Abstract
The relatively rapid development of microbial resistance after the entry of every new antimicrobial into the marketplace necessitates a constant supply of new agents to maintain effective pharmacotherapy. Despite extensive efforts to identify novel lead compounds from molecular targets, only the peptide deformylase inhibitors (PDIs) have shown any real promise, with some advancing to phase I human trials. Bacterial peptide deformylase, which catalyzes the removal of the N-formyl group from N-terminal methionine following translation, is essential for bacterial protein synthesis, growth, and survival. The majority of PDIs are pseudopeptide hydroxamic acids and two of these (IV BB-83698 and oral NVP LBM-415) entered phase I human trials. However, agents to the present have suffered from major potential liabilities. Their in vitro activity has been limited to gram-positive aerobes and some anaerobes and has been quite modest against the majority of such species (MIC90 values ranging from 1–8 mg/L). They have exerted bacteriostatic, not bacteriocidal, activity, thus reducing their potential usefulness in the management of serious infections in the immunocompromised. The relative ease with which microorganisms have been able to develop resistance and the multiple available mechanisms of resistance (mutations in fmt, defB, folD genes; AcrAB/TolC efflux pump; overexpression of peptide deformylase) are worrisome. These could portend a short timespan of efficacy after marketing. Despite these current liabilities, further pursuit of more potent and broader spectrum PDIs which are less susceptible to bacterial mechanisms of resistance is still warranted.
Natural products have played pivotal roles in the development of antimicrobials since the early years of the twentieth century. However, the relatively rapid onset of microbial resistance with every new antimicrobial introduced into the marketplace requires a constant supply of new agents for effective therapy of infectious diseases. illustrates natural product-derived antimicrobial compounds undergoing investigation (including natural product templates and new templates present in recently-discovered lead antimicrobials) [CitationButler and Buss 2006]. Despite extensive efforts to identify novel lead compounds from molecular targets, only the peptide deformylase inhibitors (PDIs) are currently in clinical trials.
Table 1 Natural product-derived antimicrobial compounds under investigationTable Footnotea
Bacterial peptide deformylase (EC 3.5.1.31) is an enzyme responsible for catalyzing the removal of the N-formyl group from N-terminal methionine following translation. This enzyme is encoded by the def gene, which is present in all pathogenic bacteria, including Mycoplasma and Chlamydia species, and which does not share a functionally equivalent gene in mammalian cells. The def gene is an essential gene for bacterial growth and survival (this has been validated for Streptococcus pneumoniae and Escherichia coli) [CitationMazel et al 1994; CitationChan et al 2003]. The enzyme contains three highly conserved catalytic domains and belongs to the matrix metallo-protease (MMP) family of enzymes. Blockade of bacterial peptide deformylase produces inhibition of protein synthesis (similar to the mechanism of the tetracyclines, macrolides, streptogramins, lincosamides, and chloramphenicol).
The only naturally-occurring PDIs are actinonin and macrolactin N [CitationChen et al 2000; Yoo et al 2006]. Although actinonin is too weak an inhibitor to use clinically, it did form the framework for the synthesis, purification, and evaluation of more potent PDIs [CitationChen et al 2000].
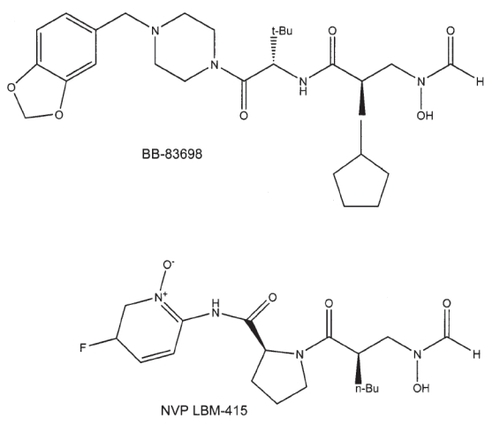
Individual papers have reviewed the discoveries of BB-3497 [CitationClements et al 2001], VRC 3852 [CitationHackbarth et al 2002], VRC 3375 [CitationJain et al 2003; CitationChen et al 2004], Ro 66-0376 and Ro 66-6976 [CitationApfel et al 2000], and PDF-611 (LBK611) [Yoo et al 2006]. outlines the peptide deformylase inhibitors of greatest potential. The pseudopeptidic hydroxamic acids (or N-formyl-N-hydroxylamines) constitute the largest group and two of these compounds were investigated for some time in human clinical trials (intravenous BB-83698 and oral LBM-415). The chemical structures of these latter two PDIs are illustrated in the .
Table 2 Peptide deformylase inhibitors
Chemistry
Peptide deformylase inhibitors are selective for bacterial enzyme and exhibit activity against similar mammalian (including human) enzymes only at extremely high concentrations unlikely to be achievable in vivo. For example, BB-81384 inhibits the peptide deformylase (Ni complex) of Streptococcus pneumoniae, Haemophilus influenzae, E. coli, and Staphylococcus aureus with IC50 values of 9, 11, 60, and 300 nM, respectively [CitationGross et al 2004], where IC50 refers to the concentration inhibiting enzyme activity by 50 percent. Corresponding IC50 values for the human metalloenzymes collagenase (MMP-1), gelatinase (MMP-2) and angiotensin converting enzyme (ACE) were 10000, 60000, and 5000 nM, respectively [CitationGross et al 2004]. In the development of VRC 3852, 20/21 related compounds (95%) exhibited IC50 values of 100 nM or less for E. coli peptide deformylase and 18/20 (90%) exhibited IC50 values of 75 nM or less for S. pneumoniae peptide deformylase. All 21 compounds were very selective, with IC50 values for tested human metalloenzymes being 200000 nM or greater [CitationHackbarth et al 2002]. Two PDIs exhibited potent activity against the peptide deformylase of Mycobacterium bovis BCG: PDF-611 (IC50 of 69.5 nM) and BB-3497 (IC50 of 24.9 nM) [CitationTeo et al 2006].
