Abstract
Hyperprolactinemia is a common endocrinological disorder that may be caused by several physiological and pathological conditions. Several drugs may determine a significant increase in prolactin serum concentration that is frequently associated with symptoms. The so-called typical antipsychotics are frequently responsible for drug-related hyperprolactinemia. Risperidone is one of the atypical neuroleptics most likely to induce hyperprolactinemia, while other atypical drugs are unfrequenlty and only transiently associated with increase of prolactin levels. Women are more sensitive than men to the hyperprolactinemic effect of antipsychotics. Classical and risperidone-induced hyperprolactinemia may be revert when a gradual antipsychotic drug discontinuation is combined with olanzapine or clozapine initiation. Antidepressant drugs with serotoninergic activity, including selective serotonin reuptake inhibitors (SSRI), monoamine oxidase inhibitors (MAO-I) and some tricyclics, can cause hyperprolactinemia. A long list of other compounds may determine an increase in prolactin levels, including prokinetics, opiates, estrogens, anti-androgens, anti-hypertensive drugs, H2-receptor antagonists, anti-convulsivants and cholinomimetics. Finally, hyperprolactinemia has also been documented during conditioning and after autologous blood stem-cell transplantation and during chemotherapy, even though disturbances of prolactin seem to occur less frequently than impairments of the hypothalamus-pituitary-gonad/thyroid axis after intensive treatment and blood marrow transplantation.
Regulation of prolactin secretion
Prolactin (PRL) is a 23 kDa polypeptide hormone secreted by the lactotroph cells of the anterior pituitary gland. It is released with a circadian trend, in 4 to 14 daily secretory pulses of increasing amplitude after sleep onset, with a decline short after waking and nadir around noon. Prolactin secretion responds to physiological stimuli: it increases after food intake and breast mechanical stimulation. Prolactin main biological role is milk induction and lactation, but it probably exerts also metabolic effects and takes part in reproductive mammary development, parental behavior (CitationBenker et al 1990) and immune responsiveness stimulation (CitationHalbreich et al 2003).
Even though monomeric 23 kDa form is predominant, big variants of 50 kDa and prolactin-IgG complexes of 150 kDa, with high immunogenic properties but poor biological effects, can circulate in large amount (up to 85% of total prolactin). This condition, referred to as “macroprolactinemia”, is characterized by normal levels of biological prolactin and lack of clinical symptoms (CitationGibney et al 2005).
Accurate definition of normal prolactin serum concentration can be difficult, because of high inter-individual variability and the frequent occurrence of “macroprolactinemia”, that can be revealed by polyethylene glycol precipitation of serum samples (CitationSuliman et al 2003).
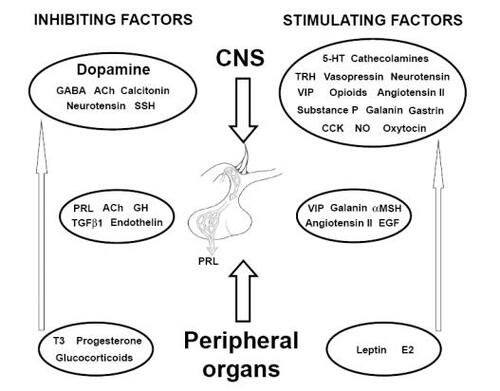
Prolactin homeostasis is the result of a complex balance between positive and negative stimuli, derivating from both external and endogenous environment. A plenty of mediators, of central, pituitary and peripheral origin (CitationFreeman et al 2000, ), take part in regulating prolactin secretion, through a direct or indirect effect on lactotroph cells.
The main physiologic control of prolactin secretion is exerted by the inhibiting action of dopamine. Dopamine, secreted in hypothalamic periventricular zone (periventricular nucleus and arcuates nucleus) and released from neuronal projections in the median eminence, reaches the anterior pituitary gland through portal vessels (system known as “tuberoinfundibular dopamine pathway” or “TIDA”). The dopamine-mediated inhibition of prolactin secretion occurs through the binding of D2 receptors on the membrane of lactotroph cells and involves several signal transduction systems, resulting in inhibition of prolactin gene transcription, reduction of prolactin synthesis and release.
Other prolactin inhibiting factors of CNS derivation include GABA, somatostatin, acetylcholine and norepinephine; an autocrine regulation is exerted by prolactin through a short loop negative feedback on its own release, by stimulating TIDA cells through prolactin receptors binding, while GH, TGFβ1 and endothelin have a paracrine regulating role at pituitary level.
Prolactin secretion mostly results from removal of dopamine inhibiting pathway. Hence, most “prolactin-relasing factors (PRFs)” act indirectly trhough the disactivation of TIDA system, though direct effects on the lactotrophs and other mechanisms of action, not completely clarified, are certainly present.
Serotonin physiologically mediates nocturnal surges and suckling-induced prolactin rises and is a potent, though indirect, modulator of prolactin secretion. The serotoninergic neurons project from the dorsal raphe nucleus to the medial basal hypothalamus and exert their action via 5HT1A and 5HT2 receptors mechanisms; Paraventricular Nucleus (one of the prominent nuclei of the Medial Hypothalamus), proved by pharmacologic and anatomic data to be a major regulatory site of serotonin-induced prolactin release, contains different populations of neurosecretory cells, producing oxytocin, vasopressin, vasoactive intestinal peptide (VIP), thyreotropin releasing hormone (TRH) and other neuropeptides. It is known that serotonin affects prolactin levels through the action of one or more of these PRFs, among which VIP pathway is the best studied. VIP (and a fragment of its precursor named peptide his-isoleucine) acts both via hypothalamic afferents and direct paracrine and autocrine mechanisms, through lactotroph cell receptors binding, enhancing adenylate cyclase activity and increasing prolactin gene transcription. Oxytocin seems to participate in VIP-induced prolactin release and may act through the inhibition of TIDA. However, there is little synaptic contact between serotonin-fibers and dopamine cells. Hence, if direct inhibition of dopamine cells occurs, it is rather through serotonin volume transmission. A larger body of evidence of direct stimulation of GABAergic neurons in the vicinity of dopamine cells exists. This pathway, referred to as “tuberoinfundibular-GABA (TI-GABA) system”, has been shown to modulate prolactin secretion in humans, possibly through serotoninergic stimulation of GABA interneurons (that express the 5HT1A membrane receptor) resulting in inhibition of TIDA cells and prolactin secretion (CitationEmiliano and Fudge 2004).
TRH physiologic role is unresolved, but it is thought to stimulate both prolactin gene expression (via protein kinase C pathway) and prolactin release from anterior pituitary.
Estrogens modulate prolactin secretion in response to reproductive events through different mechanisms: amplification of mitotic activity of the lactotrophs, enhancement of prolactin gene transcription and translation through ERβ-receptor binding, indirect simulation of prolactin synthesis through VIP and OT gene expression enhancement. Estrogens have also an indirect stimulating action on prolactin release through inhibition of hypothalamic dopamine synthesis and reduction in the number of pituitary D2 receptors. The net effect is an elevation of prolactin levels through increase in amplitude of prolactin bursts, release and storage (CitationHalbreich et al 2003).
Histamine has a predominantly stimulatory effect, due to the inhibition of the dopaminergic system, which is mediated via H2-receptors following central administration and via H1-receptors following systemic infusion. During blockade of the receptor mediating the stimulatory effect, histamine may also exert a minor inhibitory effect on prolactin secretion, which involves transmitters other than dopamine and is mediated via H1-receptors following central administration and via H2-receptors following systemic administration (CitationKnigge 1990). Histamine-induced prolactin production is mediated by dopaminergic as well as serotoninergic neurons, but other PRFs (eg, beta-endorphin, VIP, vasopressin or TRH) may be involved (CitationKnigge 1990).
Opioids regulate prolactin secretion through unknown mechanisms, perhaps acting on the synchronization of pulsatile pattern of prolactin (CitationLafuente 1994) and may have a role in stress-induced hyperprolactinemic response (CitationVan Vugt and Meites 1980; CitationBenker et al 1990).
A number of other neurotrasmitters and neuropeptides can also modulate prolactin secretion, among which galanin, endothelin, TGFbeta1, angiotensin, somatostatin, substance P, neurotensin, calcitonin, EGF, natriuretic atrial peptide, bombesin, colecistokinine, acetylcholine, vasopressin ().
Hyperprolactinemia, usually defined as fasting levels at least 2 hours after waking above 20 ng/ml in men and above 25 ng/ml in women (CitationHalbreich et al 2003), is one of the most common endocrinologic disorders of the hypothalamic-pituitary axis.
Several physiologic and pathologic conditions can result in increased plasma prolactin levels (): during pregnancy, prolactin is secreted under estrogen stimuli and induces lactation; prolactin is a major stress-induced hormone, and its secretion follows psychological, environmental or physical stress; conditions of impaired prolactin metabolism are advanced liver dysfunction or chronic renal failure. Among central nervous system diseases, the most common are anterior pituitary disorders (above all prolactin-secreting adenomas, but also GH-secreting adenomas and empty sella syndrome), and any space – occupying lesion involving hypothalamus (meningiomas, craniopharingiomas, sarcoidosis, vascular impairments) or disrupting pituitary stalk connection; systemic diseases, such as severe hypothyroidism, epathic cirrhosis, chronic renal failure, polycystic ovary syndrome, estrogenic tumors from granulosa cells (CitationSantala et al 2001), pseudocyesis.
