Abstract
Patients with myelodysplastic syndromes (MDS) are challenging to treat, given the advanced median age and comorbidities of the population. For most patients, the standard therapy is supportive care, including broad-spectrum antibiotics, red blood cell/platelet transfusions, and growth factors. Decitabine, a hypomethylating agent that allows for the re-expression of tumor suppressor genes, represents an exciting new treatment option for MDS patients. In phase 2 and 3 studies, decitabine has been associated with durable responses in MDS patients and delayed time to acute myeloid leukemia (AML) transformation or death compared with supportive care. Decitabine has been shown to be well tolerated with a toxicity profile expected for this class of agent. Recent studies also suggest that lower dose schedules of decitabine may result in additional improvements in response. As more is learned about the mechanism of hypomethylating agents, new roles are emerging for decitabine in combination therapy for MDS and in other hematologic malignancies such as AML.
Myelodysplastic syndromes
Myelodysplastic syndromes (MDS) are a group of bone marrow disorders characterized by ineffective hematopoiesis resulting in anemia, neutropenia, and thrombocytopenia (CitationAmerican Cancer Society 2005; CitationMyelodysplastic Syndromes Foundation 2006; CitationAmerican Cancer Society 2006). MDS can be classified as de novo, arising from no apparent cause, or as secondary, resulting from exposure to a mutagen such as chemotherapy or benzene (CitationAmerican Cancer Society 2005; CitationAplastic Anemia & MDS International Foundation 2005). Chromosomal abnormalities are common in both types of MDS (CitationKurzrock 2002; CitationList et al 2004; CitationAmerican Cancer Society 2005; CitationAmerican Cancer Society 2006). In patients with de novo MDS, chromosomal abnormalities have been reported in approximately 40%–70% of cases, whereas in patients with secondary MDS, chromosomal abnormalities are observed in almost 95% of cases (CitationList et al 2004). MDS occurs primarily in the elderly and is rare in young adults (CitationWilliamson et al 1994; CitationAul et al 1998; CitationAmerican Cancer Society 2005; CitationAmerican Cancer Society 2006).
Although the exact number of MDS cases is unknown owing to the lack of a central United States registry, it is estimated that, in the general population, MDS occurs in 5 per 100,000 people (CitationNational Comprehensive Cancer Network 2006). Estimates range between 10,000 and 20,000 new cases of MDS per year in the United States (CitationAmerican Cancer Society 2005; CitationAplastic Anemia & MDS International Foundation 2005). The number of MDS cases is believed to be increasing as a result of the aging population and the increased survival rate of patients who have received chemotherapy (CitationAmerican Cancer Society 2005).
The greatest risk factor for MDS appears to be advancing age, with 80%–90% of all patients over 60 years old (CitationAmerican Cancer Society 2005). Other risk factors for MDS are previous chemotherapy, exposure to environmental toxins, tobacco and cigarette smoke, congenital disorders, familial disorders, and being of male gender (CitationAmerican Cancer Society 2005). Clinical symptoms may include fatigue, weakness, and serious infections; however, approximately half of MDS patients are asymptomatic at the time of initial diagnosis and are diagnosed only after routine laboratory tests show abnormalities (CitationHofmann and Koeffler 2005; CitationAmerican Cancer Society 2005; CitationAplastic Anemia & MDS International Foundation 2005). Although MDS can eventually result in neutropenia and/or thrombocytopenia, anemia is the most common characteristic at the time of initial diagnosis. In the early course of the disease, a hemoglobin value of less than 10 g/dL has been observed in approximately 80% of patients (CitationHofmann and Koeffler 2005).
Prognosis can depend on many variables, including morphology, number of cytopenias, blast count, and cytogenetics. Left untreated, the median survival ranges from 0.4 years for high-risk MDS patients to 5.7 years for low-risk MDS patients (CitationGreenberg et al 1997). In the majority of MDS patients, death typically results from complications of bone marrow failure, such as chronic anemia, infections, or severe bleeding (CitationKurzrock 2002; CitationMyelodysplastic Syndromes Foundation and Bennett 2006). Approximately one-third of adult MDS patients progress to acute myeloid leukemia (AML), with a median survival of 6–12 months (CitationGanser and Hoelzer 1992; CitationGreenberg et al 1997).
Classification systems
At the present time there are two MDS classification systems that have been used to determine expected median survival and median time to AML transformation. The French-American-British (FAB) Co-operative Group Classification recognizes five subgroups of MDS based on cell morphology and the percentage of blasts (CitationBennett et al 1982). In order to improve the prognostic value, the World Health Organization (WHO) subsequently made modifications to FAB that included the reduction of the blast threshold for the diagnosis of AML and refinements of the categories of refractory anemia (RA) and refractory anemia with ringed sideroblasts (RARS) (CitationVardiman et al 2002).
There has also been the development of a widely used prognostication tool, the International Prognostic Scoring System (IPSS), which takes into account cytogenetics and number of cytopenias as well as morphology (CitationGreenberg et al 1997; CitationKurzrock 2002). The IPSS scoring system subdivides MDS into four distinct subgroups for predicting survival and risk of transformation to AML () (CitationGreenberg et al 1997).