The majority of PDIs are hydroxamic acid derivatives ), in which the PDI coordinates with the active-site metal atom [CitationHuo et al 1999; CitationClements et al 2001; CitationGuilloteau et al 2002; CitationHackbarth et al 2002]. These data have been obtained during crystallography studies of enzyme-substrate complexes at resolutions of 2Å or less. Two PDIs produce time-dependent inhibition of peptide deformylase: actinonin and BB-3497 (including its 15-membered macrocyclic ring analogue) [CitationHu et al 2003, Citation2004; CitationVan Aller et al 2005]. With these agents, binding to the enzyme occurs in two steps, wherein the initial encounter complex tightens into a final encounter complex with an extremely slow rate of dissociation (half-life for dissociation ≥0.77 days [actinonin] and ≥1.9 days [BB-3497]) [CitationVan Aller et al 2005].
The effect of NVP LBM 415 on the proteomes of S. aureus and S. pneumoniae has been studied using two-dimensional electrophoresis. During exposure to PDI, similar findings were noted with both microorganisms. Many N-terminal formylated peptides/proteins were seen, their accumulation being time-dependent and the degree of accumulation differing for different peptides/proteins. Upon removal of PDI, these peptides/proteins underwent deformylation in a time-dependent manner. However, if sub-MIC (minimum inhibitory concentration) concentrations of the PDI were maintained over time, high levels of formylated peptides/proteins were present for a longer period and this correlated with a prolonged post-antibiotic effect (PAE) in vitro [CitationWang et al 2006].
The PDIs may also work via stimulation of the innate immune system. The innate immune system uses the formylation of bacterial proteins as a target and professional phagocytes express receptors for bacterial-derived formylated peptides. Activation of formyl peptide receptors (FPR) mediates phagocyte (neutrophil) migration and release of free radicals and other antimicrobial substances from phagocytes. Theoretically, PDIs should enhance this response and, hence, innate immunity. This has been demonstrated with actinonin in animal models. In subtherapeutic doses, actinonin enhances the production and secretion of neutrophil-activating peptides that work via FPR [CitationFu et al 2003].
In vitro antibacterial activity
Peptide deformylase inhibitors are much less active against intact bacteria than predicted by their kinetics of inhibition of purified enzyme, likely due to the barrier effects of the cell wall and outer membrane and the presence of active efflux pump mechanisms [CitationApfel et al 2000].
These agents generally lack useful activity against Enterobacteriaceae and non-fermentative gram-negative bacilli [CitationJones and Rhomberg 2003]. However, BB-3497 was active against single isolates of E. coli, Enterobacter cloacae, and Klebsiella pneumoniae (MIC = 8 mg/L for each) [CitationClements et al 2001]. In addition, Ro 66-0376 and Ro 66-6976 were active against one isolate of Stenotrophomonas maltophilia (MIC < 0.25 mg/L and 4 mg/L, respectively) [CitationApfel et al 2000]. Structure-activity relationship studies with the VRC series of compounds suggest the feasibility of extending the spectrum of activity of the PDIs to include gram-negative microorganisms [CitationHackbarth et al 2003].
and illustrate the in vitro antibacterial activities of the British Biotech (BB) and Vicuron/Novartis (NVP) series of PDIs, respectively [CitationWootton et al 2001; CitationWise et al 2002; CitationBowker et al 2003; CitationJones and Rhomberg 2003; CitationRoblin and Hammerschlag 2003; CitationCredito et al 2004; CitationCynamon et al 2004; CitationEdnie et al 2004; CitationJones et al 2004; CitationLofland et al 2004; CitationBell et al 2005; CitationEdelstein et al 2005; CitationFritsche et al 2005; CitationJones et al 2005; CitationSnydman et al 2005; CitationWaites et al 2005; CitationTeo et al 2006; CitationWatters et al 2006].
Table 3 Antibacterial activity of British Biotech (BB) series of peptide deformylase inhibitorsTable Footnotea
Table 4 Antibacterial activity of NVP series of peptide deformylase inhibitorsTable Footnotea
Within a series of five British Biotech compounds evaluated for in vitro activity against C. pneumoniae (strains TW 183, CT 815, CT 712), the activity was quite variable: for TW 183 and CT815, MIC range of 0.5 to 2 mg/L and minimum lethal concentration (MLC) range of 0.5 to 4 mg/L and for CT 712, MIC range of 0.25 to 4 mg/L and MLC range of 0.25 to 8 mg/L [CitationWise et al 2002].
NVP LBM-415 has demonstrated modest-moderate activity against linezolid-resistant staphylococci (N = 6, MIC range of 0.25–2 mg/L), Streptococcus oralis (N = 1, MIC 0.5 mg/L), E. faecalis (N = 3, MIC range of 2–4 mg/L), and E. faecium (N = 10, MIC range of 0.5–4 mg/L) [CitationJones et al 2004a]. It has also demonstrated a similar degree of activity against quinupristin/dalfopristin-resistant E. faecium(N = 6, MIC range of 1–2 mg/L) and staphylococci (N = 19, MIC range of 0.12–2 mg/L) [CitationJones et al 2004a]. Two PDIs exhibit reasonable in vitro activity against mycobacteria: PDF-611 (MIC90 = 0.25 mg/L vs. M. bovis BCG) and BB-3497 (MIC90 = 0.5–1 mg/L vs. M. tuberculosis) [CitationCynamon et al 2004; CitationTeo et al 2006].
An inoculum effect has been noted with NVP LBM-415, with 10- to 100-fold increases in inoculum producing 2- to 4-fold increases in MIC [CitationFritsche et al 2005]. However, varying the incubation environment, pH, or calcium concentration and medium supplementation have negligible effects on NVP LBM-415 activity [CitationFritsche et al 2005]. Alterations in medium cation content and inoculum (over a range of 102 to 106 colony-forming units [CFU] per mL) do not have a significant effect on BB-83698 activity [CitationLofland et al 2003]. Alteration in medium pH does not affect its activity against S. pneumoniae but does reduce it against S. aureus and H. influenzae. For example, a medium pH of 6 increases S. aureus MIC’s about four-fold while the identical pH increases the MIC’s of H. influenzae between four- and eight-fold [CitationLofland et al 2003]. The postantibiotic effect (PAE) duration of LBM-415 ranged from 0.3 to 1.4 hours for S. pneumoniae. The PAE duration of LBM-415 for S. pneumoniae was not affected by strain susceptibility to β-lactams or macrolides [CitationKosowska-Shick et al 2007].