Table 1 Major physiologic and pathologic causes of hyperprolactinemia
Most symptoms of hyperprolactinemia involve the reproductive system and are due to both a direct action of prolactin on target tissues and indirect effects mediated by the decrease in gonadotropin pulsatile secretion, that leads to gonadal dysfunction. The most frequent symptoms of chronic hyperprolactinemia include reproductive dysfunction (anovulation, menstrual irregularity, sub-fertility, decreased estrogen and testosterone production), sexual impairment (diminished libido, erectile dysfunction, retrograde or painful ejaculation, orgasmic dysfunction, impotence), breast pathology (galactorrhea, breast enlargement, possible prolactin – sensitive dysplasia with increased risk for breast cancer), abnormalities associated with chronic hypogonadism (decreased bone mineral density and osteoporosis, increased cardiovascular risk), behavioral and mood alterations (depression, anxiety, hostility, memory deficit, psychosis) (CitationKinon et al 2003b), possible immunologic depression (CitationHalbreich et al 2003).
Drug-induced hyperprolactinemia
Pharmacologic hyperprolactinemia is a problem of underestimated prevalence. This is due to lack of externally visible symptoms, patients’ reluctance for embarrassing disturbs and/or clinicians’ lack of awareness.
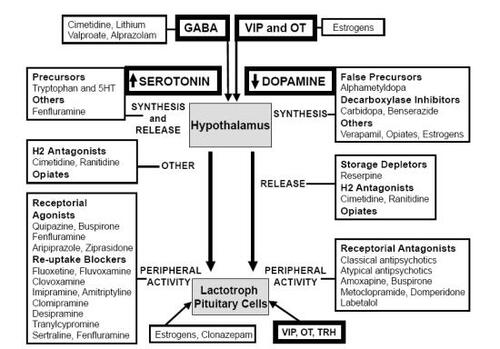
A large group of medications can raise prolactin levels ( and ): drugs variably able of impairing central nervous system (CNS) dopaminergic function, such as false dopamine precursors, inhibitors of L-aromatic aminoacids decarboxylase and dopamine receptor antagonists, drugs enhancing serotoninergic neurotransmission, such as serotoninergic precursors, direct and indirect serotonin agonists and blockers of serotonin reuptake, histamine H2 receptor antagonists (CitationSteiner et al 1976; CitationPolleri et al 1980; CitationMuller et al 1983; CitationDi Renzo et al 1989; CitationMolitch 2005).
Table 2 Drugs inducing sustained hyperprolactinemia
Most of the above mentioned medications lead to an increase in prolactin plasma concentration through an influence on prolactin secretion control, primarily removing inhibitor pathways or directly stimulating prolactin production by the lactotroph cells.
In a pharmacoepidemiological analysis conducted on French Pharmacovigilance Database, recording 182,836 adverse drug reactions from 1985 to 2000, Petit et al reported 159 cases of hyperprolactinaemia (CitationPetit et al 2003). In that study (CitationPetit et al 2003), the female/male ratio was 5.9 (136 women and 29 men) and mean age was 40 (range 14–85) years. The rates of hyperprolactinaemia according to therapeutic drug classes were as follow: 31% were associated with neuroleptics, 28% with neuroleptic-like drugs, 26% with antidepressants, 5% with H2-receptor antagonists, and 10% with other drugs (CitationPetit et al 2003). Among the latter group, veralipride, a benzamide derivated with anti-dopaminergic action (CitationVercellini et al 1994), indoramin (CitationPradalier et al 1998) and sertraline are reported in the literature as inducing hyperprolactinemia.
Psychotropic drugs
Antipsychotics
Antipsychotics are the most common cause of pharmacologic hyperprolactinemia, and the majority of antipsychotic agents cause hyperprolactinemia (CitationMolitch 2005).
The early age of onset of schizophrenia and related disorders and the need for long-term therapy make antipsychotic chronic adverse effects, such as hyperprolactinemia, a major therapeutic problem (CitationHalbreich and Kahn 2003).
In female schizophrenic patients, menstrual irregularities can be partly due to an illness-related dysfunction of the hypothalamic-pituitary-gonadal axis with subsequent hypoestrogenic state (CitationHaddad and Wieck 2004) and to abnormalities of neurotransmitters or their receptors, including D1, D2 serotoninergic alfa1 e 2, histamine, GABA and sigma opiates (CitationGoldstein SR 1999). However, since the prolactin secretory pattern of drug-free psychiatric patients, measured throughout the day, is not different from that observed in healthy control subjects, the elevation of baseline prolactin in treated psychiatric patients seems to be more related to drug effects rather than to the illness itself (CitationHaddad and Wieck 2004).
During the last ten years, a new class of neuroleptic drugs, usually referred to as “atypical antipsychotics”, has been developing, to avoid the side effects related to the complete dopaminergic blockade exerted by the oldest drugs (extrapyramidal symptoms and hyperprolactinemia). Actually, neither receptorial binding characteristics, nor clinical criteria, enable a clear cut definition of “atypical” behavior, a possibility being a positive relation between antipsychotic efficacy and degree of side effects due to antagonism of other dopamine pathways (CitationKuhn et al 2000).
In this regard, classification of antipsychotic drugs may be based on their prolactin-elevating aptitude: classical antipsychotics are traditionally “prolactin-raising”, while the newest class is usually “prolactin-sparing”.
A study of 422 psychotic patients on neuroleptic treatment showed that antipsychotic therapy is strongly associated with hyperprolactinemia, with a significantly higher prevalence of hyperprolactinemia in classic antipsychotics-treated patients compared to the “atypical”-treated group (CitationMontgomery et al 2004). Furthermore, neuroleptic-induced hyperprolactinemia is often a dose-related side effect (CitationSmith et al 2002; CitationMontgomery et al 2004).
The extent of prolactin increase is not only dependent on drug characteristics (ie, class of antipsychotic and dose administered), but also on patient’s sex and age. Clinical consequences associated with hyperprolactinemia are well documented in women on antipsychotics, in whom the prevalence of symptomatic hyperprolactinemia reaches 50% or more, the most common symptoms being galactorrhea and menstrual irregularities (CitationWieck and Haddad 2002; CitationHalbreich et al 2003). However, little information is available on the gender-specific response to prolactin elevating antipsychotics. On equal doses, women on chronic prolactin-raising antypsichotic seem more likely to develop hyperprolactinemia than men, and they reach significantly higher prolactin levels during the treatment (CitationSmith et al 2002; Kinon et al 2003). Among male patients, age was not found to influence prolactin concentration, while in women a younger age was associated with higher prolactin levels, as expected for their reproductive status (CitationHalbreich and Kahn 2003; CitationMontgomery et al 2004).
Regarding age, data from studies performed with new atypical drugs in elderly patients and in youths are consistent with previous findings in adult patients: olanzapine appears to be a prolactin-sparing antipsychotic medication in the elderly (CitationKinon et al 2003c), and quetiapine produced significantly less frequent hyperprolactinemic events and lower mean elevation of prolactin levels than the conventional drug risperidone in children (CitationStevens et al 2005).
Classical antipsychotics
Classical antipsychotics are the most common cause of drug-induced hyperprolactinemia (CitationMolitch 2005). The antipsychotic action of these dopamine-receptor blockers is based on the dopamine pathogenetic hypothesis of schizophrenic disorders. These drugs act as nonselective dopamine receptors antagonists and interfere with all four dopamine pathways (). Therapeutic effects on negative and positive psychotic symptoms occur through dopamine D2 and D4 receptors binding in the mesolimbic area, while side effects are mediated by D2 blockade in the striatal area (extrapyramidal effects) and in the hypothalamic infundibular system (hyperprolactinemia) (CitationWieck and Haddad 2002).
Prolactin is reported to reach higher levels on neuroleptics than during most of other prolactin-raising treatments and seem to increase proportionally to the antipsychotic therapeutic efficacy (CitationGruen et al 1978; CitationGreen and Brown 1988).
Although prolactin does not usually reach the levels typically associated with prolactin-secereting pituitary tumors, cases of huge elevation are reported, above 300 ng/ml (CitationRivera et al 1976; CitationPollock and McLaren 1998; CitationSmith et al 2002).
Plasma prolactin levels have been reported to increase in a dose-dependent manner (CitationSmith et al 2002; CitationMontgomery et al 2004; CitationMeltzer and Fang 1976), but even low daily dosages of classical antipsychotics can cause significant elevations (CitationWieck and Haddad 2002).
The increase begins after a few hours and persists during the rest of the treatment, the total effect depending on therapy duration: a medium-term treatment (3–9 weeks) has been found to increase baseline levels up to 10-fold (CitationMeltzer and Fang 1976), while during chronic treatment, partial tolerance may lead to prolactin normalization, though after long-term therapy prolactin remains above normal in most cases (CitationRivera et al 1976).
After suspension of the oral treatment, prolactin returns to normal range within two to three weeks, depending on the half-life of the drug and its metabolites and on the storage in the fatty tissue, but remains above pre-treatment values for six months after discontinuation in case of intramuscular depot administration (CitationWieck and Haddad 2002).
Butyrrophenones
Haloperidol is a butyrrophenone used for the treatment of schizophrenia, tics, stutter or delirium. Endocrinologic side effects (amenorrhea, galactorrhea, gynecomastia, sexual dysfunction, mastalgia) occur at undefined frequency.