Table 1 International prognostic scoring system (IPSS) score and prognosis
Clinical practice guidelines
Evidence-based treatment guidelines for MDS have recently been published by the Italian Society of Hematology, the United Kingdom, and the United States (National Comprehensive Cancer Network [NCCN]) (CitationAlessandrino et al 2002; CitationBowen et al 2003; CitationNational Comprehensive Cancer Network. 2006). According to these guidelines, the treatment strategy for MDS should be determined by IPSS risk category, as well as age and performance status. The recently updated NCCN guidelines recommend hypomethylating agents, such as decitabine or azacitidine, for the treatment of higher risk MDS patients and for lower risk MDS patients who are nonresponsive to growth factor therapy or who are HLA-DR15 negative (CitationNational Comprehensive Cancer Network. 2006).
Treatment options
The MDS patient population presents many challenges when considering an appropriate treatment strategy, including advanced age, comorbidities, and an inability to tolerate certain types of intensive therapy. Therapy should be selected based on the patient’s performance status, disease classification, IPSS score, and treatment tolerance. In patients with a low-risk or intermediate-1 IPSS score, the goals of therapy are to improve blood counts and ensure age-related quality of life (CitationNational Comprehensive Cancer Network. 2006), whereas for intermediate-2 and high-risk patients, the goals of therapy are to prolong survival and delay leukemic progression (CitationNational Comprehensive Cancer Network. 2006). The only potentially curative treatment for MDS is hematopoietic stem cell transplantation (HSCT), however, this option is available for only a small number of patients (ie, younger age, histocompatible donor, no significant comorbidities) (CitationAlessandrino et al 2002; CitationBowen et al 2003; CitationNational Comprehensive Cancer Network. 2006). Some high-risk MDS patients who are not candidates for HSCT may be eligible for intensive antileukemic chemotherapy (CitationAlessandrino et al 2002; CitationBowen et al 2003; CitationNational Comprehensive Cancer Network. 2006). Nevertheless, the vast majority of MDS patients are managed with supportive care, including red blood cell (RBC)/platelet transfusions, growth factors such as recombinant erythropoietin and colony-stimulating factors, and antibiotics, including broad coverage in neutropenic patients as infection occurs.
A number of emerging therapeutic options are currently being evaluated for the treatment of MDS that will, it is hoped, add to the treatment options for patients who are ineligible to receive HSCT or intensive chemotherapy. Lenalidomide, an immunomodulatory drug derived from thalidomide, has been recently approved by the Food and Drug Administration (FDA) and is indicated for the treatment of MDS in patients with chromosome 5q deletion. Other agents such as imatinib and tipifarnib are currently being evaluated in clinical trials (CitationCortes et al 2003; CitationFeldman 2005; CitationSekeres 2005; CitationJabbour and Giles 2005). Some of the therapies farthest along in development are the hypomethylating agents decitabine and azacitidine, both of which have been recently approved by the FDA for the treatment of MDS.
Hypermethylation in cancer
DNA methylation is a common epigenetic modification that plays an important role in gene expression in mammalian cells (CitationLeone et al 2002; CitationDas and Singal 2004). As part of normal development, certain genes may be silenced through methylation of cytosine residues in their promotor regions (CpG islands). However, in some hematopoietic neoplasms including MDS, DNA hypermethylation can inactivate genes essential for the control of normal cell growth, differentiation, or apoptosis. A group of enzymes called DNA methyltransferases (DNMTs) catalyze the methylation of cytosine residues in newly synthesized DNA, thus replicating the methylation signal. In recent years, there has been interest in pharmacologic therapies that target this mechanism by inhibiting DNMT, resulting in hypomethylation of the DNA and re-expression of tumor suppressor genes. Cytosine analogues such as decitabine have been shown to inhibit DNMT and are being used against MDS, as well as AML and other cancers (CitationLeone et al 2002; CitationDas and Singal 2004).
Multiple genes appear to be hypermethylated in MDS, including p15INK4B, which encodes a cell-cycle inhibitor. Evidence suggests that p15INK4B methylation is correlated with blastic bone marrow involvement and that it increases during disease progression to AML (CitationQuesnel et al 1998; CitationQuesnel and Fenaux 1999). Methylation of the p15INK4B gene may allow leukemic cells to escape the inhibitory signals in the bone marrow (CitationQuesnel et al 1998; CitationQuesnel and Fenaux 1999). Decitabine treatment has been shown to reverse hypermethylation of p15INK4B, allowing for re-establishment of normal p15INK4B protein expression (CitationDaskalakis et al 2002). In addition, hypomethylation of p15INK4B has been associated with hematologic response, supporting pharmacologic demethylation as a possible mechanism for clinical response (CitationDaskalakis et al 2002).