BB-81384, BB-83698, NVP LBM-415, and PDF-611 exhibit primarily bacteriostatic activity against pneumococci, staphylococci, mycobacteria, and M. catarrhalis, even at medium concentrations up to and exceeding tenfold the MIC [CitationCredito et al 2004; CitationEdnie et al 2004; CitationGross et al 2004; CitationLofland et al 2004; CitationFritsche et al 2005; CitationTeo et al 2006]. However, bactericidal activity has been noted against C. pneumoniae (with NVP PDF 386) [CitationRoblin & Hammerschlag 2003], H. influenzae (with BB-83698) [CitationLofland et al 2004] and S. pneumoniae (in 5/16 [31%] strains with BB-83698) [CitationLofland et al 2003]. NVP LBM-415 is active both intra- and extra-cellularly against a variety of Legionella species but such activity is bacteriostatic in nature [CitationEdelstein et al 2005]. Preliminary data from combination studies with NVP LBM-415 demonstrate rare synergy with the vast majority of combinations resulting in indifference [CitationFritsche et al 2005].
The in vitro growth curves of S. pneumoniae after exposure to linezolid and NVP LBM-415 reveal marked differences in the time to onset of effect. The inhibitory effect of linezolid was already apparent at the 1 hour sampling timepoint while that of the PDI was delayed until the 2 hour timepoint [CitationAzoulay-Dupuis et al 2004]. Dry-form broth microdilution panels for susceptibility testing in NVP LBM-415 clinical trials have been validated (99.2% of dry-form MIC results were within ±1 log2 dilution of the reference standards with between-day and within-day reproducibility being 96.7% and 98.9%, respectively) [CitationFritsche et al 2004a]. Quality control guidelines have also been established for this same PDI for disk diffusion and broth microdilution testing, each using 4 strains of American Type Culture Collection (ATCC) microorganisms [CitationAnderegg et al 2003, Citation2004].
Provisional MIC and disk diffusion zone size criteria have been published for NVP LBM-415. Sensitive corresponds to an MIC value of 4 mg/L or less (zone size of 20 mm or greater) while resistance is denoted by an MIC value of 16 mg/L or greater (zone size of 16 mm or less) [CitationFritsche et al 2004]. In a collection of 2625 isolates, no strains of staphylococci, S. pneumoniae, other streptococci, or enterococci had MIC/zone size values corresponding to resistant. The authors thus felt that until resistant isolates of streptococci and staphylococci are seen, the only interpretive category for these microorganisms should be sensitive. For enterococci and H. influenzae, all three interpretive criteria should be developed, based on pharmacokinetic/pharmacodynamic and MIC population considerations [CitationFritsche et al 2004].
The development of resistance to PDIs may be a worrisome issue as these agents enter clinical trials and, eventually, the marketplace. Resistant mutants of S. pneumoniae and S. aureus were selected at medium concentrations of 2 to 32 times the MIC and their frequencies were 10−8 to 10−10 and 2 × 10−6 to 2 × 10−8, respectively [CitationLeeds et al 2004]. Multistep resistance selection testing yielded resistant clones with fourfold or greater increases in MIC in 11/12 (92%) strains of S. pneumoniae after 14–50 daily passages. MICs rose from 0.125–1.0 mg/L (parents) to 2–>16.0 mg/L (mutants) [CitationKosowska-Shick et al 2007]. For H. influenzae, two distinct mutants were selected, one at medium concentrations of 8 to 32 times the MIC (frequency of 10−8 to 10−9) and the other at medium concentrations of 2 to 4 times the MIC (frequency of 6 × 10−8) [CitationLeeds et al 2004]. At a medium concentration of 10 times the MIC, resistant mutants of M. bovis BCG arose at frequencies of 5 × 10−7 or less [CitationTeo et al 2006]. In a global surveillance study conducted between 2002 and 2004, one S. aureus isolate was isolated with high-level resistance (MIC ≥ 1024 mg/L) to NVP LBM-415. Resistance was due to multiple sequence changes in resistance phenotype genes (defB [1 nucleotide change] and fmt [3 nucleotide changes]). At the time of collection of this isolate, PDIs had not been used in humans at all and in vitro and in vivo (animal) exposures had been limited to a small number of clinical laboratories [CitationWatters et al 2006].
Mutations in the formyl transferase gene (fmt) reduce the susceptibilities of S. aureus, H. influenzae, M. bovis BCG and E. coli for PDIs. These mutations also produce cross-resistance to all PDIs [CitationMargolis et al 2000, CitationApfel et al 2001; CitationLeeds et al 2004; CitationTeo et al 2006]. These mutations also put these organisms at a disadvantage as their growth rates fall, morphologic changes occur (only with H. influenzae), and their virulence attenuates compared to wild-type strains [CitationMargolis et al 2000; CitationApfel et al 2001; CitationLeeds et al 2004; CitationTeo et al 2006]. Mutations in the defB gene reduce the susceptibility of S. pneumoniae to PDIs [CitationKosowska-Shick et al 2007]. In contrast to the fmt gene, mutations of the defB gene may not produce cross-resistance across all PDIs [CitationMargolis et al 2001]. Another potential mechanism of resistance is overexpression of the target enzyme due to the def gene amplification by the microorganism-of-interest and/or surrounding microorganisms (“bystanders”) [CitationApfel et al 2001]. This has been noted as a resistance mechanism for H. influenzae [CitationDean et al 2007].
Another potential mechanism of resistance to the PDIs involves efflux pumps located in the cell membrane/wall which act to actively pump antimicrobials out of the cell. Inactivation of the AcrAB-TolC efflux pump of H. influenzae enhances susceptibility to NVP LBM-415 [CitationDean et al 2005; CitationFritsche et al 2005]. In strains with MICs of 16 mg/L or greater, genetic deletion of AcrAB produces hypersusceptible strains and inactivation of AcrAB or TolC significantly enhances susceptibility [CitationDean et al 2005; CitationFritsche et al 2005]. In contrast to the effect of mutations in the fmt gene, the presence of AcrAB or TolC does not alter the growth rate or virulence of H. influenzae [CitationNeckerman 2005]. Perhaps the presence of this efflux pump may account for the modest-marginal intrinsic activities of PDIs against H. influenzae ( and ). In addition, the presence of efflux pumps also result in reduced susceptibility to the macrolides [CitationLeeds et al 2004].