A study of haloperidol (10–20 mg) versus placebo showed association of the treatment with prolactin elevation, persistent at sixth week of therapy (CitationCrawford et al 1997). Substantial increases, up to nine-fold above basal levels, have been found after single injections of haloperidol, with persistent, though less consistent, elevation (three-fold) after weeks of treatment (CitationGoodnick et al 2002). Prolactin response to haloperidol, studied in 15 patients, with prolactin sampling taken every 3 days, showed a distinct pattern: prolactin concentration quickly increased in the first 6–9 days, reaching a peak between 30 and 50 ng/mL, and then a plateau that remained mainly constant throughout the study, always below 77 ng/mL. The pattern and amplitude of the increase were not influenced by the dose administered (CitationSpitzer et al 1998).
Phenothiazines
Acute increases, of two- to tenfold, have also been observed after single parenteral or oral doses of phenothiazines. Prolactin elevation induced by a single fluphenazine injection was found to last up to 28 days. Long term studies have documented an initial increase in prolactin levels (three-fold above normal range) in the first three days of treatment, with a subsequent further increase (up to two-fold) during the following weeks of treatment. In this case, no gender-dependent response was observed (CitationGoodnick et al 2002).
A great, dose-dependent increase in prolactin has been observed in 40% to 90% of patients treated with phenothiazines (CitationRivera et al 1976; CitationKinon et al 2003a). Chlorpromazine-induced hypeprolactinemia is reported to develop earlier during the treatment and to reach higher levels in women than in men (CitationMeltzer et al 1983). The increase begins hours after the first i.m. or oral administration (CitationMannisto et al 1978; CitationBusch et al 1979) and high levels persist during treatment period (CitationKolakowska et al 1976).
Thioridazine 50 mg was found to increase prolactin at level similar to those observed after chlorpromazine, within the first two hours following administration (CitationSachar et al 1975).
Thioxanthenes
In a study that compared the effects of thiothixhene and thioridazine (CitationCrowley and Hydinger-Macdonald 1981), prolactin levels increased equally with the two drugs, but another study documented exaggerated hyperprolactinemic response to thiothixene in one subject (CitationAsh and Bouma 1981).
Unlike other “classical” thioxanthenes, flupenthixol shows an “atypical” receptorial profile, with a mixed dopamine D1/D2, serotonin and histamine H1 receptorial antagonism. In this regard, flupentixol could be referred to as “partial atypical” neuroleptic (CitationKuhn 2000). Indeed, prolactin levels during prolonged therapy with flupenthixol were found to increase two- to three-fold above baseline during the first month of therapy, with reduction at month 3 and 6 and normalization over the next few months (CitationSchlosser et al 2002).
Others
The effect on prolactin levels of pimozide administration seems to be the result of an impaired dopamine secretion to portal vessels, rather than the consequence of a reduction of hypothalamic dopamine production (CitationAguilar et al 1985).
Atypical antipsychotics
Atypical antipsychotics are characterized by increased antipsychotic efficacy and fewer neurological and endocrine-related side effects as compared to classical antipsychotic drugs. Most of them elicit poor hyperprolactinemic response or no hyperprolactinemia at all. There are several hypotheses to explain the “atypical” behavior on prolactin levels: regional limbic selectivity (CitationGoldstein 1999), preferential binding to D3 and D4 (CitationKuhn 2000), peculiar binding dynamics at the D2 receptors or differences in affinity to D2 (CitationSeeman 2002), combined antagonism of dopamine and serotonin receptors (CitationKuhn 2000).
Receptor binding dynamics have been widely investigated and are now completely identified: while classical drugs are complete dopamine antagonists, the newest antipsychotics exert a mixed dopamine-agonistic/antagonistic activity (CitationLieberman 2004). Partial agonists have a lower intrinsic activity at receptors than full agonists, and that allows them to act either as a functional antagonist or agonist depending on the surrounding levels of naturally neurotransmitters, that are fully agonists (CitationLieberman 2004). This results in an early dissociation from the receptor, that enables a normal dopaminergic neurotransmission in tuberoinfundibular pathway and avoids hyperprolactinemia (CitationLieberman 2004).
Most clinical studies on atypical drugs have been limited by blood sampling after 12 to 24 hours from the drug administration, not allowing the detection of early hyperprolactinemia. Indeed, clinical studies of pharmacokinetics have shown a transient D2 blockade by atypical antipsychotics with an early hyperprolactinemic effect after acute administration. Accordingly, only monitoring of prolactin levels within the first 24 hours after oral administration of atypical antipsychotics may reveal early modifications: prolactin serum concentration increases within the first six hours after drug administration (up to two-fold from basal level) with a variable mean peak time, also in relation to the specific drug (120 min for risperidone, 180 min for clozapine and 290 min for olanzapine), and return to baseline values within 12–24 hours. More specifically, only clozapine and risperidone result in a pathologic elevation, while olanzapine-induced prolactin increase is always within normal range (CitationTurrone et al 2002).
The hypothesis of a transient blockade of D2 receptors has been supported by PET scans showing that haloperidol constantly binds to D2 for 24 hours, while receptors occupation by atypical drugs is significantly shorter and mostly expired at the 24th hour (CitationSeeman 2002), supporting the hypothesis of a transient D2 blockade.
Indeed, partial agonistic activity was observed with bifeprunox and aripiprazole. Other antipsychotics, such as olanzapine, ziprasidone and clozapine, have been associated with attenuated dopamine-induced ERK phosphorylation (CitationBruins et al 2006).
A second theory is that of a simultaneous binding at D2 and 5-HT2A receptors, leading to a more physiologic serotonin-dopamine balance (CitationSeeman 2002). The effects of the new antipsychotics on dopamine D2/serotonin 5-HT1A receptors have been recently investigated: antipsychotics, with the exception of olanzapine, exhibited agonist properties at serotonin 5-HT1A receptors, the greatest E-max values being found for bifeprunox, efficaciously stimulating both serotonin 5-HT1A and dopamine D2 receptors, followed by aripiprazole, ziprasidone and clozapine (CitationBruins et al 2006).
Risperidone
Risperidone is one of the atypical antipsychotics most likely to induce hyperprolactinemia. This drug is used for the treatment of schizofrenia, bipolar disturb, acute mania, dementia, Tourette syndrome and autism. Risperidone has a dose-dependent serotonin and dopamine antagonistic action. It binds with very high affinity to serotoninergic 5-HT2 receptors, in the CNS and in periphery, and to dopamine D2 receptors, with an affinity 20-fold lower, but relatively higher compared to other atypical antipsychotics. Risperidone causes more marked elevations in prolactin than other atypical antipsychotics because it does not fully cross the blood-brain barrier, hence D2 receptor occupancy is higher at the level of the pituitary than in the striatum. However, the effect on prolactin levels is unlikely related to 5HT2 receptors binding, which would result in inhibition of prolactin secretion. Endocrinologic side effects, such as amenorrhea, galactorrhea, gynecomastia, sexual dysfunction in patients on risperidone are documented with a frequency of 1%–10%.
Current data show that, similarly to conventional antipsychotics, high doses of risperidone (>6 mg/day) increase prolactin levels to the range typically associated with sexual dysfunction in otherwise healthy patients (CitationPetty 1999). Some studies report that risperidone would raise prolactin levels even more than the classic antipsychotics: comparative studies showed a significantly higher prevalence and higher prolactin mean levels in risperidone-treated patients, as compared to patients treated with conventional (CitationKleinberg et al 1999; CitationKinon et al 2003a) or atypical antipsychotics such as clozapine (CitationKearns et al 2000). However, in other studies (CitationKearns et al 2000), the rise in prolactin levels was lower with risperidone than with classical neuroleptics. In the same study by Kearns et al, prolactin increase under risperidone was present in 12 out of 12 patients and was influenced by age and gender (but not by duration of the therapy), being higher in young females.
On risperidone and haloperidol therapy, prolactin serum concentration increases within a few hours after acute treatment, and remains permanently high during an extended period (up to 54 weeks for risperidone and 52 for haloperidol) (CitationTollefson et al 1997; CitationPurdon et al 2000). Cases of successful treatment with dopamine agonists of risperidone-induced hyperprolactinemia are reported (CitationTollin 2000).
Amisulpride
Amisulpride is a substituted benzamide derivative, characterized by little extrapyramidal symptoms (EPS) but great prolactin elevation, similar to that of conventional antipsychotics andoften clinically relevant (CitationFric and Laux 2003). Since amisulpride is a highly selective D2/D3 receptor antagonist, its “atypical properties” cannot be explained by combined 5-HT2/D2 antagonism (CitationLeucht et al 2002), but rather by a preferential occupancy of dopamine receptors in limbic than in striatal regions (CitationMcKeage and Plosker 2004). High dosages of amisulpride seem to preferentially antagonize postsynaptic D2/D3 receptors, resulting in reduced dopamine transmission, while low dosages preferentially block presynaptic D2/D3 receptors, resulting in enhanced dopamine transmission (CitationMcKeage and Plosker 2004). To explain prolactin elevation with low extrapyramidal symptoms, it has recently been hypothesized that amisulpride may determine a selective higher occupancy of D2/D3 at the pituitary level than at central regions, because of its poor brain barrier permeability (CitationBressan et al 2004).