Decitabine is believed to have a dual mechanism of action depending on dose. At both lower and higher doses, decitabine incorporates into DNA; however, at higher doses, decitabine inhibits cell proliferation through nonreversible covalent linking with DNA methyltransferase and blocking of DNA synthesis (CitationLeone et al 2002). At lower doses, decitabine induces hypomethylation, thereby promoting cell differentiation, re-expression of tumor suppressor genes, stimulation of immune mechanisms, and suppression of tumor growth (CitationLeone et al 2002; CitationMund et al 2005).
Description and structure of decitabine
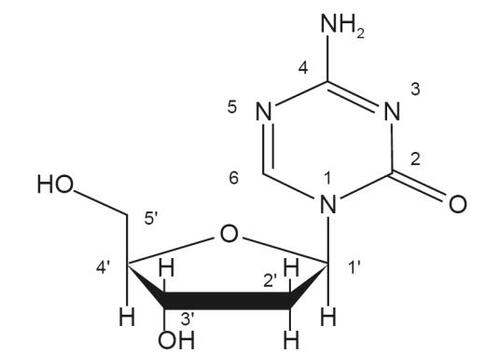
Decitabine (5-aza-2’-deoxycytidine) is a cytosine analogue modified in position 5 of the pyrimidine ring (). Decitabine is slightly soluble in ethanol/water (50/50), methanol/water (50/50), and methanol; sparingly soluble in water; and soluble in dimethylsulfoxide (DMSO). Decitabine (Dacogen™ for Injection) is a white to almost-white sterile lyophilized powder supplied in a clear, colorless glass vial (CitationDacogen 2006).
Pharmacokinetics
Decitabine distributes extensively throughout human tissues. In a phase 1 pharmacokinetic study of decitabine in 21 patients with advanced solid tumors, the mean value of volume of distribution was found to be 4.59 L/kg ± 1.42 (Citationvan Groeningen et al 1986). Although the exact route of elimination and metabolic fate of decitabine is unknown in humans, high total body clearance values and a total urinary excretion of less than 1% of the administered dose suggest that decitabine is eliminated rapidly and primarily through enzymatic metabolism (Citationvan Groeningen et al 1986).
In a more recent pharmacokinetic phase 1 study, 16 patients with MDS/AML were administered decitabine at a dose of 15 mg/m2 as a 3-hour infusion every 8 hours for 3 consecutive days of a 6-week cycle for two cycles (CitationCashen et al 2005). Preliminary results suggest that repeated administration of decitabine does not result in systemic accumulation of the drug. For the five patients who received decitabine for two cycles, maximum concentration (Cmax) values for Cycle 1 (49.0 ± 22.2 ng/mL) and Cycle 2 (62.7 ± 45.2 ng/mL) were comparable, suggesting that decitabine pharmacokinetics remain unchanged from cycle to cycle.
Clinical studies of decitabine
Phase 2 studies of decitabine in MDS patients yielded encouraging response rates, including overall responses (complete response [CR] + partial response [PR]) of 26%–45% and complete responses of 21%–28% (CitationWijermans et al 1997; CitationWijermans et al 2000; CitationSaba et al 2005; CitationSaba and Wijermans 2005). These results led to a North American, multicenter phase 3 study of decitabine compared with supportive care in 170 MDS patients, which formed the basis for the FDA approval of decitabine (CitationSaba et al 2004; CitationKantarjian et al 2006). Patients were stratified by IPSS risk group and type of MDS (de novo or secondary) and randomly assigned to receive either supportive care alone or decitabine at a dose of 15 mg/m2 as a 3-hour infusion every 8 hours for 3 days, repeated every 6 weeks, plus supportive care. Primary endpoints were overall response rate (ORR) and time to AML transformation or death. Responses were assessed using the International Working Group (IWG) criteria (CitationCheson et al 2000), which defined a CR as normalization of peripheral counts and bone marrow for at least 8 weeks with serial bone marrow blasts less than 5% without dysplastic changes, hemoglobin greater than 11 g/dL, a neutrophil count 1.5 × 109/L or greater, and a platelet count of 100 × 109/L or greater. A PR was defined similarly to CR except for the reduction of ≥50% of blasts that remained above 5%, or a downgrade in the FAB criteria. Response criteria had to be met for at least 8 weeks. The study design dictated that patients be removed from therapy after two cycles of a maintained CR.
The results of the phase 3 study indicate that decitabine is clinically effective in patients with MDS. Patient baseline characteristics were well balanced between the two study arms. Responses were defined according to strict IWG criteria (CitationCheson 2000). The ORR of patients in the decitabine arm was 17% compared with 0% in the supportive care only arm (p < 0.001) (). In decitabine-treated patients considered evaluable for response (ie, those patients with pathologically confirmed MDS at baseline who received at least two cycles of treatment), the ORR was 21% (12/56) (CitationMcKeage and Croom 2006). Responses were observed across all IPSS risk groups and were found to be durable, with a median duration of response of 10.3 months (). Median time to first response (PR or CR) was 3.3 months (). Hematologic improvement (HI) was observed in an additional 13% of patients in the decitabine group versus 7% in the supportive care arm. The overall improvement rate for patients receiving decitabine was 30% versus 7% for patients receiving supportive care. Patients in the decitabine arm had a median time to AML or death that was 4.3 months greater than that of patients in the supportive care only arm (p = 0.16) (). When patient subgroups were analyzed, patients receiving decitabine experienced a longer time to AML or death than patients receiving supportive care only (treatment-naïve [12.3 vs 7.3 months; p = 0.08] [], IPSS risk of intermediate-2/high-risk patients [12.0 vs 6.8 months; p = 0.03] [], IPSS high-risk patients [9.3 vs 2.8 months; p = 0.01], or de novo MDS [12.6 vs 9.4 months; p = 0.04]).