Lastly, mutations in/near the folD gene have a similar effect to that of mutations in the fmt gene since both genes are necessary to encode the proteins needed for the formylation of methionyl initiator transfer RNA. The folD gene encodes the bifunctional enzyme methylene tetrahydrofolate-dehydrogenase and -cyclohydrolase. Again, bacterial fitness suffers in acquiring this form of resistance, leading to greatly reduced growth rates and virulence [CitationNilsson et al 2006].
Concerns regarding resistance issues played a significant role in suspension of further development of Ro 66-0376 and Ro 66-6976 (Roche).
In vivo (animal) pharmacodynamics
BB-81384 has been evaluated in three animal infection models: murine systemic infection (sepsis) model, neutropenic mouse thigh infection model, and murine lung infection model [CitationGross et al 2004]. In the murine sepsis model, an inoculum of S. pneumoniae was injected into the peritoneal cavity followed one hour later by a single oral dose of antimicrobial. Five day survival rates (endpoint) were 0, 50, 100, 100, and 100 percent following BB-81384 10, 30, and 90 mg/kg, amoxicillin/clavulanate 10 mg/kg, and azithromycin 10 mg/kg, respectively. When BB-81384 was administered twice on the day of inoculation (ie, 1 and 5 hours post-inoculation), the ED50 (dose effective at prolonging survival to at least 5 days in 50% of the group) fell from 30 to 20 mg/kg. In the neutropenic mouse thigh infection model, an inoculum of S. pneumoniae was injected IM into mice rendered neutropenic by intraperitoneal injections of cyclophosphamide followed 2 hours later by a single oral dose of antimicrobial. BB-81384 30 and 60 mg/kg and amoxicillin/clavulanate 10 mg/kg produced log10 thigh CFU reductions of 3.8, not done and 5.5 at 5 hours post-inoculation, respectively and 2.6, 3.3, and 5.0 at 24 hours post-inoculation, respectively. In the murine lung infection model, an inoculum of S. pneumoniae was instilled intranasally followed by 3 days of antimicrobials. BB-81384 100 mg/kg once daily for 3 days produced 100 percent survival at 3 days but none at 6 days. Three-day regimens of 50 mg/kg twice daily and 100 mg/kg twice daily both produced 100 percent survival at 3 days and 40 and 67 percent survival at 6 days, respectively. In addition, the latter two regimens reduced the 22 hr log10 lung CFU counts by 4.3 and 5.6 and the 50 hr log10 CFU counts by 4.5 and 6.0, respectively [CitationGross et al 2004].
Oral and subcutaneous BB-83698 has also been evaluated in the neutropenic (and normal) mouse thigh infection model. Eight strains of S. pneumoniae were investigated, 5 being penicillin-resistant and 3 being multi-resistant. Regimens evaluated included single 20 and 80 mg/kg doses and 24 hour treatment with 5 to 640 mg/kg/day given at dosing intervals of 3, 6, 12, and 24 hours. Based on thigh CFU values, BB-83698 was bactericidal and exhibited in vivo post-antibiotic effect (PAE) values of 6 to 13 hours. The pharmacodynamic parameter which best correlated with efficacy in neutropenic mice was the ratio of 24 hour area under the plasma concentration versus time curve (AUC)/MIC (r2 = 0.91). Efficacy was predicted when AUC/MIC was 133 or higher. The ratio of peak plasma concentration (Cmax)/MIC and proportion of the dosing interval where plasma concentration exceeded the MIC had lower r2 values of 0.50 and 0.60, respectively. The dosing interval had no effect on the total dose needed to exert a bacteriostatic effect in neutropenic mice. These data supported evaluation of a once-daily dosing regimen for the treatment of pneumococcal infections [CitationCraig 2001].
In a murine model of pneumococcal pneumonia (MICs of the 4 test strains were 0.06 to 0.25 mg/L), BB-83698 80 mg/kg twice daily SC or 160 mg/kg once daily SC protected 70 to 100 percent of animals at 10 days post-inoculation. Bacterial burden in the bloodstream and lungs both fell in a dose-dependent fashion [CitationAzoulay-Dupuis et al 2004]. After a single 80 mg/kg SC dose, mean serum and lung tissue Cmax/MIC ratios were 238 and 1032 to 1, respectively. Corresponding mean 24 hour AUC/MIC ratios were 957 and 3823 to 1 [CitationAzoulay-Dupuis et al 2004].
BB-3497 (IV, oral) was evaluated in the murine sepsis model using S. aureus Smith. The ED50’s were 7 mg/kg (IV) and 8 mg/kg (oral). With a methicillin-resistant S. aureus test strain, the ED50 for oral drug rose to 14 mg/kg [CitationClements et al 2001]. NVP LBM-415 was evaluated in a M. pneumoniae pneumonia model in mice. Drug was dosed as 50 mg/kg once daily SC. As compared to untreated mice, drug-treated mice exhibited significant reductions in bronchoalveolar lavage (BAL) fluid M. pneumoniae CFU counts on days 6 and 13 on treatment and lung histopathology scores on days 3, 6, and 13 on treatment and 7 days after treatment. Airway obstruction fell significantly in active-treated mice on days 1, 3, and 6 on treatment and 7 days after treatment while airway hyperresponsiveness fell only on day 3 on treatment. Significant reductions were found in the BAL fluid concentrations of most, but not all, cytokines and chemokines (eg, tumor necrosis factor alpha, interferon gamma, interleukins −6 and −12, functional interleukin −8, monocyte chemotactic protein −1, macrophage inflammatory protein 1±, monokine induced by interferon-gamma, and interferon-inducible protein 10) in active-treated mice [CitationFonseca-Aten et al 2005].
VRC 3375 was evaluated in the murine sepsis model (S. aureus Smith). The ED50 values were 32 mg/kg (IV), 17 mg/kg (SC), and 21 mg/kg (oral) [CitationChen et al 2004]. In this same model, the ED50 for VRC 4307 was 17.9 mg/kg (SC) (no efficacy was seen with oral VRC 4307, even at 30 mg/kg) [CitationHackbarth et al 2002]. For VRC 4232, the ED50 was 29.7 mg/kg (SC) [CitationHackbarth et al 2002].