Amisulpride-induced hyperprolactinemia is reported after both acute and chronic treatment and does not seem to be strongly dose-related: samples taken during the first eight hours following 20 or 100 mg i.v. single administration in ten healthy subjects revealed a significant elevation of prolactin levels (8–10 fold above baseline) (CitationWetzel et al 1994). Long-term amisulpride treatment was found to lead to a constant but slow decline in prolactin levels, that remained significantly elevated until the 12th month of treatment and then significantly decreased, but still above normal range, during the first three months after suspension (CitationSchlosser et al 2002). Therefore, amisulpride chronic therapy in patients treated with antidepressants or benzodiazepines can potentially worsen hyperprolactinemia, even at low doses (CitationKopecek et al 2004). However, the safety profile of amisulpride, as reported from a comparison of 18 clinic trials, appears to be better than traditional neuroleptics (CitationLeucht et al 2002), but it is reported more likely to cause hyperprolactinaemia than risperidone or olanzapine, amenorrhoea occurring in about 4% of treated women (CitationMcKeage and Plosker 2004). A recent study reported the rapid and complete reversibility of amisulpride-induced prolactin elevation in 17 psychiatric patients, with significant reduction after 14 to 51 days after discontinuation, independently from dosage (50–800 mg/day) or treatment duration. (CitationPaparrigopoulos et al 2006). In that study (CitationPaparrigopoulos et al 2006), mean prolactin increase was significantly greater in women than in men.
Prolactin pattern after 100 mg i.m. of the benzamide sulpiride, analyzed in 10 healthy patients through blood sampling at 30, 60 and 120 minutes has showed a quick, marked and permanent increase (CitationMancini et al 1976).
Molindone is another atypical new drug able to cause sustained hyperprolactinemia. In a study by Pandurangi et al, prolactin showed a positive dose-effect response in 14 psychiatric patients receiving high doses of molindone (CitationPandurangi et al 1989).
Problems related to hyperprolactinaemia occur less often with some atypical antipsychotics than with typical drugs, even though risperidone and amisulpride appear to have no advantages on this regard. Some other atypical antipsychotics, such as clozapine, olanzapine, quetiapine, sertindole, and ziprasidone cause only mild and transient hyperprolactinemia (CitationStanniland and Taylor 2000).
Clozapine
Clozapine, the prototypic atypical antipsychotic drug, antagonizes serotonin and dopamine receptors. It has been proposed that the selective interaction of clozapine with dopamine D1, D2 and D4 and 5HT2 receptors results in a distinctive alteration in the function of pre- and post-synaptic dopamine elements, with the hyperprolactinemic effect being mediated by supra-pituitary action of the drug (CitationMeltzer and Gudelsky 1992). The lack of prolactin increase following clozapine administration could be due to both the sparing of dopamine-mediated inhibition of prolactin release and the direct stimulatory effect on TID neurons (CitationMeltzer and Gudelsky 1992). Moreover, clozapine does not interfere with norepinephrine-mediated inhibition of prolactin secretion (CitationLamberts et al 1990). In in vitro cultured pituitary tumor cells, clozapine at high concentration appeared to directly inhibit prolactin release and DNA content, suggesting anti-mitotic action on the lactotrophs (CitationLamberts et al 1990). Early single-dose trials reported that clozapine reduced hyperprolactinemia by 16%–80% during 6 weeks of drug administration (CitationGoodnick et al 2002).
Olanzapine
Olanzapine is a potent 5-HT2 blocker that shows higher affinity for 5-HT2 than D2 at all doses. D2 occupancy is dose-dependent and seems to be similar to risperidone pattern, greater than clozapine. At the usual clinical dose range of 10–20 mg/day, receptor occupancy varies from 71% to 80%, a restricted range that may explain the modest extrapyramidal side effects and prolactin elevation. However, doses greater than 30 mg/day are associated with more than 80% D2 occupancy and may induce prolactin elevation (CitationKapur et al 1998). A double-blind placebo and haloperidol controlled trial of three doses of olanzapine, enabled the analysis of the temporal trend of serum prolactin among 137 patients. After two weeks of treatment, only the groups of medium and high olanzapine dosage (7,5–17,5 mg/day) differed significantly from the placebo group, with prolactin elevation being less frequent, lower in magnitude and duration compared to the haloperidol-treated group. At week 6, all olanzapine-treated groups exhibited an incidence of prolactin elevation comparable to that of the placebo group. Rates of elevation were approximately one-third to half those observed with haloperidol and were more transient (CitationCrawford et al 1997). Another large comparison study with haloperidol by Tollefson et al on 1996 patients confirmed these data both during acute and extended treatment (up to 52 weeks) (CitationTollefson et al 1997). Olanzapine was associated with a favorable safety profile and significantly fewer discontinuations of treatment due to adverse events (CitationTollefson et al 1997).
David et al documented significantly higher prolactin levels under risperidone than under olanzapine or haloperidol, in a side by side analysis of three independent multicenter, double blind randomized clinical trials, with a moderate increase in serum prolactin concentration for olanzapine (1–4 ng/ml), intermediate for haloperidol (17 ng/ml) and high for risperidone (45–80 ng/ml) (CitationDavid et al 2000). No consistent dose-response relationship was observed for any of the drugs; the time course and sex dependency of the response differed among the three studies, though risperidone was found to be associated with early peaks and the mean change in prolactin was found to be greater in women treated with haloperidol or risperidone (CitationDavid et al 2000).
In 2003, Kinon et al collected the results of five clinical trials on the factors that could influence serum prolactin levels in antipsychotic treated subjects, such as type of treatment, gender, time course and age. In addition, the potential reduction or reversibility of the endocrinologic side effect was also studied. From the comparison of two contemporary studies of olanzapine vs haloperidol (CitationTollefson et al 1997) and of olanzapine vs risperidone (CitationTran et al 1997), prolactin levels raised several fold above baseline in patients treated with risperidone and haloperidol but not in those treated with olanzapine, with the highest levels observed for risperidone group and females. Similar treatment- and gender-dependent effects were observed in a study directly comparing olanzapine, risperidone and haloperidol (CitationPurdon et al 2000). The switch patterns comparison attested the reversibility of classical and risperidone-induced hyperprolactinemia when previous drug is gradually discontinued and a prolactin-sparing drug is simultaneously initiated (CitationKinon et al 2000).
Melkersson reported the following prevalence of treatment-induced hyperprolactinemia in 75 patients: risperidone (medium daily dosage 3 mg/day) 89%, olanzapine (medium daily dosage 10 mg/day) 24% and clozapine (medium daily dosage 400 mg/day) 0%. Mean prolactin levels were significantly higher in risperidone and olanzapine-treated subjects as compared to clozapine-treated group (CitationMelkersson 2005).
Although higher affinity for 5-HT2 receptors than for D2 dopamine receptors is a common feature of atypical neuroleptics, considerable differences in their clinical and pharmacological properties are present. At clinical doses, atypical neuroleptics occupy serotoninergic receptors near to saturation, but show considerable differences on D2 receptor occupancies, with clozapine showing the lowest degree of occupation, as confirmed by blunted prolactin responses to the acute administration of the dopaminergic drug haloperidol after olanzapine but not after clozapine administration (CitationMarkianos et al 2002). The fact that prolactin responses to haloperidol were not altered after treatment with clozapine, but were significantly reduced after the olanzapine treatment indicates that there is a difference between the two drugs in their capacity to block dopamine receptors at the hypothalamus-pituitary level, consistently with SPECT receptor binding results on striatal dopamine receptors (CitationMarkianos et al 2002).
Other atypical drugs
Quetiapine, aripiprazole and ziprasidone are reported either to cause no prolactin increase at all or to increase it transiently and mildly: serial blood samples showed a rapid raise in prolactin levels (up to 1.5- to 2.5-fold above basal levels) during the first two-four hours after drug administration and normalization within the following eight hours (CitationTurrone et al 2002).
Quetiapine interacts with a wide range of neurotransmitter receptors and has a high affinity for serotonin 5HT2A receptors and lower affinity for dopamine D2 receptors. Furthermore, quetiapine seems to be selective for mesolimbic and mesocortical dopamine receptors, with relative sparing of TIDA system (CitationGoldstein JM 1999). Quetiapine transient action on prolactin elevation corresponds to a transient dopamine D2 occupancy: a single administration leads to receptor occupancy that ranges from 58%–64% after two to three hours, with D2 occupancy decreasing to minimum (0%–27%) within 12 hours. This high, but transient, D2 occupancy, which decreases to very low levels by the end of the dosing interval, can explain its efficacy and lack of prolactin elevation (CitationKapur et al 2000). This conclusion has been confirmed by clinical data acquired from a comparison study with haloperidol, carried out in 35 schizophrenic patients, that showed persistently and significantly lower prolactin concentration at the 6th week control in the quetiapine treated group (CitationAtmaca et al 2002). These data confirm preliminary clinical studies that documented a quetiapine effect on prolactin levels not different from placebo at week 6 (CitationHamner et al 1996).
Aripiprazole, a new atypical antipsychotic, has a unique receptor binding profile, resulting in a potent (ie, active at low dose) but “partial” agonistic effect at D2 and 5HT1A (CitationBuckley 2003; CitationBruins et al 2006) and complete antagonistic effect at 5HT2A receptors (CitationTaylor 2003; CitationLieberman. 2004) (). In vitro partial agonism at D2 receptor coincides with a reduced prolactin release in vivo, as confirmed in a comparison study of haloperidol vs aripiprazole oral administration (CitationCosi et al 2006).