Figure 2 Time to acute myeloid leukemia (AML) or death: (A) all patients; (B) treatment-naïve patients; (C) International Prognostic Scoring System subgroups intermediate-2 to high-risk patients. (Reprinted, with permission, from CitationKantarjian et al 2006).
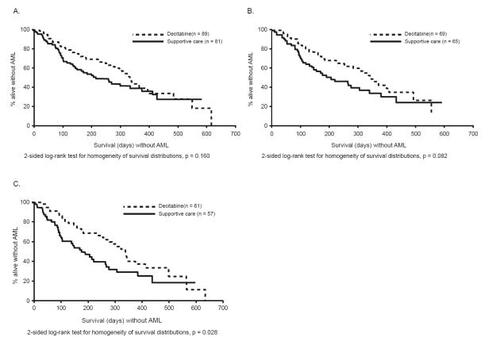
Table 2 Response to decitabine (ITT) using the FDA approved dose of 15 mg/m2 over 3 hours every 8 hours ×3 days every 6 weeks (Adapted, with permission, from CitationKantarjian et al (2006))
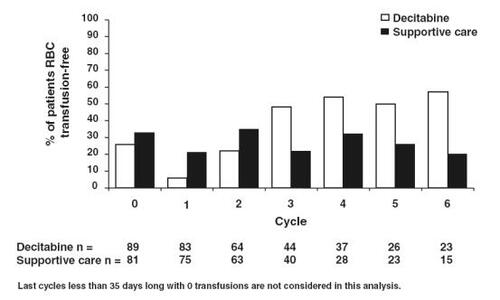
All responders in the phase 3 study, defined as patients achieving a CR or PR, became RBC and platelet transfusion independent in the absence of growth factors during the time of the response (CitationSaba et al 2004; CitationSaba and Wijermans 2005; CitationKantarjian et al 2006). The percentage of patients in the decitabine arm who became RBC transfusion independent increased with increased number of treatment cycles, while the percentage of patients on supportive care who required RBC transfusions did not change (). All eight responders who had cytogenetic abnormalities at baseline and were evaluable for cytogenetic response achieved a cytogenetic response (seven major responses and one minor response). The median number of cycles delivered was three, with 43 of 89 patients receiving two or more cycles. Of the 15 patients who responded after decitabine treatment, the median number of courses was six. In contrast, the median number of cycles in the phase 2 studies was four, which may in part explain the slightly higher response rates in the phase 2 studies (CitationSaba et al 2005). The authors of the phase 3 study speculate that greater benefit may have been observed if the study design had allowed patients to continue receiving decitabine therapy for a longer period of time.
Figure 3 Percentage of patients red blood cell (RBC) transfusion free per cycle (Reprinted, with permission, from CitationKantarjian et al 2006).
Safety data were evaluated for 83 patients treated with decitabine and 81 patients who received supportive care only. Overall, decitabine therapy was well tolerated with manageable adverse effects. The most common adverse effects included myelosuppression (neutropenia, thrombocytopenia, and anemia), pyrexia, fatigue, nausea, cough, petechiae, diarrhea, and constipation (). Febrile neutropenia occurred in 28% of patients who received decitabine. The authors noted that neutropenia, thrombocytopenia, anemia, and leukopenia appeared to diminish in incidence over the first four cycles of decitabine treatment; however, these toxicities remained frequent, most likely owing to the continuing presence of underlying disease and myelosuppression.
Table 3 Most common adverse events of decitabine
Decitabine has also been studied in MDS patients with disease recurrence who had previously responded to the drug. Rüter and colleagues reported recently on 22 patients who received decitabine retreatment at the time of disease relapse (CitationLubbert et al 2004; CitationRuter et al 2006). These patients had initially received a median of six courses of decitabine (range 2–6 courses), which resulted in a CR in 55% (12 of 22 patients), a PR in 27% (6 of 22), and a hematologic improvement in 18% (4 of 22). Decitabine retreatment was initiated at a median of 11 months after the last course of initial treatment. In the retreatment stage, patients received a median of three courses, resulting in 45% (10 of 22 patients) of patients responding (7 HI, 2 PR, and 1 CR). The median duration of second response was 4 months. Because the quality and duration of the second response were inferior to those of the first response, the authors suggest that decitabine responders may derive more clinical benefit from a longer period of initial treatment.
