP is also used to estimate the possible etiology of CAP and guides the selection of empirical therapy. The approach to empirical antimicrobial drug selection in CAP is presented in .
Antimicrobial therapy
Ideally, the choice of antibacterial therapy for the empirical treatment of CAP will be one that is highly effective against the common respiratory pathogens, in particular S. pneumoniae, has a good safety profile with few adverse effects, and is formulated to achieve adequate dosing to eradicate the infecting pathogen.
β-lactam antibiotics
The relationship between the inappropriate use of antimicrobials and resistance might suggest that β-lactams would have reduced effectiveness in CAP caused by S. pneumoniae. Although this is the case for meningeal infections, the clinical relevance of resistance in the treatment of pneumonia remains controversial. Several studies have shown no difference in outcomes, including mortality, between patients with penicillin-susceptible and those with penicillin-resistant S. pneumoniae as the cause of CAP (CitationFriedland 1995; CitationPallares et al 1995; CitationChoi and Lee 1998; CitationDeeks et al 1999). It is likely that, in cases in which isolates have intermediate or low-level resistance to penicillin, the drug concentrations achieved in serum and in the lungs are adequate to eradicate these strains. However, strains for which the minimum inhibitory concentrations (MICs) of penicillin are higher (≥4 mg/L) may affect outcomes, and therapeutic failures are more likely to be seen as more strains with high-level penicillin resistance emerge (CitationPallares et al 2003). Although the impact of PRSP in CAP has been evaluated in several studies, the impact of cephalosporin resistance is less well studied. Several studies reported that cephalosporin resistance negatively affected the clinical outcomes of patients with CAP (CitationAilani et al 2002; CitationYu et al 2003; CitationGarau 2005). In pneumonia caused by other respiratory pathogens, such as H. influenzae and Moraxella catarrhalis, the impact of resistance caused by β-lactamase production can be overcome by the use of a β-lactam/β-lactamase inhibitor combination or a β-lactamase-stable cephalosporin (CitationGarau 2005).
Macrolides
Although the global increase in macrolide resistance in isolates of S. pneumoniae is disturbing, the clinical impact of these in vitro results has not been determined (CitationGotfried 2000). In vitro macrolide resistance may not translate into therapeutic failure if high tissue concentrations are achieved at the site of infection. This may be the case for infections caused by strains of S. pneumoniae with low-level macrolide resistance (MIC <8 μg/mL) through increased active efflux of antimicrobials (CitationShortridge et al 1999). On the other hand, the majority of pneumococci harboring the ermB gene exhibit high levels of resistance that cannot be overcome by clinical use of macrolides; therefore, failure is predictable if a macrolide is used in infections caused by strains harboring the ermB gene (CitationOster et al 1999). Several studies have examined macrolides in the treatment of CAP in outpatients who were subsequently hospitalized (CitationKelley et al 2000; CitationLonks et al 2002; CitationVan Kerkhoven et al 2003). The results of these studies suggest that breakthrough bacteremia is likely to occur during macrolide therapy for pneumonia due to the presence of macrolide-resistant S. pneumoniae strains. In addition, there have been several case reports and case series concerning macrolide failures in patients with pneumonia caused by macrolide-resistant strains (CitationFogarty et al 2000; CitationKelley et al 2000; CitationMusher et al 2002).
Based on current rates and level of macrolide resistance, continued use of this drug class is warranted for most patients with CAP. A macrolide alone should be an adequate treatment option for mild-to-moderate CAP in patients who have been healthy and are without risk factors for antibiotic resistance (recent macrolide use, age <5 or >65 years, daycare attendance, recent hospitalization, residence in a high prevalence area). Given the inability to predict antibiotic resistance, however, macrolides are not recommended for the treatment of complicated or life- threatening severe CAP.