Ziprasidone exerts agonistic properties at serotonin 5-HT1A receptors (CitationBruins et al 2006). In one controlled study of ziprasidone vs haloperidol 15 mg, only transient elevations in prolactin were observed, with return to the normal range within the dosing interval, at every dose of ziprasidone administered (4–160 mg/day) (CitationGoff 1998).
Zotepine is another drug reported to cause prolactin elevation during both acute and chronic administration (CitationVon Bardeleben et al 1987).
Perospirone impact on serum prolactin levels was investigated in 41 schizophrenic patients receiving clinically effective doses: blood samples obtained 10–14 hours after perospirone administration showed median levels of prolactin within normal range (in both female and male patients), still normal after more than 4 weeks. These results suggest that in contrast to risperidone, where baseline prolactin levels were elevated 5.3-fold in female and 4.2-fold in male patients, baseline prolactin levels were not elevated after treatment with perospirone (CitationTogo et al 2003). However, these results should be carefully interpreted, because drug-by-time interactions are reported in antipsychotic-induced hyperprolactinemia (CitationTogo et al 2003).
In summary, effects of antypsychotic medications on serum prolactin seem to be multifactorial. It seems that both drug type and gender influence the risk for developing hyperprolactinemia, with women being more sensitive than males and risperidone and typic drugs more likely to induce hyperprolactinemia than olanzapine (CitationKinon et al 2003b). The elderly age seemed to influence the prolactin response, leading to a blunted reaction to conventional antipsychotics (CitationStreet et al 2000) when compared with the subjects of the study by CitationPurdon et al 2000, aged 28.87 ± 8.36, as reported by CitationKinon et al 2003b. Among antypsychotic, classical drugs, and more specifically haloperidol, chlorpromazine, thioridazine and thiothixene, induce sustained hyperprolactinemia. Atypical drugs show a better pharmacological profile under this respect, even though risperidone, amisulpride and zotepine induce hyperprolactinemia with frequencies and levels similar to those observed with classical drugs. Other atypical drugs, such olanzapine, clozapine or aripiprazole can be used in switching protocols to correct an antipsychotic-induced hyperprolactinemia.
Management of antipsychotic-induced hyperprolactinemia
The importance of monitoring for health problems, among which amenorrhea, in patients on psychotropic treatment, has been stressed during an expert consensus on the pharmacologic treatment of psychotic disorders (CitationKane et al 2003). For their peculiar activity on dopamine receptors, atypical antipsychotics are usually associated with a broader spectrum of clinical efficacy and are better tolerated as compared to conventional agents. However, adverse effects such as weight gain and metabolic changes are cause for concern, even in patients treated with some of the newest drugs (CitationCassano et al 2007). The management of antipsychotic-induced hyperprolactinemia should include subsequent measures, in order to minimize the side effects, thus optimizing therapy compliance and reducing the risk of subsequent psychotic relapses. Avoiding hyperprolactinemia and its long-term complication means improving treatment outcomes and enhancement of quality of life.
A safe and effective approach to drug-induced hyperprolactinemia should consist in prolactin measurement in symptomatic patients on antipsychotic treatment and, routinely, in all the patients taking conventional drugs or risperidone (CitationMiller 2004). Patients showing plasma prolactin levels ≥100 ng/ml should undergo hypophiseal NMR to rule out the presence of a prolactinoma. In case of hyperprolactinemia during antipsychotic treatment, once other potential causes have been excluded, prolactin normalizing strategies should be attempted when clinically significant hyperprolactinemia occurs, specifically amenorrhea in women or testosterone deficiency in men (CitationHaddad and Wieck 2004; CitationMiller 2004).
Traditional pharmacological approach of hyperprolactinemia in schizophrenic patients can raise some problems, even when no concomitant antipsychotic treatment is present, because of the potential worsening effect of dopaminergic drugs on psychosis. The hypothesis that the pathophysiology of negative symptoms in schizophrenia may involve relative hypoactivity of central dopaminergic neurotransmission, however, led to the exploration of dopamine agonist strategies in the treatment of this condition (CitationLevi-Minzi et al 1991). Although the use of dopamine agonists in otherwise unmedicated schizophrenic patients often leads to the exacerbation of psychosis, trials of dopamine agonists in combination with neuroleptic agents warrant investigation (CitationLevi-Minzi et al 1991).
The effects of bromocriptine (5.0–7.5 mg/day) on antipsychotic-induced prolactin-related endocrinological disturbances (amenorrhea, galactorrhea and impotence) were investigated in psychiatric patients receiving classical neuroleptics (CitationMatsuoka et al 1986), with the following results: menstrual cycles recurred in 7 of 10 patients with amenorrhea, a decrease in lactation appeared in 5 of 6 patients with galactorrhea and a significant increase in the serum levels of testosterone was observed after 8 weeks of treatment in six out of six male patients. In the same study (CitationMatsuoka et al 1986), no remarkable deterioration of psychotic symptoms was reported in 6 schizophrenic patients. In another study (CitationSmith 1992), one woman out of 6 developed worsened psychiatric symptoms while taking bromocriptine (daily dosage 5–10 mg) and had to discontinue the therapy.
Concerning atypical drugs, the optimal management of risperidone-induced hyperprolactinemia has not been clarified, though it is widely assessed that risperidone can cause clinically significant hyperprolactinemia. Tollin (CitationTollin 2000) reported 4 patients on risperidone, with significant hyperprolactinemia (65.5 to 209 ng/ml) that showed reduction of prolactin level and alleviated hypogonadism after dopamine agonists (bromocriptine or cabergoline) were added, without deterioration of psychotic symptoms.
A more recent pilot study (CitationCavallaro et al 2004) attested the safety and efficacy of a low dose cabergoline (0.125 to 0.250 mg/week) administered for 8 weeks in 19 male and female schizophrenic patients with risperidone-induded symptomatic hyperprolactinemia. Mean plasma prolactin levels, assessed at baseline and at the end of the study, were significantly decreased in all the patients, and within the normal range in 11 patients, with remission of clinical signs; in this study no side effects or changes in the patients’ psychopathology were observed.
Cases of successful treatment of risperidone-induced hyperprolactinemia with cabergoline in youths are also reported (CitationCohen and Biederman 2001): the addition of cabergoline (initial mean dose 2.13 +/– 0.09 mg/week) in four male children on risperidone with plasma prolactin ranging 57.5–129 ng/ml, led to serum prolactin levels normalization. Cabergoline was well tolerated without adverse effects during the whole treatment (13–21 months) on maintenance dose of 1 mg/week.
A case report has been reported of bromocriptine efficacy in resolving amisulpride-induced hyperprolactinemia (CitationBliesener et al 2004).
Although dopamine-agonists have successfully been used in patients with antipsychotic–induced hyperprolactinemia, bromocriptine treatment is reported to be associated with exacerbation of acute psychotic state in psychotic women (CitationFrye et al 1982; CitationDorevitch et al 1991). Discontinuation of the bromocriptine treatment and increase in antipsychotic dosage resulted in complete remission. Caution is advisable in the use of bromocriptine especially in patients with a pre-existing psychiatric history and monitoring for changes in mental status when bromocriptine is prescribed is recommended. (CitationDorevitch et al 1991).
In conclusion, dopamine agonist therapy is not generally advisable (CitationMiller 2004), even though successful cases are reported of risperidone-induced hyperprolactinemia treated with the addition of either cabergoline or bromocriptine, without worsening psychotic symptoms (CitationTollin 2000). More specifically, bromocriptine, confirmed to be effective in reducing hyperprolactinemia and resolving amenorrhea/oligomennorrhea in schizophrenic women, should be cautiously condidered as a drug potentially exacerbating acute psychosis (CitationSmith 1992), thus leading to the conclusion that other treatment approaches should be suggested for neuroleptic-induced hyperprolactinemia and associated manifestations (CitationFrye et al 1982).
Since the discontinuation of a neuroleptic therapy, or the addition of dopamine-agonists, may worsen the psychosis, and the reduction of the neuroleptic dose may not necessarily lead to decrease in prolactin, switching to prolactin sparing agents acquires great relevance.
CitationKinon et al (2000) studied the reversibility of hyperprolactinemia by comparing four medication switching paradigms from classical drugs or risperidone to olanzapine and showed the return of prolactin within normal limit three weeks after switching. The switch patterns comparison attested that reversibility of classical and risperidone-induced hyperprolactinemia is successful when a gradual antipsychotic drug discontinuation is combined with olanzapine initiation (CitationKinon et al 2000). These results were confirmed a few years later, with olanzapine found to be able to reverse hyperprolactinemia in conventional or risperidone-treated female schizophrenic patients, to decrease amenorrhea, to improve cycle regularity and to reduce sexual side effects, thus creating a safe and effective alternative method for patients with antipsychotic-induced hyperprolactinemia (CitationKim et al 2002; CitationKinon et al 2006). In this study by CitationKinon et al 2006, male patients who switched to olanzapine treatment experienced significantly (p = .03) increased free testosterone levels, even with no significant improvement in total testosterone levels; some female patients experienced improved menstrual cycling, as well as resolution of galactorrhea and gynecomastia, and sexual functioning was significantly improved in both genders. Patients switching to olanzapine, as well as those remaining on their pre-study medication, maintained clinical stability, their symptoms continued to improve, although there were no significant between-treatment differences in improvement. Treatment-emergent adverse events occurred in both treatment groups, with no significant differences between groups.