Optimal dosing of decitabine
As previously described, decitabine is believed to have a dual mechanism of action depending on dose, with higher doses associated with cytotoxicity and lower doses associated with demethylation. Because of this dose-dependent mechanism of action, lower-dose schedules of decitabine may be safer and more effective than higher dose schedules. Indeed, early studies of decitabine using high doses of the drug showed activity in various types of hematologic malignancies but with significant prolonged myelosuppression (CitationSantini et al 2001). In a more recent study, decitabine appeared significantly more active at lower doses compared with higher doses (CitationIssa et al 2004).
In order to further optimize therapy with decitabine, Kantarjian and colleagues performed a randomized study of three low-dose schedules in patients with MDS and chronic myelomonocytic leukemia (CMML) (CitationO’Brien et al 2005; CitationKantarjian et al 2005; CitationKantarjian et al 2007). The decitabine dose per course was reduced from the FDA approved dose of 135 mg/m2 to 100 mg/m2. In addition, the doses of decitabine were delivered every 4 weeks (rather than every 6–8 weeks), as long as there was persistent disease and no significant myelosuppression-associated complications, and therapy was continued for at least three courses before response was evaluated. The three dosing schedules were as follows: 20 mg/m2 intravenously (IV) over 1 hour daily for 5 days (arm A), 10 mg/m2 IV over 1 hour daily for 10 days (arm B), and 10 mg/m2 subcutaneously (SQ) given twice daily for 5 days (arm C).
The results of the study by Kantarjian and colleagues indicate that lower dose schedules of decitabine have higher efficacy in MDS patients (CitationO’Brien et al 2005; CitationKantarjian et al 2005; CitationKantarjian et al 2007). At least one course of therapy was received by 95 patients. Response criteria for CR and PR were the same as for AML (PR also requiring a decrease in blasts by >50%) but required response durability for at least 4 weeks. In total, 32 patients had a CR (34%), 1 patient had a PR (1%), 23 patients (24%) had marrow CR without (n = 10, 11%) or with other HI responses (n = 13, 14%), and 13 patients had an HI (14%). When analyzed by schedule, the complete response rates were 39% for arm A, 24% for arm B, and 21% for arm C (). The median number of courses to reach CR for all treatment groups was three (range, 1–7). Myelosuppression was the primary toxicity reported and it occurred more with arm B (). In summary, the schedule of 20 mg/m2 IV over 1 hour daily for 5 days was found to be superior to the other two regimens and to offer an excellent therapeutic option in addition to the FDA approved dose ().
Table 4 Efficacy and side effects of three alternative decitabine dosing schedules (Adapted, with permission, from CitationKantarjian et al(2007))
Table 5 Decitabine dosing schedules
Approved indications for decitabine
Decitabine is approved for the treatment of patients with MDS, including previously treated or untreated, de novo or secondary MDS of all FAB subtypes (RA, RARS, RAEB, RAEB-T, and CMMoL) and intermediate-1, intermediate-2, and high-risk IPSS groups (CitationDacogen [package insert] 2006). Decitabine dosing for MDS is 15 mg/m2 via a 3-hour continuous infusion three times a day for 3 days for the first treatment cycle, repeated every 6 weeks. It is recommended that patients be treated for a minimum of four cycles; however, it is noted that a complete or partial response may take longer than four cycles. Treatment may be continued as long as the patient continues to benefit. Patients may be premedicated with standard antiemetic therapy.
Decitabine treatment is associated with myelosuppression, so complete blood counts are recommended before each cycle of therapy, or as needed to assess toxicity (CitationDacogen 2006). After the first cycle of therapy, dose adjustments and delays may be required and are outlined in the package labeling. Clinicians should consider the early administration of growth factors and/or antimicrobial agents for prevention or treatment of infections. Myelosuppression and worsening neutropenia may occur more frequently in the first or second treatment cycles, and may not necessarily indicate progression of underlying MDS. Decitabine is contraindicated in patients with a known hypersensitivity to decitabine and carries a pregnancy category D rating.
Preparation and stability
Decitabine should be aseptically reconstituted with 10 mL of Sterile Water for Injection (USP) (CitationDacogen 2006). Immediately after reconstitution, the solution should be further diluted with 0.9% sodium chloride injection, 5% dextrose injection, or lactated Ringer’s injection to a final drug concentration of 0.1–1.0 mg/mL. Unless used within 15 minutes of reconstitution, the diluted solution must be prepared using cold (2 °C–8 °C) infusion fluids and stored at 2 °C–8 °C (36 °F–46 °F) for up to a maximum of 7 hours until administration.
Azacitidine
Azacitidine is the only other approved hypomethylating agent for the treatment of MDS. Although similar in structure to decitabine, azacitidine contains ribose rather than deoxribose and is incorporated primarily into RNA and to a much lesser extent into DNA. This difference may account for the approximately 10-fold higher potency of decitabine compared with azacitidine (CitationCreusot et al 1982).