The acute toxicology has been described for one PDI, VRC 3375. The LD50 (lethal dose for 50% of mice) was 447 mg/kg (IV), with 3 deaths occurring immediately post-injection. No deaths were reported after SC and oral dosing. Day 8 necropsy results were unremarkable except in 3 mice in the 500 mg/kg SC groups (all had dark red skin plaques at the injection site) [CitationChen et al 2004].
Animal pharmacokinetics
The BB series of PDIs are quantified in biological samples using high performance liquid chromatography (HPLC) with tandem mass spectrometry (MS) [CitationClements et al 2001; CitationAzoulay-Dupuis et al 2004; CitationGross et al 2004; CitationRamanathan-Girish et al 2004]. The VRC series of PDIs are also quantitated using HPLC with tandem MS as well as HPLC with ultraviolet detection [CitationHackbarth et al 2002, CitationChen et al 2004a;CitationTeo et al 2006]. illustrates the mean pharmacokinetic parameters of a variety of peptide deformylase inhibitors in mice and rats [CitationClements et al 2001; CitationCraig 2001; CitationHackbarth et al 2002; CitationAzoulay-Dupuis et al 2004; CitationChen et al 2004a; CitationGross et al 2004; CitationRamanathan-Girish et al 2004; CitationTeo et al 2006].
Table 5 Mean pharmacokinetic parameters for selected peptide deformylase inhibitors in mice and rats
Absorption
BB-83698 is reasonably well-absorbed by the oral route, with a mean bioavailability of 50 percent in neutropenic mice [CitationCraig 2001]. Absorption proceeds rapidly after oral administration, with a mean time to peak plasma concentration (Tmax) of about 1 hour [CitationAzoulay-Dupuis et al 2004]. BB-81384 is also reasonably well-absorbed after oral administration, with mean bioavailabilities of 55 percent (10 mg/kg orally) and 88 percent (50 mg/kg orally) [CitationGross et al 2004]. Of the VRC series of compounds, only VRC 3375 is reasonably well-absorbed, with a mean bioavailability of 64 percent and Tmax of 0.167 to 0.33 hours [CitationChen et al 2004a]. The mean oral bioavailabilities of VRC 4232 and VRC 4307 are 3.2 and 0.1 percent, respectively [CitationHackbarth et al 2002]. Absorption proceeds rapidly with VRC 4307, the mean Tmax being 0.25 hours [CitationHackbarth et al 2002]. The antimycobacterial PDF-611 is also reasonably well-absorbed, with a mean bioavailability of 45 percent and Tmax of 0.25 h [CitationTeo et al 2006].
For NVP LBM-415, the mean Tmax after oral dosing was the same in rats and mice (0.5 hr) [CitationChen et al 2004a]. Oral bioavailability averaged 65% in mice but ranged from 21 to 94% in rats over a dose range of 12 to 436 mg/kg (latter being an effect of saturable metabolism, not altered bioavailability) [CitationChen et al 2004a].
Distribution
For VRC 3375 in mice, concentrations in heart, kidney, and lung tissue exceeded those in serum while those in muscle and serum were comparable at 3 minutes following IV dosing of 100 mg/kg [CitationChen et al 2004a].
For BB-81384, after a single 10 mg/kg oral dose, lung tissue and plasma concentrations were approximately equivalent over a five hour period following dosing while thigh muscle concentrations were approximately one-third those in plasma over the same time period. Peak concentrations were achieved within 20 minutes in all 3 compartments [CitationGross et al 2004]. For BB-83698, after an 80 mg/kg SC dose, lung tissue concentrations exceeded those of serum by approximately four-fold, whether measured by Cmax or AUC () [CitationAzoulay-Dupuis et al 2004]. For NVP LBM-415, lung tissue concentrations were double those in plasma after both oral and IV dosing in mice [CitationChen et al 2004a].
Metabolism and excretion
VRC 4232/4307 are rapidly metabolized in mouse and rat hepatic microsomes, in contrast to their metabolism in human microsomes [CitationHackbarth et al 2002]. For VRC 4232, metabolism by mouse, rat, and human microsomes is extensive, with hydroxylation predominating but also oxidation, hydrolysis, and reduction, all followed by glucuronidation [CitationHackbarth et al 2002]. As all metabolites exhibit an altered hydroxamic acid moiety, all should be microbiologically inactive [CitationHackbarth et al 2002]. The pattern of metabolites varied with species and with the model used (ie, in vivo animal versus in vitro liver microsomes). Thus, the patterns of the three major metabolites were different for the in vivo mouse, mouse liver microsome, rat liver microsome and human liver microsome models. In human liver microsomes, the three major metabolic products (in descending order of amount) were the product of proline hydroxylation and hydrolysis, the product of hydrolysis alone, and the glucuronide conjugate of the parent compound [CitationHackbarth et al 2002].
Multiple-dose studies with BB-83698 have been conducted in rats and dogs at 3 dose levels (10, 22, and 50 mg/kg IV once daily × 28 days). In rats, mean day 1 peak plasma concentrations rose 2.5-fold (expected 2.2-fold from 10 to 22 mg/kg) and 4.9-fold (expected 5-fold from 10 to 50 mg/kg) in males with corresponding values in females of 2.6- and 6.6-fold. Mean day 1 AUC rose 2.2-fold and 6.6-fold in males and 3.1-fold and 8.8-fold in females. Mean day 28 peak plasma concentrations rose 3.4-fold and 3.3-fold in males and 2.6-fold and 4.2-fold in females. Mean day 28 AUC rose 3.4-fold and 14.4-fold in males and 2.7-fold and 9.4-fold in females. In rats, BB-83698 exhibited non-linear pharmacokinetics in both genders, especially at the higher 50 mg/kg dose and when quantitated using AUC data (ie, the rise in AUC was disproportionately high compared to the rise in dose). In the dog, non-linear pharmacokinetics were also noted in both genders. However, this was apparent with both Cmax and AUC data and with the 22 and 50 mg/kg dose. In the case of the dog, the rises in Cmax and AUC were disproportionately low compared to the rises in dose (dog data not shown) [CitationRamanathan-Girish et al 2004].