A case report confirmed the successful strategy of switching to olanzapine while tapering risperidone: one month of titrated dose (15 mg) olanzapine normalized serum prolactin, restored menstrual regularity, resolved galactorrhea and improved the psychiatric condition (CitationCanuso et al 1998).
Six months olanzapine was reported effective in reducing prolactin levels from 116 ng/ml to 72 ng/ml also in a woman receiving phenothiazines. This patient recovered from galactorrhea and menstrual irregularities and her psychiatric condition remained stable (CitationCanuso et al 1998).
Bunker et al reported a case of correction of serum prolactin levels after conversion to clozapine therapy in a 16-year-old female patient who had developed hyperprolactinemia with galactorrhea and amenorrhea while receiving thioridazine 300 mg daily and after a period of treatment with bromocriptine (CitationBunker et al 1997).
Successful management of antipsychotic-induced hyperprolactinemia with switching to quetiapine has also been reported (CitationKeller and Mongini 2002; CitationKunwar and Megna 2003).
Aripiprazole provides to clinicians another treatment option, both as a first-line antipsychotic agent and a switching possibility from maintenance therapy, as confirmed by the report of an expert consensus meeting of October 2005 that aimed to agree on a set of guidelines for best-practice use of aripiprazole in the acute and long-term management of schizophrenia in Italy (CitationCassano et al 2007). A recent pilot study reported the successful replacement of amisulpride and risperidone with aripiprazole, with normalization of prolactin concentration at the end of week 4 (CitationLee et al 2006).
Given that it may be advantageous to avoid the use of direct dopaminergic agents in psychotic patients, for the risk of worsening psychosis, it is still unclear whether this risk is lower when using a partial agonist agent as aripiprazole in combination with a full antagonist (CitationCassano et al 2007). Indeed, because of the coexistence of high dopamine receptor affinity and partial agonist properties, aripiprazole may act as a dopamine agonist and may restore tonic inhibition to anterior pituitary lactotrophs and correct dopamine hypoactivity induced by risperidone (Whal and Ostroff 2005).
A single case of aripiprazole used in combination with another antipsychotic (risperidone) for the treatment of symptomatic hyperprolactinemia was reported by Wahl and Ostroff (2005). In a 17-year-old adolescent diagnosed with schizophrenia, treated with risperidone long acting formulation (25 mg i.m. every 2 weeks), introduction of aripiprazole (15 mg/day) determined a gradual resolution of bilateral breast pain, swelling, and galactorrhea and normalization of serum prolactin from 119 ng/ml to 18 ng/ml (Whal and Ostroff 2005). However, the safety and efficacy of the combined use of aripiprazole with other antipsychotics, for the treatment of drug-induced hyperprolactinemia, need to be demonstrated in large, controlled trials (CitationCassano et al 2007).
In conclusion, a systematic evaluation of atypical neuroleptics as an alternative treatment for conventional drug-induced hypeprolactinemia is recommended. Switching to olanzapine has been proven to be an advantageous treatment of hyperprolactinemia in women with schizophrenia (CitationKinon et al 2000, Citation2006; CitationKim et al 2002). Also clozapine (CitationBunker et al 1997), quetiapine (CitationKunwar and Megna 2003) and aripiprazole (CitationLee et al 2006; CitationCassano et al 2007) seem to be reasonable substitutive treatment options for patients suffering from old neuroleptic- and risperidone-induced hyperprolactinemia. As for the use of aripiprazole in combination with other antipsychotics, large clinical trials are needed.
Antidepressant drugs
Only a few data concerning the effect of antidepressant drugs on prolactin secretion are currently available. Unlike neuroleptics, the action of antidepressant drugs on the neuroendocrine system is highly variable and not strictly related to their therapeutic efficacy (CitationMeltzer et al 1982). This heterogeneity reflects the complexity of the aminergic control on pituitary hormone secretion and the role of antidepressants on these pathways (CitationGoyot et al 1985). Antidepressant drugs with serotoninergic activity, including selective serotonin reuptake inhibitors (SSRI), monoamine oxidase inhibitors (MAO-I) and some tricyclics, can cause modest and generally asymptomatic hyperprolactinemia (CitationWieck and Haddad 2003; CitationMolitch 2005) ( and ). Monotherapy-treated patients rarely reported symptoms due to increased prolactin secretion, while in patients on antipsychotic drugs that stimulate prolactin secretion, serotoninergic antidepressants may elevate prolactin levels above symptomatic levels or worsen pre-existing symptoms (CitationHaddad and Wieck 2000). Most serotonin reuptake inhibitors can slightly elevate prolactin, with the exception of sertraline (CitationFoley and Kast 2006). The atypical (ie, alone in their class) antidepressants mirtazapine and bupropion, are prolactin neutral (CitationFoley and Kast 2006) the latter being previously reported to even decrease serum prolactin, maybe through a dopamine re-uptake blockade (CitationMeltzer et al 1982).
Heterocyclic antidepressants
Heterocyclic antidepressants are reported to induce only mild hyperprolactinemia, but further studies are needed. Some of them, such as the tertiary amines imipramine (CitationNutt et al 1987), amitriptyline (CitationSchlienger et al 1980) and clomipramine (CitationAnderson and Cowen 1986) and the secondary amine desipramine (CitationPrice et al 1989), are thought to share a serotoninergic mechanism with SSRI.
A study on 14 patients on the tertiary amine amitriptyline showed an increase of 100% above pre-treatment prolactin level in 14% of the patients (CitationMeltzer et al 1982), while a moderate (about 25%), though statistically significant, increase in plasma prolactin was reported in 17 patients daily treated with the secondary amine nortriptyline for three weeks, even though it was never above the symptomatic threshold (CitationNielsen 1980).
Prolactin levels, evaluated in depressive patients after acute and chronic administration of tricyclic antidepressants clomipramine and amitriptyline, were found to temporary rise during the first day of treatment in 6 patients out of 11, with a delay in relation to the drug plasma peak. After 28 days of therapy, a significant increase was observed in the clomipramine-treated group and a significant decrease in the amitriptyline group (CitationGoyot et al 1985).
The abolishment of prolactin response to clomipramine after pre-treatment with serotonin receptor blockers clozapine and olanzapine (CitationMarkianos et al 2002) and its enhancement after L-tryptophan (CitationAnderson and Cowen 1986) confirm the involvement of serotoninergic pathway in clomipramine-induced prolactin increase. Therapeutic doses of clomipramine had been associated with non-puerperal lactation and elevated plasma prolactin, both disturbances recovering within weeks after drug discontinuation (CitationFowlie and Burton 1987). Treatment with either clomipramine or maprotiline in 17 patients with major depressive disorder and in healthy subjects increased significantly basal prolactin levels, that were also significantly higher in responders than in non-responders. This increase is possibly the result of a weak inhibition of prolactin secretion, due to down-regulation of beta-adrenergic receptors, and/or enhanced activity of prolactin stimulating serotoninergic receptors (CitationBaumgartner et al 1988). However, Schlienger et al showed that basal plasma levels of prolactin and delta prolactin response to TRH were increased in women on clomipramine but remained normal under maprotiline, in agreement with the different pharmacologic effect of these two classes of drugs, tricyclics mainly inhibiting serotonin, while tetracyclics rather norepinephrine recaptation (CitationSchlienger et al 1980).
No evidence for a hyperprolactinemic effect of mianserine is reported (CitationMeltzer et al 1982).
Desipramine administration was found not to affect prolactin levels in 24 patients on short term treatment (one week), but significantly enhanced prolactin levels after four weeks (CitationPrice et al 1989).
The effect of traditional tricyclic antidepressant imipramine on serum prolactin levels is controversial: oral administration of 100 mg, but not of 40 mg, led to consistent rise in prolactin levels, as measured in nine healthy young men, the effect being the result of serotonin enhancement following reuptake inhibition (CitationNutt et al 1987). A previous five-week double-blind study on depressed patients did not show any significant change in serum prolactin levels after imipramine therapy (CitationCooper et al 1981). The lack of prolactin raising effect and of antipsychotic properties is possibly explained with imipramine and other traditional tricyclic antidepressants not affecting dopamine transmission (CitationCooper et al 1981).
Amoxapine
Amoxapine is an antidepressant known to have neuroleptic properties. Its in vitro profile receptor occupancy pattern and preclinical effects are very similar to atypical antipsychotics, and it has also shown antipsychotic efficacy in clinical trials (CitationAntor et al 1983; CitationApiquian et al 2005). In agreement with such properties, amoxapine was found to increase serum prolactin in 10 major depressed men significantly more than the secondary amine desipramine in the control group (CitationAntor et al 1983). However a randomized, double-blind 6-week trial performed by CitationApiquian et al in 2005 to compare the antipsychotic and side effect profile of amoxapine (up to 250 mg/day) and risperidone (up to 5 mg/day) in 39 schizophrenic patients, showed that amoxapine was associated with less extrapyramidal effects and lower prolactin elevation than risperidone (CitationApiquian et al 2005). This prolactin raising effect, that amoxapine shares with loxapine, a related compound widely used as neuroleptic (CitationRobertson et al 1982), is reported in both female and male patients and may be explained with the blockade of dopamine receptors in central tuberoinfundibular pathways (CitationCooper et al 1981) or in the anterior pituitary gland (CitationRobertson et al 1982) ().
Monoamine-oxidase inhibitors
The precise mechanism underlying monoamine-oxidase inhibitors (MAO-I)-induced hyperprolactinemia is still unknown. These drugs interact with several possible stimulatory pathways (CitationMeltzer et al 1982; CitationPrice et al 1985).