In a randomized phase 3 trial of azacitidine in patients with MDS, azacitidine produced results similar to decitabine, with an ORR (CR + PR) in the azacitidine arm of 16.2% compared to 0% in the supportive care arm (p < 0.0001) (CitationSilverman et al 2002; CitationKaminskas et al 2005). The CR rate in patients treated with azacitidine was 6.1% compared with 0% for patients treated with supportive care. Median time to AML or death was significantly increased with azacitidine treatment (21 months compared with 13 months for supportive care). As expected for this class of agent, the most common treatment-related toxicity was myelosuppression.
Because of the lack of any head-to-head trials, it is difficult to compare the efficacy of decitabine and azacitidine. Differences in study design between the two completed phase 3 trials add to this difficulty. Patients in the phase 3 azacitidine study were able to stay on treatment longer, resulting in a median of nine treatment cycles (CitationSilverman et al 2002), compared with those in the phase 3 decitabine trial who received a median of three treatment cycles (CitationKantarjian et al 2006). The median duration of MDS was 7.3 months in the decitabine study compared with 2.8 months in the azacitidine trial, suggesting that the decitabine patients had more aggressive disease (CitationSilverman et al 2002; CitationKantarjian et al 2006). In addition, response criteria in the azacitidine trial were less rigorous, requiring a CR or PR for at least 4 weeks and not requiring disappearance of dysplastic changes (CitationSilverman et al 2002), compared with the decitabine study in which response was determined using IWG criteria (CitationKantarjian et al 2006).
Future considerations
Clinical studies are now under way to evaluate combination therapy with decitabine and other agents. Farthest along in development is the combination of decitabine and histone deacetylase (HDAC) inhibitors such as valproic acid. Results from a recent phase 1/2 study of decitabine and valproic acid suggest that this combination has significant activity in patients with AML and MDS and is associated with changes in histone acetylation and DNA hypomethylation (CitationGarcia-Manero et al 2006). Other agents that are being studied in combination with decitabine include amsacrine, idarubicin, daunorubicin, topotecan, cisplatin, carboplatin, and imatinib (CitationPlumb et al 2000; CitationGarcia-Manero and Gore 2005).
Decitabine therapy, alone and in combination with other agents, has shown encouraging results in other studies involving AML patients (CitationRivard et al 1981; CitationMomparler et al 1985; CitationPinto et al 1989; CitationPetti et al 1993; CitationLubbert et al 2005). Preliminary results from a recent phase 2 study in AML patients not eligible for induction chemotherapy suggest that decitabine is an active first-line treatment (CitationLubbert et al 2005). Decitabine was administered at a dose of 135 mg/m2 IV over 72 hours repeated every 6 weeks for up to four courses, with all-transretinoic acid (ATRA) administered at a dose of 45 mg/m2 per day for 28 days during the second course in decitabine-sensitive patients. In the 29 fully evaluable patients, a CR was observed in four patients (14%) and a PR was observed in five patients (17%). Toxicities with decitabine were similar to those described for MDS, and no unexpected toxicities were observed with the combination of decitabine plus ATRA.
Although not within the scope of this review, decitabine is also being evaluated in chronic myelogenous leukemia (CML) (CitationSacchi et al 1999; CitationKantarjian et al 2003; CitationIssa et al 2005) and in some solid tumors including renal, ovarian, colorectal, and cervical cancer (Citationvan Groeningen et al 1986; CitationAbele et al 1987; CitationSessa et al 1990; CitationClavel et al 1992; CitationMomparler et al 1997; CitationThibault et al 1998; CitationSchwartsmann et al 2000; CitationPlumb et al 2000; CitationSamlowski et al 2005; CitationStewart et al 2005; CitationGollob et al 2006).
Summary
Results from phase 2 and phase 3 studies indicate that decitabine is effective in the treatment of MDS, resulting in durable clinical responses and delayed time to AML transformation or death.
Decitabine has been shown to be well tolerated, with a toxicity profile expected for this class of agents. Recent studies suggest that the efficacy of decitabine may be further optimized by allowing for multiple treatment cycles and by using lower-dose schedules such as a schedule of 20 mg/m2 IV over 1 hour daily for 5 days. Future directions for decitabine include its use in combination therapy with agents such as HDAC inhibitors and in AML.