Quinolones
Quinolones inhibit bacterial DNA gyrase and topoisomerase IV, thereby causing bacterial cell death (CitationLewis et al 1996; CitationO’Donnell and Gelone 2000). Topoisomerase IV is a key target for quinolones that have activity against Gram-positive organisms (CitationO’Donnell and Gelone 2000). The early quinolones, ciprofloxacin and ofloxacin, are not considered suitable for the treatment of CAP because of their low activity against Gram-positive bacteria (CitationO’Donnell and Gelone 2000). Recent years have seen the development of newer quinolones such as levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin, which have significantly improved activity against Gram-positive organisms (including PRSP) and macrolide-resistant strains (CitationGarcia-Rodriguez and Munoz Bellido 2000). These agents have enhanced activity against topoisomerase IV. They have rapid bactericidal activity and desirable pharmacokinetic and pharmacodynamic features (CitationGarcia-Rodriguez and Munoz Bellido 2000). These newer quinolones are also active against bacteria causing atypical pneumonia and against β-lactamase producing and non-producing H. influenzae and M. catarrhalis. Moreover, they have a good activity against other other Gram-negative bacilli, similar or even higher, in some cases, to ciprofloxacin. Current resistance is very low in these agents and so, from a theoretical point of view, their spectrum and intrinsic activity are suitable for the treatment of CAP.
Gemifloxacin
Gemifloxacin was approved by the FDA in 2003 to treat mild-to-moderate CAP caused by a range of pathogens. It is a fluoronaphthyridone possessing a C-7 pyrrolidine substitution. The commercially available product is gemifloxacin mesylate salt in the sesquihydrate form. Gemifloxacin is available in tablet formulation, with each tablet containing gemifloxacin mesylate equivalent to 320 mg of gemifloxacin (CitationHong 2001).
Mechanism of action and resistance
Gemifloxacin possesses a strong affinity for topoisomerase IV, which is likely to be responsible for its potent in vitro activity against S. pneumoniae (CitationMorrissey and George 2000). It is a dual targeting quinolone and retains activity against mutations in either or both targets (CitationHeaton et al 2000; CitationGillespie et al 2002; CitationYague et al 2002). The high affinity for gemifloxacin for both targets accounts for its high potency and continued activity.
Organisms with a single mutation in the ParC subunit usually remain susceptible to these agents. S. pneumoniae becomes resistant to gemifloxacin through mutations in gyrA. Because mutations in parC arise at a much higher rate than in gyrA in S. pneumoniae, resistance to gemifloxacin may be expected to emerge at a slower rate than for quinolones that become resistant through mutations in parC (CitationGillespie et al 2003). In fact, despite a variety of resistance mechanisms, quinolone-resistant strains of S. pneumoniae remain susceptible to gemifloxacin (CitationHeaton et al 1999; CitationBroskey et al 2000).
Gemifloxacin was also shown to have a significant in vitro post-antibiotic effect against strains of S. pneumoniae and H. influenzae (CitationDavies et al 2000a). In another study, the PAE at 4 x MIC was greater than 6 hours for H. influenzae, Pseudomonas aeruginosa, and Proteus vulgaris and 0.1–2.5 hours for the other Gram-positive and Gram-negative organisms tested (CitationDavies et al 2000b).
In vitro activity
Compared with other quinolones, it possesses enhanced in vitro activity against S. pneumoniae, including isolates resistant to β-lactams, macrolides, and quinolones, while retaining activity against Gram-negative and atypical pathogens. The in vitro activity of gemifloxacin, compared to other quinolones, against commonly isolated respiratory pathogens is shown in (CitationDeshpande and Jones 2000; CitationMarchese et al 2000; CitationMcCloskey et al 2000; CitationFile and Tillotson 2004; CitationOncu et al 2004; CitationBhavnani and Andes 2005).