Tranylcypromine was proved to enhance prolactin production after administration of the serotonin precursor tryptophan, thus confirming its role in serotonin function. The study by Price et al (CitationPrice et al 1985) showed little increase in prolactin concentration (3 ng/ml) after 2 weeks of tranylcypromine 10–40 mg/day treatment (CitationPrice et al 1985). Instead, hyperprolactinemia was certainly documented for old drugs currently not in use, such as pargyline and clorgyline, which acted through unknown mechanisms (CitationSlater et al 1977), a hypothesis being the production of a prolactin releasing factor (CitationMeltzer et al 1982).
SSRI
The development of SSRI as antidepressant drugs derived from the “serotonin hypothesis” of depression. These drugs have the ability to enhance serotonin activity, by inhibition of CNS neuron serotonin reuptake. Although several SSRI interact with other neurotransmitters (for example sertraline with dopamine and paroxetine with norepinephrine), prolactin stimulation probably involves only serotoninergic pathways, since hyperprolactinemia is a constant class-related effect, regardless of other pharmacological interactions (rev. by CitationEmiliano and Fudge 2004).
SSRI were reported to be the most frequent cause of drug-induced hyperprolactinemia (CitationCohen and Davies 1998), but other data do not confirm this conclusion. SSRI actually cause little, if any, increase in prolactin secretion. Several uncontrolled studies assessed prolactin rise during SSRI treatment, though only paroxetine-treated patients exhibited statistically significant elevations, while all subjects on fluoxetine, sertraline or venlafaxine showed not significant elevations of basal prolactin (CitationUrban and Veldhuis 1991; CitationSpigset and Mjorndal 1997; CitationCowen and Sargent 1997).
Thirteen case reports were collected to revise the effect of chronic serotonin stimulation on prolactin in female patients on chronic SSRI therapy: prolactin raised between 28 and 60 ng/ml in all the patients, with most of them developing galactorrhea, associated with amenorrhea, soon after initiation of the therapy. In all the reports, hyperprolactinemia promptly subsided after discontinuation of the drug (CitationEmiliano and Fudge 2004).
Sertraline, used for depressive illness, is the strongest dopamine reuptake blocker among SSRIs. Although one study of 15 female patients on 42.5 mg/day sertraline reported no difference in prolactin concentration, as compared to 16 control subjects, evaluated up to 24 weeks of treatment (CitationŠsagud 2002), a large French review on the pharmacological causes of hyperprolactinemia, conducted on 159 patients, reported 27 cases (17%) due to SSRI administration (CitationPetit et al 2003) with the highest incidence of SSRI-associated hyperprolactinemia for sertraline, followed by fluoxetine, paroxetine, and fluvoxamine. In the same study (CitationPetit et al 2003) only citalopram was not linked to a significant increase in prolactin levels (CitationPetit et al 2003), differently from previous findings of an increase by 40% from basal prolactin level after 10 days of treatment (CitationLaine et al 1997).
Fluoxetine is indicated in major depression and panic disorders with agoraphobia, ossessive-compulsive disorders, binge eating, bulimia, premenstrual syndrome. Fluoxetine clinical efficacy is due to enhanced serotonin pathway mainly via postsynaptic mechanisms, with minimal effect on dopamine reuptake. Fluoxetine induces a 5HT receptor–mediated stimulation of prolactin secretion and is reported to increase prolactin levels more than tricyclics (CitationMeltzer et al 1997). The most common endocrinologic side effects associated with fluoxetine are reduced libido (1%–11%) impaired ejaculation (<1%–7%) and impotence (<1%–7%). Since fluoxetine half-life varies upon duration of the treatment (1–3 days after short term intake, 4–6 days after chronic treatment) and its metabolite norfluoxetine washout is complete only after 4–16 days, the resolution of symptoms after discontinuation may be slow. Frequent blood sampling after 60 mg fluoxetine administered daily for 6 days to 7 female patients showed a significant increase of mean 24-hour serum prolactin concentrations (16% above basal level), due to an increase in maximal serum prolactin peak height, without alteration of prolactin pulse circadian frequency (CitationUrban and Veldhuis 1991).
Paroxetine is used for depression, panic disorder with agoraphobia, obsessive-compulsive disorder, binge eating. The most frequent endocrinologic side effects are impaired libido (6%–15%), anorgasmia (2%–9%) and dismenorrhea (5%). In a study by Cowen and Sargent on 11 healthy subjects treated with paroxetine 20 mg/daily, prolactin levels showed no increase above pre-treatment values within the first week, but a slight increase (by 35%) was observed at the 3rd week, not correlated with an increased drug plasma concentration (CitationCowen and Sargent, 1997). These data have been confirmed by another study that reported significant hyperprolactinemia in female patients treated with paroxetine for at least 2 months (CitationAmsterdam et al 1997). These clinical results support experimental data suggesting that SSRI produce a delayed increase in brain serotoninergic neurotransmission (CitationCowen and Sargent 1997; CitationPorter et al 1999).
In the same study by Amsterdam et al, venlafaxine-treated patients showed no increase in prolactin concentration after two months (CitationAmsterdam et al 1997).
Concerning fluvoxamine, mild hyperprolactinemia was reported in 30 depressed patients after both short (one week) and long term (four weeks) treatment (CitationPrice et al 1989) and in two out of eight healthy subjects treated with increasing dosage up to 200 mg/day for 4 weeks (CitationSpigset and Mjorndal 1997).
Unlike other antidepressants, mirtazapine does not inhibit the reuptake of serotonin or norepinephrine but acts as an antagonist at presynaptic and, presumably, postsynaptic alpha 2-receptors as well as an antagonist of postsynaptic 5-HT2 and 5-HT3-receptors. Prolactin levels, measured before and after administration of mirtazapine i.v. did not show any significant difference from placebo (CitationLaakmann et al 1999). Schule found significant lowering in plasma prolactin following acute oral administration of 15 mg mirtazapine in 12 healthy male subjects, as compared to placebo (CitationSchule et al 2002).
Trazodone administration was found to increase significantly prolactin concentrations, when measured at baseline and after 12 hours, 1 week and 2 weeks treatment, the means ± S.D. of plasma prolactin concentrations increasing from 9.1 ± 5.6 ng/ml to a maximum of 15.3 +/− 8.5 ng/ml at one week (CitationOtani et al 1995). These recent results are at variance with those of older studies that documented a decrease in prolactin concentration in ten healthy patients treated with trazodone (CitationRolandi et al 1981).
In summary, controversial data are available on antidepressant-induced hyperprolactinemia. Sustained and symptomatic hyperprolactinemia has been demonstrated with the heterocyclic antidepressants amitriptyline, desipramine, clomipramine and amoxapine. However, SSRI have been reported to be the most frequent cause of drug-induced hyperprolactinemia. Among those, sertraline appears to be the most frequent cause of sustained hyperprolactinemia, but also fluoxetine and paroxetine may induce pathologic and symptomatic increases in prolactin levels. Most of the other anti-depressants do not induce hyperprolactinemia or induce only transient or within normal range variations with no or little clinical relevance.
Other psychotropic drugs
Other psychotropic drugs such as lithium, valproic acid, buspirone, carbamazepine, and benzodiazepines rarely produce clinically relevant increases in prolactin concentrations (CitationMarken et al 1992).
Buspirone is an anxiolytic drug with mixed dopamine agonist-antagonist and 5-HT1A agonist properties. Hyperprolactinemia after acute buspirone administration is reported in both depressive and healthy subjects, responses being significantly higher in women than in men (CitationMeltzer and Maes 1994). Prolactin levels raised in a dose-dependent manner (up to 320% of basal value) after administration of a dose of buspirone higher than that required for the anxiolytic effect (100 mg) (CitationSeppala et al 1987). However, it has not been entirely clarified whether the serotoninergic or the dopaminergic system is especially accountable for the buspirone-induced prolactin secretive response (CitationMaskall et al 1995) ().
No published reports have so far documented hyperprolactinemia as a side effect of carbamazepine treatment. In a study by CitationBonuccelli et al (1985), on normal and epileptic subjects, no appreciable change in prolactin spontaneous secretion or in prolactin secretory circadian rhythm was observed, though a small increase in early sleep values was reported. These results were interpreted to indicate that prolactin changes induced by carbamazepine are mediated by serotoninergic activity.
Clonazepam (a central type benzodiazepine agonist) and diazepam (a mixed agonist) are reported to decrease prolactin levels, possibly through a direct action on the anterior pituitary gland (CitationJarvinen et al 1992) ().
Prolactin levels are differentially influenced by GABA-ergic drugs, depending on their potency (ie, receptor affinity) and dose. The robust increase in prolactin levels found in response to the GABA agonist alprazolam is not consistent with previous data on traditional benzodiazepines: Zemishlany et al reported that plasma prolactin levels increased by 100% two to eight hours after a single dose (3 mg) of alprazolam (CitationZemishlany et al 1990).
Short-term GABA-mimetic valproate treatment in eight healthy male volunteers was found not to alter hypothalamic or pituitary 5-HT1A or dopamine receptor function (CitationDelva et al 2002). In 1985, during studies on tuberoinfundibular-GABA (TI-GABA) system, 800 mg oral valproate was found to decrease prolactin concentrations in 20 healthy women, the lack of effect in schizophrenic patients supporting the hypothesis of a defect of the TI-GABA system in chronic schizophrenia (CitationMonteleone et al 1985).