From day 1 to day 28, the mean degrees of accumulation as measured by change in Cmax were 1.2-, 1.6-, and 0.8-fold in males and 1.5-, 1.6-, and 1.0-fold in females (with doses of 10, 22, and 50 mg/kg, respectively). The corresponding degrees of accumulation as measured by change in AUC were 0.8-, 1.4-, and 1.9-fold in males and 0.8-, 0.7-, and 0.8- fold in females. In both rats and dogs, accumulation over 28 days occurred to a minor extent, if at all, whether quantitated using Cmax or AUC data (dog data not shown) [CitationRamanathan-Girish et al 2004].
After IV administration of NVP LBM-415, the mean total body clearances in mice and rats were 5.19 and 1.27 L/h/kg, respectively [CitationChen et al 2004]. Peak plasma concentration and AUC did not rise in proportion to dose in rats due to (presumed) saturable metabolism [CitationChen et al 2004]. In the first 24 hours after IV drug administration, mean urinary excretion of parent compound was 19 percent of the dose in mice and 48 percent of the dose in rats [CitationChen et al 2004a]. Mean biliary excretion in the first 7 hours after IV dosing was 23 percent in rats [CitationChen et al 2004a].
Human pharmacokinetics
Few published data exist regarding the pharmacokinetics of PDIs in humans. One published phase I clinical trial evaluated the pharmacokinetics and tolerability of ascending single intravenous doses (10, 25, 50, 100, 200, 325, 400, 475 mg) of BB-83698 in healthy male volunteers. All doses were administered over 15 minutes. No significant adverse events (AEs) occurred at any dose level. The overall mean ± SD clearance was 238 ± 130 mL/min, with CL’s ranging from 189 mL/min (475 mg) to 521 mL/min (10 mg). Linearity was evident with Cmax and AUC data, although these data did appear to deviate somewhat at the lowest and highest doses. Further studies are necessary to resolve this issue. However, Vdss and t 1/2 did vary significantly with dose (ranging from 57–106 L and 4.8–16.7 h, respectively). Plasma protein binding ranged from 78 to 82 percent [CitationRamanathan-Girish et al 2004].
Similar single and multiple dose pharmacokinetic studies have also been conducted with oral NVP LBM-415. After single oral doses of 100, 250, 500, 1000, 2000, and 3000 mg, the median Tmax was 1 hr or less in all dosing groups. Linearity was noted for dose-normalized 12-h AUC data. The mean t 1/2 ranged from 2 to 3 h except in the 2000 mg group (where the value was 4.2 h). When a 1000 mg dose was taken immediately after finishing breakfast, as compared to ingestion in the fasting state, the Tmax was prolonged (median rose from 0.5 to 2 h) and Cmax fell (mean fell from 15.5 to 6.7 mg/L) but the AUC was statistically unchanged. After multiple oral dosing (250, 500, and 1000 mg twice daily for 11 d), no accumulation was noted and steady-state was achieved after one day of dosing. These data supported the evaluation of a twice daily regimen in clinical trials [CitationJain et al 2005].
Timelines for development of BB-83698 and NVP LBM-415
BB-83698
In October 2002, BB-83698 (developed by British Biotech in collaboration with Genesoft) entered phase I studies in humans. Although dose-limiting central nervous system adverse events (AEs) such as trauma, unsteady gait and seizures had occurred in dogs, no significant AEs were seen in humans. The probable therapeutic dose was judged to be 475 mg, based on an AUC/MIC value of 184 (assuming, in turn, an MIC for S. pneumoniae of 0.25 mg/L). Development was terminated for unknown reasons when British Biotech became Vernalis. Oscient has now purchased this compound and one must wait to see the drug’s ultimate fate.
NVP LBM-415
In October 2003, NVP LBM-415 (developed by Vicuron in collaboration with Novartis) entered phase I studies in humans. Multiple dose pharmacokinetic studies used dosage regimens of 250 and 500 mg twice daily. No significant AEs were seen after administration of single doses up to and including 3g. However, Novartis and Vicuron discontinued its development in April 2004. Vicuron was purchased by Pfizer in June 2005. However, Novartis appears to be still pursuing PDI’s, most recently the antimycobacterial NVP PDF-611 (LBK611).
Conclusion
The relatively rapid development of microbial resistance after the entry of every new antimicrobial into the marketplace necessitates a constant supply of new agents to maintain effective pharmacotherapy. Despite extensive efforts to identify novel lead compounds from molecular targets, only the peptide deformylase inhibitors (PDIs) have shown any real promise, with some advancing to phase I human trials. Bacterial peptide deformylase, which catalyzes the removal of the N-formyl group from N-terminal methionine following translation, is essential for bacterial protein synthesis, growth, and survival. The majority of PDIs are pseudopeptide hydroxamic acids and two of these (IV BB-83698 and oral NVP LBM-415) entered phase I human trials. However, agents to the present have suffered from major potential liabilities. Their in vitro activity has been limited to gram-positive aerobes and some anaerobes and has been quite modest against the majority of such species (MIC90 values ranging from 1–8 mg/L). They have exerted bacteriostatic, not bacteriocidal, activity, thus reducing their potential usefulness in the management of serious infections in the immunocompromised. The relative ease with which microorganisms have been able to develop resistance and the multiple available mechanisms of resistance (mutations in fmt, defB, folD genes; AcrAB/TolC efflux pump; overexpression of peptide deformylase) are worrisome. These could portend a short timespan of efficacy after marketing. Despite these current liabilities, further pursuit of more potent and broader spectrum PDIs which are less susceptible to bacterial mechanisms of resistance is still warranted.
Note in proof
The recent discovery of a peptide deformylase homologue (mitochondrial PDF or mPDF) in humans has raised major objections to using PDF as a target of antimicrobial drugs. Indeed, mPDF is functional, displaying PDF activity in the human mitochondrion; is involved in the same essential pathway as in bacteria; is inhibited in vitro and in vivo by actinonin (see above); and, when inhibited, produces an antiproliferative effect triggered by mitochondrial dysfunction leading to cell death. Fortunately, PDF exists in 3 forms: PDF1B and PDF2 (both are bacterial PDFs) and PDF1A (human mPDF). Thus, the search is now on for compounds that selectively inhibit PDFs 1B and 2 and have no effect on PDF1A. At least one compound with this spectrum of enzyme activity is now known: 2-(5-bromo-1H-indol-3-yl)-N-hydroxyacetamide [CitationBoularot et al 2007].