Table 2 The MIC90 (μg/mL) values of gemifloxacin and other quinolones against common CAP pathogens
Pharmacokinetic and pharmacodynamic properties
Gemifloxacin given orally is rapidly absorbed, with the peak concentration being observed in 30–120 minutes, and it is widely distributed throughout the body (2004). Compared with other quinolones, gemifloxacin achieves higher concentrations in bronchial mucosa, epithelial lining fluid, and bronchoalveolar macrophages than in plasma (CitationAppelbaum et al 2004; CitationBhavnani and Andes 2005). It has also the highest area-under-the-curve (AUC)/MIC and Cmax/MIC values among quinolones (CitationFirsov et al 2000).
Gemifloxacin and its metabolites are dually excreted via urine and feces. Approximately 20%–30% of the administered dose is excreted unchanged in the urine, and the plasma half-life is approximately 6–8 hours (CitationAllen et al 2001; CitationZhanel and Noreddin 2001). Dosage adjustments are not necessary in patients with mild renal or any level of hepatic insufficiency, nor in the elderly.
Drug–drug interactions
Gemifloxacin has been investigated for interactions with various other substances (CitationFile and Tillotson 2004). Gemifloxacin can be taken with or without food, should be taken either 2 hours before sucralfate or ferrous sulfate, or at least 3 hours after ferrous sulfate, and should be administered 2 hours or more prior to or 3 hours or more after cation-containing compounds (CitationAllen et al 1999, Citation2000a, Citationb).
Adverse effects
The most frequently reported adverse effects in the clinical trials were diarrhea, nausea, and rash (CitationFile et al 2001; CitationFile and Tillotson 2004). The potential of phototoxicity caused by gemifloxacin is similar to that of ciprofloxacin (CitationAllen et al 1999). Gemifloxacin has been reported to demonstrate small, non-significant QTs interval prolongation (CitationYoo et al 2004). Liver failure does not appear to be associated with gemifloxacin, but mild and reversible elevations of liver enzymes may occur (CitationLode et al 2002).
On the whole, the following advantages are more prominent in gemifloxacin compared with other drugs usable in CAP (CitationBall 2000; CitationFile and Tillotson 2004):
Enhanced activity against S. pneumoniae (including PRSP)
Improved activity against atypical pathogens
Active against Gram-negative pathogens except P. aeruginosa
Favorable pharmacokinetics, once-daily dosing, balanced elimination requiring very little dosage modifications
Based on pharmacokinetics, highly effective in respiratory infections
Few drug–drug interaction
Few adverse drug reactions
Clinical experience of gemifloxacin in CAP
Six clinical trials of gemifloxacin have been performed in CAP (CitationBall et al 2001; CitationFile et al 2001; CitationLode et al 2002; CitationAppelbaum et al 2004; CitationLeophonte et al 2004). The clinical success rates were all close to or above 90% for gemifloxacin (87.6%–94.0%) and were similar to the comparators (87.6%–93.4%). Also, bacteriological success rate with gemifloxacin was good in all six studies (87.2%–90.6%) and comparable with that recorded by the comparators in the four comparative studies (88.9%–89.3%). summarizes the clinical and bacteriological efficacy of gemifloxacin and comparator agents.
Table 3 Clinical and bacteriological efficacy of gemifloxacin and comparator antibiotics in CAP
Conclusion
CAP remains a frequent and important clinical entity. Nearly 80% of the treatment for this condition is provided in the outpatient setting. Antimicrobial treatment of CAP must cover S. pneumoniae, H. influenzae, and M. catarrhalis and in many circumstances should also cover the intracellular atypical pathogens. The β-lactams have been considered standard therapy for the treatment of CAP. However, rising resistance rates among CAP pathogens are now a primary concern. Recent antibiotic usage and the presence of comorbidities are among the accepted risk factors for CAP caused by resistant pathogens. Patients without any risk factor should be treated effectively with a narrow spectrum β-lactam agent, like amoxicillin, or a macrolide. For patients with risk factors broader-spectrum β-lactam agents and a macrolide or a new generation of quinolones should be started empirically. Quinolones are broad-spectrum antibiotics that exhibit high levels of penetration into the lungs and low levels of resistance. Gemifloxacin is a new quinolone antibiotic that targets pneumococcal DNA gyrase and topoisomerase IV and is highly active against S. pneumoniae including penicillin-, macrolide-, and quinolone-resistant strains, as well as H. influenzae and the atypical pathogens. Among the quinolones, it has the best in vitro activity. In clinical trials in CAP, gemifloxacin has been shown to be as effective as the comparators and demonstrates an adverse event profile that is in line with comparator agents. Its improved bioavailability, pharmacokinetic–pharmacodynamic properties, and safety profile make this agent an excellent option for the treatment of CAP. But it should be kept in mind that unnecessary use of these agents will facilitate the emergence of resistant strains.