References
- AbeleRClavelMDodionPThe EORTC Early Clinical Trials Cooperative Group experience with 5-aza-2’-deoxycytidine (NSC 127716) in patients with colo-rectal, head and neck, renal carcinomas and malignant melanomasEur J Cancer Clin Oncol198723192142449354
- AlessandrinoEPAmadoriSBarosiGEvidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of HematologyHaematologica200287128630612495903
- American Cancer SocietyMultiple myeloma detailed guide2006 8/3/06
- American Cancer SocietyWhat are myelodysplastic syndromes and myelodysplastic/myeloproliferative diseases?2005 19/5/06 L:\\65589.pdf
- Aplastic Anemia & MDS International FoundationMyelodysplastic syndromes basic explanations2005 15/2/06
- AulCBowenDTYoshidaYPathogenesis, etiology and epidemiology of myelodysplastic syndromesHaematologica19988371869542325
- BennettJMCatovskyDDanielMTProposals for the classification of the myelodysplastic syndromesBr J Haematol198251189996952920
- BowenDCulliganDJowittSGuidelines for the diagnosis and therapy of adult myelodysplastic syndromesBr J Haematol200312018720012542475
- CashenAShahAHelgetAA phase I pharmacokinetic trial of decitabine administered as a 3-hour infusion to patients with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS)Blood2005106527a8a
- ChesonBDBennettJMKantarjianHReport of an international working group to standardize response criteria for myelodysplastic syndromesBlood2000963671411090046
- ClavelMMonfardiniSFossaS5-Aza-2’-deoxycytidine (NSC 127716) in non-seminomatous testicular cancer. Phase II from the EORTC Early Clinical Trials Cooperative Group and Genito-Urinary GroupAnn Oncol199233994001377488
- CortesJGilesFO’BrienSResults of imatinib mesylate therapy in patients with refractory or recurrent acute myeloid leukemia, high-risk myelodysplastic syndrome, and myeloproliferative disordersCancer2003972760612767088
- CreusotFAcsGChristmanJKInhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-Aza-2’ deoxycytidineJ Biol Chem1982257204186173384
- Dacogen [package insert]2006MGI Pharmaceuticals Inc
- DasPMSingalRDNA methylation and cancerJ Clin Oncol20042246324215542813
- DaskalakisMNguyenTTNguyenCDemethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2’-deoxycytidine (decitabine) treatmentBlood200210029576412351408
- FeldmanEJFarnesyltransferase inhibitors in myelodysplastic syndromeCurr Hematol Rep200541869015865870
- GanserAHoelzerDClinical course of myelodysplastic syndromesHematol Oncol Clin North Am19926607181613009
- Garcia-ManeroGGoreSDFuture directions for the use of hypomethylating agentsSemin Hematol200542S50916015506
- Garcia-ManeroGKantarjianHMSanchez-GonzalezBPhase I/II study of the combination of 5-aza-2’ -deoxycytidine with valproic acid in patients with leukemiaBlood20061083271916882711
- GollobJASciambiCJPetersonBLPhase I trial of sequential low-dose 5-aza-2’-deoxycytidine plus high-dose intravenous bolus interleukin-2 in patients with melanoma or renal cell carcinomaClin Cancer Res20061246192716899610
- GreenbergPCoxCLeBeauMMInternational scoring system for evaluating prognosis in myelodysplastic syndromesBlood1997892079889058730
- HofmannWKKoefflerHPMyelodysplastic syndromeAnnu Rev Med20055611615660498
- IssaJPGarcia-ManeroGGilesFJPhase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in hematopoietic malignanciesBlood200410316354014604977
- IssaJ-PJGharibyanVCortesJPhase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylateJ Clin Oncol20052339485615883410
- JabbourEJGilesFJNew agents in myelodysplastic syndromesCurr Hematol Rep20054191915865871
- KaminskasEFarrellAAbrahamSApproval summary: azacitidine for treatment of myelodysplastic syndrome subtypesClin Cancer Res2005113604815897554
- KantarjianHIssaJPRosenfeldCSDecitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized studyCancer2006106179480316532500
- KantarjianHO’BrienSGilesFDecitabine low-dose schedule (100 mg/m2/course) in myelodysplastic syndrome (MDS). Comparison of 3 different dose schedulesBlood200510611 pt 1708a
- KantarjianHOkiYGarcia-ManeroGResults of a randomized study of three schedules of low-dose decitabine in higher risk myelodysplastic syndrome and chronic myelomonocytic leukemiaBlood200710952716882708
- KantarjianHMO’BrienSCortesJResults of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemiaCancer200398522812879469
- KurzrockRMyelodysplastic syndrome overviewSemin Hematol200239182512214289
- LeoneGTeofiliLVosoMTDNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemiasHaematologica20028713244112495905
- ListAFVardimanJIssaJPMyelodysplastic syndromesHematology2004Washington DCAmerican Society of Hematology297317
- LubbertMRuterBSchmidMContinued low-dose decitabine (DAC) is an active first-line treatment of older AML patients: first results of a multicenter phase II studyBlood2005106527a
- LubbertMWijermansPWRuterBHRe-treatment with low-dose 5-Aza-2’ deoxycytidine (decitabine) results in second remissions of previously responsive MDS patientsBlood2004104405a406a