References
- AndereggTRBiedenbachDJJonesRNQuality Control Working GroupQuality control guidelines for MIC susceptibility testing of NVP PDF-713: a novel peptide deformylase inhibitorInt J Antimicrob Agents200322846 letter12842335
- AndereggTRJonesRNQuality Control Working GroupDisk diffusion quality control guidelines for NVP-PDF-713: a novel peptide deformylase inhibitorDiagn Microbiol Infect Dis20044855714761722
- ApfelCBannerDWBurDHydroxamic acid derivatives as potent peptide deformylase inhibitors and antibacterial agentsJ Med Chem20004323243110882358
- ApfelCMLockerHEversSPeptide deformylase as an antibacterial drug target: target validation and resistance developmentAntimicrob Agents Chemother20014510586411257016
- Azoulay-DupuisEMohlerJBedosJPEfficacy of BB-83698, a novel peptide deformylase inhibitor, in a mouse model of pneumococcal pneumoniaAntimicrob Agents Chemother20044880514693522
- BellJMTurnidgeJDInoueMActivity of a peptide deformylase inhibitor LBM415 (NVP PDF-713) tested against recent clinical isolates from JapanJ Antimicrob Chemother2005552768 letter15649992
- BoularotAGiglioneCPetitSDiscovery and refinement of a new structural class of potent peptide deformylase inhibitorsJ Med Chem200750102017201406
- BowkerKENoelARMacGowanAPIn vitro activities of nine peptide deformylase inhibitors and five comparator agents against respiratory and skin pathogensInt J Antimicrob Agents2003225576114659651
- ButlerMSBussADNatural products–the future scaffolds for novel antibiotics?Biochem Pharmacol2006719192916289393
- ChanPFO’DwyorKMPalmerLMCharacterization of a novel fructose-regulated promoter (PfcsK) suitable for gene essentiality and antibacterial mode-of-action studies in Streptococcus pneumoniaeJ Bacteriol20031852051812618474
- ChenDZPatelDVHackbarthCJActinonin a naturally occurring antibacterial agent is a potent deformylase inhibitorBiochemistry20003912566210684604
- ChenDHackbarthCNiZJPeptide deformylase inhibitors as antibacterial agents: identification of VRC3375 a proline-3-alkylsuccinyl hydroxamate derivative by using an integrated combinatorial and medicinal chemistry approachAntimicrob Agents Chemother2004482506114693547
- ChenDTembeVCramerJLBM415 (VIC-104959), a novel peptide deformylase inhibitor with favorable pharmacokinetic profile in rodentsPresented at the 44th Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapyNovember 2004aWashington, D.C. abstract F-1965–2004
- ClementsJMBeckettRPBrownAAntibiotic activity and characterization of BB-3497 a novel peptide deformylase inhibitorAntimicrobial Agents Chemother20014556370
- CraigWAIn vivo pharmacodynamics of BB-83698, a deformylase inhibitorPresented at the 41st Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 2001Chicago, IL abstract F-355
- CreditoKLinGEdnieLMAppelbaumPCAntistaphylococcal activity of LBM415, a new peptide deformylase inhibitor compared with those of other agentsAntimicrob Agents Chemother2004484033615388473
- CynamonMHAlvirez-FreitesEYeoAETBB-3497 a peptide deformylase inhibitor, is active against Mycobacterium tuberculosisJ Antimicrob Chemother2004534035 letter14688038
- DeanCRNarayanSDaigleDMRole of the AcrAB-TolC efflux pump in determining susceptibility of Haemophilus influenzae to the novel peptide deformylase inhibitor LBM415Antimicrob Agents Chemother20054931293516048914
- DeanCRNarayanSRichardsJReduced susceptibility of Haemophilus influenzae to the peptide deformylase inhibitor LBM415 can result from target protein overexpression due to amplified chromosomal def gene copy numberAntimicrob Agents Chemother20075110041017220413
- EdelsteinPHHuBEdelsteinMACIn vitro and intracellular activities of LBM415 (NVP PDF-713) against Legionella pneumophilaAntimicrob Agents Chemother2005492533515917565
- EdnieLMPankuchGAppelbaumPCAntipneumococcal activity of LBM415, a new peptide deformylase inhibitor, compared with those of other agentsAntimicrob Agents Chemother20044840273215388472
- Fonseca-AtenMSalvatoreCMMejiasAEvaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumoniaAntimicrob Agents Chemother20054941283616189089
- FritscheTRMoetGJJonesRNCommerical broth microdilution panel validation and reproducibility trials for NVP PDF-713 (LBM415), a novel inhibitor of bacterial peptide deformylaseClin Microbiol Infect2004108576015355422
- FritscheTRRhombergPFJonesRNComparisons of the inter-method susceptibility testing accuracy for LBM415 (NVP PDF-713) using 2,625 recent clinical isolatesPresented at the 44th Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapyNovember 2004aWashington, D.C. abstract D-1919–2004
- FritscheTRSaderHSCleelandRJonesRNComparative antimicrobial characterization of LBM415 (NVP PDF-713), a new peptide deformylase inhibitor of clinical importanceAntimicrob Agents Chemother20054914687615793128
- FuHDahlgrenCBylundJSubinhibitory concentrations of the deformylase inhibitor actinonin increase bacterial release of neutrophil-activating peptides: a new approach to antimicrobial chemotherapyAntimicrob Agents Chemother20034725455012878517
- GrossMClementsJBeckettRPOral anti-pneumococcal activity and pharmacokinetic profiling of a novel peptide deformylase inhibitorJ Antimicrob Chemother2004534879314963065
- GuilloteauJ-PMathieuMGiglioneCThe crystal structures of four peptide deformylases bound to the antibiotic actinonin reveal two distinct types: a platform for structure-based design of antibacterial agentsJ Mol Biol20023209516212126617
- HackbarthCJChenDZLewisJGN-alkyl urea hydroxamic acids as a new class of peptide deformylase inhibitors with antibacterial activityAntimicrob Agents Chemother20024627526412183225