References
- AilaniRKAlimchandaniAHidalgoJCephalosporin-resistant pneumococcal pneumonia:does it, affect outcome?Respir Med2002968051112412980
- AllenABygateEClarkDThe effect of food on the bioavailability of oral gemifloxacin in healthy volunteersInt J Antimicrob Agents2000a16455011185412
- AllenABygateEFaesselHThe effect of ferrous sulphate and sucralfate on the bioavailability of oral gemifloxacin in healthy volunteersInt J Antimicrob Agents2000b15283910929878
- AllenABygateEVousdenMMultiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteersAntimicrob Agents Chemother200145540511158752
- AllenAVousdenMPorterAEffect of Maalox on the bioavailability of oral gemifloxacin in healthy volunteersChemotherapy1999455041110567782
- AppelbaumPCGillespieSHBurleyCJAntimicrobial selection for community-acquired lower respiratory tract infections in the 21st century:a review of gemifloxacinInt J Antimicrob Agents2004235334615194123
- ArancibiaFBauerTTEwigSCommunity-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosisArch Intern Med200216218495812196083
- BallPQuinolone generations:natural history or natural selection?J Antimicrob Chemother200046Suppl T1172410997595
- BallPFileTMTwynholmMEfficacy and safety of gemifloxacin 320 mg once-daily for 7 days in the treatment of adult lower respiratory tract infectionsInt J Antimicrob Agents200118192711463522
- BartlettJGDowellSFMandellLAPractice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of AmericaClin Infect Dis2000313478210987697
- BhavnaniSMAndesDRGemifloxacin for the treatment of respiratory tract infections:in vitro susceptibility, pharmacokinetics and pharmacodynamics, clinical efficacy, and safetyPharmacotherapy2005257174015899734
- BroskeyJColemanKGwynnMNEfflux and target mutations as quinolone resistance mechanisms in clinical isolates of Streptococcus pneumoniaeJ Antimicrob Chemother200045Suppl 195910824039
- BrueggemannABCoffmanSLRhombergPFluoroquinolone resistance in Streptococcus pneumoniae in United States since 1994–1995Antimicrob Agents Chemother200246680811850248
- ChoiEHLeeHJClinical outcome of invasive infections by penicillin-resistant Streptococcus pneumoniae in Korean childrenClin Infect Dis1998261346549636861
- CizmanMPokornMSemeKInfluence of increased macrolide consumption on macrolide resistance of common respiratory pathogensEur J Clin Microbiol Infect Dis199918522410482034
- Clavo-SanchezAJGiron-GonzalezJALopez-PrietoDMultivariate analysis of risk factors for infection due to penicillin-resistant and multidrug-resistant Streptococcus pneumoniae:a multicenter studyClin Infect Dis199724105299195057
- Corporation OPFactive (gemifloxacin) package insertMed Lett Drugs Ther200478915452464
- DaviesTAKellyLMHoellmanDBActivities and postantibiotic effects of gemifloxacin compared to those of 11 other agents against Haemophilus influenzae and Moraxella catarrhalisAntimicrob Agents Chemother2000a44633910681330
- DaviesTAKellyLMPankuchGAAntipneumococcal activities of gemifloxacin compared to those of nine other agentsAntimicrob Agents Chemother2000b443041010639354
- DeeksSLPalacioRRuvinskyRRisk factors and course of illness among children with invasive penicillin-resistant Streptococcus pneumoniae. The Streptococcus pneumoniae Working GroupPediatrics1999103409139925833
- DeshpandeLMJonesRNAntimicrobial activity of advanced-spectrum fluoroquinolones tested against more than 2000 contemporary bacterial isolates of species causing community-acquired respiratory tract infections in the United States (1999)Diagn Microbiol Infect Dis2000371394210863108