- McKeageKCroomKFDecitabine: in myelodysplastic syndromesDrugs200666951816740014
- MomparlerRLBouffardDYMomparlerLFPilot phase I-II study on 5-aza-2’-deoxycytidine (Decitabine) in patients with metastatic lung cancerAnticancer Drugs19978358689180389
- MomparlerRLRivardGEGygerMClinical trial on 5-aza-2’-deoxycytidine in patients with acute leukemiaPharmacol Ther198530277862433702
- MundCHackansonBStresemannCCharacterization of DNA demethylation effects induced by 5-Aza-2’-deoxycytidine in patients with myelodysplastic syndromeCancer Res20056570869016103056
- Myelodysplastic Syndromes FoundationFrequently asked questions about MDS [online]2006 URL: http://www.mds-foundation.org/patientinfo.htm
- Myelodysplastic Syndromes FoundationBennettJMThe myelodysplastic syndromes: a review for patients, families, friends, and healthcare professionals [online]2006 URL: http://www.mds-foundation.org/patientinfo.htm
- National Comprehensive Cancer NetworkClinical practice guidelines in oncology: myelodysplastic syndromes200642006
- O’BrienSIssaJPRavandiFDecitabine low-dose schedule (100 mg/m2/course) in myelodysplastic syndome (MDS). Comparison of 3 different dose schedulesJ Clin Oncol200523571s
- PettiMCMandelliFZagonelVPilot study of 5-aza-2’-deoxycytidine (Decitabine) in the treatment of poor prognosis acute myelogenous leukemia patients:preliminary resultsLeukemia19937Suppl 136417683355
- PintoAZagonelVAttadiaV5-Aza-2’-deoxycytidine as a differentiation inducer in acute myeloid leukaemias and myelodysplastic syndromes of the elderlyBone Marrow Transplant19894Suppl 328322483349
- PlumbJAStrathdeeGSluddenJReversal of drug resistance in human tumor xenografts by 2’-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene promoterCancer Res20006060394411085525
- QuesnelBFenauxPP15INK4b gene methylation and myelodysplastic syndromesLeuk Lymphoma1999354374310609781
- QuesnelBGuillermGVereecqueRMethylation of the p15(INK4b) gene in myelodysplastic syndromes is frequent and acquired during disease progressionBlood1998912985909531610
- RivardGEMomparlerRLDemersJPhase I study on 5-aza-2’-deoxycytidine in children with acute leukemiaLeuk Res19815453626173545
- RuterBWijermansPWLubbertMSuperiority of prolonged low-dose azanucleoside administration? Results of 5-aza-2’-deoxycytidine retreatment in high-risk myelodysplasia patientsCancer200610617445016532502
- SabaHRosenfeldCIssaJPFirst report of the phase III North American trial of decitabine in advanced myelodysplastic syndrome (MDS)Blood200410423a
- SabaHILubbertMWijermansPWResponse rates of phase 2 and phase 3 trials of decitabine (DAC) in patients with myelodysplastic syndromes (MDS)Blood2005106706a15802527
- SabaHIWijermansPWDecitabine in myelodysplastic syndromesSemin Hematol200542S233116015501
- SacchiSKantarjianHMO’BrienSChronic myelogenous leukemia in nonlymphoid blastic phase: Analysis of the results of first salvage therapy with three different treatment approaches for 162 patientsCancer1999862641
- SamlowskiWELeachmanSAWadeMEvaluation of a 7-day continuous intravenous infusion of decitabine: inhibition of promoter-specific and global genomic DNA methylationJ Clin Oncol200523389790515753459
- SantiniVKantarjianHMIssaJPChanges in DNA methylation in neoplasia: pathophysiology and therapeutic implicationsAnn Intern Med20011345738611281740
- SchwartsmannGSchunemannHGoriniCNA phase I trial of cisplatin plus decitabine, a new DNA-hypomethylating agent, in patients with advanced solid tumors and a follow-up early phase II evaluation in patients with inoperable non-small cell lung cancerInvest New Drugs200018839110830142
- SekeresMAArsenic trioxide as a treatment for myelodysplastic syndromeCurr Hematol Rep20054596315610661
- SessaCten BokkelHWStoterGPhase II study of 5-aza-2’-deoxycytidine in advanced ovarian carcinoma. The EORTC Early Clinical Trials GroupEur J Cancer19902613781691012
- SilvermanLRDemakosEPPetersonBLRandomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group BJ Clin Oncol20022024294012011120
- StewartDJKurzrockROkiYPharmacodynamics of decitabine 5 days/week × 2 weeks in advanced cancersJ Clin Oncol200523suppl 16S219s
- ThibaultAFiggWDBerganRCA phase II study of 5-aza-2’-deoxycytidine (decitabine) in hormone independent metastatic (D2) prostate cancerTumori1998848799619724
- van GroeningenCJLeyvaAO’BrienAMPhase I and pharmacokinetic study of 5-aza-2’-deoxycytidine (NSC 127716) in cancer patientsCancer Res198646483162425959
- VardimanJWHarrisNLBrunningRDThe World Health Organization (WHO) classification of the myeloid neoplasmsBlood2002100229230212239137
- WijermansPLubbertMVerhoefGLow-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patientsJ Clin Oncol2000189566210694544
- WijermansPWKrulderJWHuijgensPCContinuous infusion of low-dose 5-Aza-2’-deoxycytidine in elderly patients with high-risk myelodysplastic syndromeLeukemia199711159001409
- WilliamsonPJKrugerARReynoldsPJEstablishing the incidence of myelodysplastic syndromeBr J Haematol19948774357986716