- HackbarthCJLopezSWuCActivity of peptide deformylase (PDF) inhibitors against gram negative bacteriaPresented at the 43rd Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2003Chicago, IL abstract F-1479–2003
- HuxNguyenKTVerlindeCLMJHolWGJPeiDStructure-based design of a macrocyclic inhibitor for peptide deformylaseJ Med Chem20034637714 letter12930137
- HuXNguyenKTJiangVCLoflandDMoserHEPeiPMacrocyclic inhibitors for peptide deformylase: a structure-activity relationship study of the ring sizeJ Med Chem2004474941915369398
- HuoBGongWRajagopalanPTRZhouYPeiDChenMKStructural basis for the design of antibiotics targeting peptide deformylaseBiochemistry1999384712910200158
- JainRSundramALopezSα-substituted hydroxamic acids as novel bacterial deformylase inhibitor-based antibacterial agentsBioorg Med Chem Lett2003134223814623006
- JainRChenDWhiteRJPatelDVYuanZBacterial peptide deformylase inhibitors: a new class of antimicrobialsCurr Med Chem20051216072116022661
- JonesRNRhombergPRComparative spectum and activity of NVP-PDF386 (VRC4887), a new peptide deformylase inhibitorJ Antimicrob Chemother2003511576112493802
- JonesRNFritscheTRSaderHSAntimicrobial spectrum and activity of NVP PDF-716, a novel peptide deformylase inhibitor, tested against 1,837 recent gram-positive clinical isolatesDiagn Microbiol Infect Dis20044963515135503
- JonesRNMoetGLSaderHSFritscheTRPotential utility of a peptide deformylase inhibitor (NVP PDF-713) against oxazolidinone-resistant or streptogramin-resistant gram-positive organism isolatesJ Antimicrob Chemother2004a53804715056649
- JonesRNSaderHSFritscheTRAntimicrobial activity of LBM415 (NVP PDF-713) tested against pathogenic Neisseria spp. (Neisseria gonorrhoeae and Neisseria meningitides)Diagn Microbiol Infect Dis2005511394115698721
- Kosowska-ShickKCreditoKLPankuchGADewasseBMcGheePAppelbaumPCMultistep resistance selection and PAE studies on antipneumococcal activity of LBM415 compared to other agentsAntimicrob Agents Chemother200751770317116666
- LeedsJADeanCRFavreBIn vitro selection of decreased susceptibility to the novel peptide deformylase inhibitor LB415 in three pathogensPresented at the 44th Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapyNovember 2004Washington, D.C. abstract C1-1880–2004
- LoflandDDifuntorumSWallerAClementsJJohnsonKAntibacterial spectrum, bactericidal activity, and susceptibility testing of the peptide deformylase inhibitor GBB-83698Presented at the 43rd Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2003Chicago, IL abstract E-1712–2003
- LoflandDDifuntorumSWallerAIn vitro antibacterial activity of the peptide deformylase inhibitor BB-83698J Antimicrob Chemother200453664814973152
- MargolisPSHackbarthCJYoungDCPeptide deformylase in Staphylococcus aureus: resistance to inhibition is mediated by mutations in the formyltransferase geneAntimicrob Agents Chemother20004418253110858337
- MargolisPHackbarthCLopezSResistance of Streptococcus pneumoniae to deformylase inhibitors is due to mutations in defBAntimicrob Agents Chemother2001452432511502510
- MazelDPochetSMarlierePGenetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translationEMBO J199413914238112305
- NeckermanGDeanCRYuDManniKNarayanSDzink-FoxJRole of AcrAB-TolC efflux in reducing susceptibility to LBM-415, azithromycin, and telithromycin in a Haemophilus influenzae murine lung infection modelPresented at the 45th Annual Meeting of the Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 2005Washington, DC abstract C1-1033-2005
- NilssonAIZorzetAKanthADahlstromSBergOGAnderssonDIReducing the fitness costs of antibiotic resistance by the amplification of initiator tRNA genesPNAS200610369768116636273
- Ramanathan-GirishSMcColmJClementsJMPharmacokinetics in animals and humans of a first-in-class peptide deformylase inhibitorAntimicrob Agents Chemother20044848354215561864
- RoblinPMHammerschlagMRIn vitro activity of a new antibiotic, NVP-PDF386 (VRC4887)Antimicrob Agents Chemother2003471447812654690
- SnydmanDRJacobusNVMcDermottLAEvaluation of the in vitro activity of NVP-LBM415 against clinical anaerobic isolates with emphasis on the Bacteroides fragilis groupJ Antimicrob Chemother2005551024815824092
- TeoJWPThayalanPBeerDPeptide deformylase inhibitors as potent antimycobacterial agentsAntimicrob Agents Chemother20065036657316966397
- Van AllerGSNandigamaRPetitCMMechanism of time-dependent inhibition of polypeptide deformylase by actinoninBiochemistry2005442536015628866
- WaitesKBReddyNBCrabbDMDuffyLBComparative in vitro activities of investigational peptide deformylase inhibitor NVP LBM-415 and other agents against human mycoplasmas and ureaplasmasAntimicrob Agents Chemother2005492541215917568
- WangWWhiteRYuanZProteomic study of peptide deformylase inhibition in Streptococcus pneumoniae and Staphylococcus aureusAntimicrob Agents Chemother20065016566316641432
- WattersAAJonesRNLeedsJADenysGSaderHSFritscheTRAntimicrobial activity of a novel peptide deformylase inhibitor, LBM415, tested against respiratory tract and cutaneous infection pathogens: a global surveillance report (2003–2004)J Antimicrob Chemother2006579142316549511
- WiseRAndrewsJMAshbyJIn vitro activities of peptide deformylase inhibitors against gram-positive pathogensAntimicrob Agents Chemother20024611171811897602
- WoottonMHoweRAMacGowanAPWalshTRBennettPMIn vitro activity of BB83698 and two other peptide deformylase inhibitors compared to ciprofloxacin, moxifloxacin, gentamicin and linezolid against heterogeneous glycopeptide intermediate Staphylococcus aureus (hGISA) and GISAPresented at the 41st Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 2001Chicago, IL abstract F-352
- YeoJ-SZhengC-JLeeSKwakJ-HKimW-GMacrolactin N, a new peptide deformylase inhibitor produced by Bacillus subtilisBioorg Med Chem Lett20061648899216809037