- FileTMCommunity-acquired pneumoniaLancet20033621991200114683661
- FileTMJrNiedermanMSAntimicrobial therapy of community-acquired pneumoniaInfect Dis Clin North Am2004189931016xi15555836
- FileTMJrSchlemmerBGarauJEfficacy and safety of gemifloxacin in the treatment of community-acquired pneumonia:a randomized, double-blind comparison with trovafloxacinJ Antimicrob Chemother200148677411474633
- FileTMJrTillotsonGSGemifloxacin:a new, potent fluoroquinolone for the therapy of lower respiratory tract infectionsExpert Rev Anti Infect Ther200428314315566328
- FineMJAubleTEYealyDMA prediction rule to identify low-risk patients with community-acquired pneumoniaN Engl J Med1997336243508995086
- FirsovAAZinnerSHLubenkoIGemifloxacin and ciprofloxacin pharmacodynamics in an in-vitro dynamic model:prediction of the equivalent AUC/MIC breakpoints and dosesInt J Antimicrob Agents2000164071411118849
- FogartyCGoldschmidtRBushKBacteremic pneumonia due to multidrug-resistant pneumococci in 3 patients treated unsuccessfully with azithromycin and successfully with levofloxacinClin Infect Dis200031613510987733
- ForwardKRThe epidemiology of penicillin resistance in Streptococcus pneumoniaeSemin Respir Infect1999142435410501312
- FriedlandIRComparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal diseasePediatr Infect Dis J199514885908584317
- GarauJRole of beta-lactam agents in the treatment of community-acquired pneumoniaEur J Clin Microbiol Infect Dis200524839915696306
- Garcia-RodriguezJAMunoz BellidoJLThe role of fluoroquinolones in respiratory tract infections:community acquired pneumoniaInt J Antimicrob Agents200016281511091048
- GillespieSHVoelkerLLAmblerJEFluoroquinolone resistance in Streptococcus pneumoniae:evidence that gyrA mutations arise at a lower rate and that mutation in gyrA or parC predisposes to further mutationMicrob Drug Resist20039172412705679
- GillespieSHVoelkerLLDickensAEvolutionary barriers to quinolone resistance in Streptococcus pneumoniaeMicrob Drug Resist20028798412118521
- GotfriedMHComparison of bacteriologic eradication of Streptococcus pneumoniae by clarithromycin and reports of increased antimicrobial resistanceClin Ther20002221410688386
- HeatonVJAmblerJEFisherLMPotent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitroAntimicrob Agents Chemother2000443112711036032
- HeatonVJGoldsmithCEAmblerJEActivity of gemifloxacin against penicillin- and ciprofloxacin-resistant Streptococcus pneumoniae displaying topoisomerase- and efflux-mediated resistance mechanismsAntimicrob Agents Chemother1999432998300010582896
- HongCYDiscovery of gemifloxacin (Factive, LB20304a): a quinolone of a new generationFarmaco20015641411347965
- JohnsonAPSheppardCLHarnettSJEmergence of a fluoroquinolone-resistant strain of Streptococcus pneumoniae in EnglandJ Antimicrob Chemother2003529536014585858
- JokinenCHeiskanenLJuvonenHMicrobial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern FinlandClin Infect Dis20013211415411283803
- KelleyMAWeberDJGilliganPBreakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycinClin Infect Dis20003110081111049784
- LeophontePFileTFeldmanCGemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal originRespir Med2004987082015303634
- LewisRJTsaiFTWigleyDBMolecular mechanisms of drug inhibition of DNA gyraseBioessays199618661718760340
- LimWSMacfarlaneJTBoswellTCStudy of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital:implications for management guidelinesThorax20015629630111254821
- LimWSvan der EerdenMMLaingRDefining community acquired pneumonia severity on presentation to hospital:an international derivation and validation studyThorax2003583778212728155
- LodeHFileTMJrMandellLOral gemifloxacin versus sequential therapy with intravenous ceftriaxone/oral cefuroxime with or without a macrolide in the treatment of patients hospitalized with community-acquired pneumonia:a randomized, open-label, multicenter study of clinical efficacy and tolerabilityClin Ther20022419153612501883
- LonksJRGarauJGomezLFailure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniaeClin Infect Dis2002355566412173129
- MarcheseADebbiaEASchitoGCComparative in vitro potency of gemifloxacin against European respiratory tract pathogens isolated in the Alexander ProjectJ Antimicrob Chemother200046Suppl T111510997594
- McCloskeyLMooreTNiconovichNIn vitro activity of gemifloxacin against a broad range of recent clinical isolates from the USAJ Antimicrob Chemother200045Suppl 1132110824027
- MorrisseyIGeorgeJTPurification of pneumococcal type II topoisomerases and inhibition by gemifloxacin and other quinolonesJ Antimicrob Chemother200045Suppl 1101610824040
- MusherDMDowellMEShortridgeVDEmergence of macrolide resistance during treatment of pneumococcal pneumoniaN Engl J Med2002346630111856810
- NiedermanMSMandellLAAnzuetoAGuidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and preventionAm J Respir Crit Care Med200116317305411401897
- O’DonnellJAGeloneSPFluoroquinolonesInfect Dis Clin North Am200014489513xi10829268
- OncuSErdemHPahsaATherapeutic options for pneumococcal pneumonia in TurkeyClin Ther2005276748316117975
- OncuSPunarMEraksoyHComparative activities of beta-lactam antibiotics and quinolones for invasive Streptococcus pneumoniae isolatesChemotherapy2004509810015211085
- OsterPZanchiACrestiSPatterns of macrolide resistance determinants among community-acquired Streptococcus pneumoniae isolates over a 5-year period of decreased macrolide susceptibility ratesAntimicrob Agents Chemother1999432510210508033
- PaganinFLilienthalFBourdinASevere community-acquired pneumonia:assessment of microbial aetiology as mortality factorEur Respir J2004247798515516672
- PallaresRFenollALinaresJThe epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides and quinolonesInt J Antimicrob Agents200322Suppl 1S1524 discussion S25–614512221
- PallaresRLinaresJVadilloMResistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, SpainN Engl J Med1995333474807623879
- ShortridgeVDDoernGVBrueggemannABPrevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995Clin Infect Dis1999291186810524961
- Van KerkhovenDPeetermansWEVerbistLBreakthrough pneumococcal bacteraemia in patients treated with clarithromycin or oral beta-lactamsJ Antimicrob Chemother200351691612615872
- YagueGMorrisJEPanXSCleavable-complex formation by wild-type and quinolone-resistant Streptococcus pneumoniae type II topoisomerases mediated by gemifloxacin and other fluoroquinolonesAntimicrob Agents Chemother200246413911796351
- YooBKTrillerDMYongCSGemifloxacin:a new fluoroquinolone approved for treatment of respiratory infectionsAnn Pharmacother20043812263515187209
- YuVLChiouCCFeldmanCAn international prospective study of pneumococcal bacteremia:correlation with in vitro resistance, antibiotics administered, and clinical outcomeClin Infect Dis200337230712856216
- ZhanelGGNoreddinAMPharmacokinetics and pharmacodynamics of the new fluoroquinolones:focus on respiratory infectionsCurr Opin Pharmacol200114596311764770