The effects of lithium on prolactin secretion are controversial. It had initially been proposed that lithium would increase prolactin, possibly through a decrease in dopamine receptor sensitivity (CitationMeltzer et al 1982). A recent study showed that lithium affects variably prolactin secretion, in relation to duration of the therapy: a treatment of less than six months was found to increase prolactin levels as compared to controls, while bipolar patients on long term treatments (<six months) showed a decrease in prolactin levels (CitationBasturk et al 2001). Studies that reported that lithium enhances significantly the hyperprolactinemic response after clomipramine but not after metoclopramide or haloperidol administration indicate that the lithium-mediated prolactin release may selectively involve serotoninergic pathways (CitationMc Cance et al 1989). However, lithium-prolactin interactions are probably even more complex and involve both dopamine and serotonin pathways (CitationBasturk et al 2001).
Prokinetics
Two prokinetic drugs, that are commonly used in gastrointestinal disorders, induce hyperprolactinemia via a dopamine-antagonistic mechanism (): metoclopramide, that blocks dopamine and, at higher doses, also serotonin receptors in chemoreceptor trigger zone of CNS, and domperidone, that does not cross the blood brain barrier and is therefore a selective peripheral (extra cerebral) dopamine antagonist. These drugs have been reported to cause symptomatic hyperprolactinemia (CitationTamagna et al 1979; CitationFujino et al 1980).
Metoclopramide, a prokinetic drug used in nausea, vomiting, diabetic gastric stasis and gastroesophageal reflux, is a potent stimulator of prolactin release. Endocrine and metabolic side effects, such as amenorrhea, galactorrhea, gynecomastia and impotence are reported with undefined frequency. Serum prolactin concentration measured soon after metoclopramide administration was found acutely increased in five patients (CitationTamagna et al 1979). It has been reported that prolactin concentration may acutely increase up to six-fold over baseline after a single oral administration of 10 mg metoclopramide (CitationMc Callum et al 1976) and return to normal range after 12 hours. In these and other studies (CitationMc Callum et al 1976; CitationTamagna et al 1979; CitationBrowers et al 1980) mean serum prolactin concentration was confirmed to persist significantly high during oral chronic treatment, up to 15-fold above baseline.
Domperidone is used for gastrointestinal motility disorders and for the prevention of gastrointestinal symptoms associated with dopaminergic treatment of Parkinson’s disease. Endocrinologic side effects are reported in less than 1% of domperidone-treated subjects and include galactorrhea, gynecomastia and menstrual irregularities. Domperidone 10 mg i.v. induced acute increase in prolactin concentration in healthy subjects (CitationSowers et al 1982), the increase occurring within the first 15 minutes after administration and being higher in women than in men (CitationFujino et al 1980). In contrast to metoclopramide, however, under chronic administration of domperidone, prolactin tends to decrease significantly, even if still above the normal range (CitationBrowers et al 1980). This observation is consistent with the pharmacodynamic differences showed by these two drugs, as mentioned above.
The effect on prolactin levels of the classical antypsichotic chlorpromazine, used in clinical practice for its anti-nausea properties, has been discussed above.
Anti-hypertensive drugs
Alpha-methyldopa is an alpha-adrenergic inhibitor, which is likely to decrease dopamine synthesis acting as a false neurotransmitter and inhibits the enzymatic conversion of L-dopa to dopamine catalyzed by aromatic-L-aminoacid decarboxylase (CitationSteiner 1976). The most common endocrinologic side effect reported is gynecomastia (<1%). A single dose of 750–1000 mg of alpha-methyldopa is reported to significantly increase prolactin, with a peak concentration occurring 4–6 hours after administration; long term treatment has been associated with three- to four-fold increases in basal prolactin levels compared to normal subjects (CitationSteiner et al 1976).
The central monoamine reserpine, used in hypertension but also in psychosis, schizophrenia and tardive dyskinesia, acts by depletion of sympathetic biogenic amines (among which dopamine) inhibiting their hypothalamic storage in secretory granules (CitationLee et al 1976) (). The occurrence of gynecomastia is reported in patients treated with reserpine, with undefined frequency. Serum prolactin levels are significantly higher among hypertensive patients receiving reserpine, compared to those found six weeks after discontinuation of the treatment; prolactin elevation is proportional to the duration of the use. Increased incidence of breast cancer has also been reported among patients on antihypertensive therapy reserpine (CitationLee et al 1976).
The antiarrhythmic agent class IV verapamil is a calcium channel blocker, indicated for the treatment of hypertension, angina, arrhythmias and is reported to induce galactorrhea in <1% of patients. In this case, the mechanism sustaining hyperprolactinemia is not clear, a hypothesis being a reduction in hypothalamic dopamine generation, possibly through N-type calcium channels (CitationKelley et al 1996). However, no other calcium-antagonist, such as diltiazem (CitationVeldhuis et al 1985) or dihydropiridine (CitationKelley et al 1996) has ever been associated with prolactin increase. A study published in 1996 on 449 men receiving verapamil showed significantly higher prevalence of hyperprolactinemia in the treated group than in the control group (8.5% vs 3%); serum prolactin levels returned to normal in all patients that discontinued the therapy, while 93.3% of patients still on verapamil had persistent hyperprolactinemia, thus confirming the causal role of verapamil treatment in the development of hyperprolactinemia (CitationRomeo et al 1996).
The antihypertensive beta-blocker labetalol has been described to increase prolactin levels when administered intravenously in hypertensive patients, but not when administered orally (100 or 200 mg). This effect seems to be related to a central action, because pre-treatment with L-DOPA and carbiDOPA, reported to blunt the prolactin responses to central stimuli, avoids prolactin response (CitationBarbieri et al 1982).
Opiates
Consistently with the large body of evidence that endogenous opioid peptides participate in the regulation of pituitary secretion, though with unfocused physiological roles, morphine and related drugs have been found to exert endocrine effects through interactions with dopaminergic and serotoninergic hypothalamic pathways and consequent modulation of pituitary hormone secretion (CitationVan Vugt and Meites 1980).
Studies on both animals and humans demonstrated that endogenous opioids administered either intravenously or intraventricularly led to a rapid and dose-dependent plasma prolactin increase (CitationVan Vugt and Meites 1980; CitationRisch et al 1982). Opioids may indirectly stimulate prolactin release by inhibiting PIFs (ie, dopamine) release and/or by stimulating PRFs production (CitationShin et al 1988) (). Since it is prevented by a previous injection of the specific opiate antagonist naloxone, morphine- and methadone-induced prolactin secretion seems to be primarily mediated by μ receptor (CitationPanerai et al 1985; CitationShin et al 1988). To demonstrate whether an alternative dopamine independent mechanism, possibly involving a PRF, has a role in morphine-induced prolactin release in animals, complete blockade of dopaminergic receptors was achieved by pre-treatment with pimozide, showing that morphine (10 mg/kg) was still able to stimulate prolactin release without any functional dopaminergic receptors. In conclusion, morphine stimulatory effect on prolactin release may act through a mechanism which is not depending on dopaminergic receptors (CitationShin et al 1988), though morphine, cocaine and beta-endorphine have been long reported to increase prolactin through dopaminergic interactions (CitationGold et al 1978). Long term methadone users have normal basal prolactin levels, even though a transient increase, beginning two-four hours after each daily dose, has been shown (CitationBart et al 2003).
H2-receptor antagonists
Although histamine H2-receptor competitive antagonists cimetidine and ranitidine, used in active ulcers, ulcer prophylaxis and gastric hypersecretory conditions, have stimulatory effect on prolactin secretion (CitationPerret et al 1986; CitationKnigge 1990), hyperprolactinemia has never been systematically reported, except for isolated case reports published shortly after the approval of the drugs (CitationPetrillo et al 1977). The neurobehavioral syndrome, that appears with the withdrawal of H2 receptors blockers and subsides with the restoration of the treatment or with the administration of a potent prolactin inducing drug as domperidone, should be related to a prompt reduction of prolactin levels (CitationRampello et al 1997).
In accordance with a histamine-dependent prolactin regulatory pattern, the histamine H2-receptor competitive antagonist cimetidine can have an inhibitory (following central administration) or stimulatory (following systemic administration) effect on prolactin secretion (CitationKnigge 1990), the latter being the result of a decreased dopamine release from hypothalamus to pituitary gland (CitationMorosini et al 1979). However, high doses of cimetidine possess an additional prolactin stimulatory action, not exerted by the other H2-receptor antagonist ranitidine, that does not seem to be related to blockade of H2-receptors (CitationKnigge 1990) nor to interaction with the dopaminergic system (CitationKnigge et al 1987). The observation that systemic administration of specific histamine-H2 agonist (impromidine) is not able to avoid the cimetidine-induced prolactin release, while pre-treatment with benzodiazepines or GABA respectively suppressed or reduced prolactin response, suggested that cimetidine does not induce prolactin hypersecretion through an action on histamine H2 receptors, but through the interaction with other neurotransmitters, such as the pituitaric GABA-ergic system (CitationSibilia et al 1985) ().
Estrogens and anti-androgens
Estrogen-induced hyperprolactinemia is directly dependent on the degree of estrogenization. Physiologic amounts of estrogen in women cause a minimal increase in basal serum prolactin (CitationFrantz 1978), but may explain the greater prolactin response of women to physiologic and pharmacologic stimuli. Greater amounts of estrogens, as in pregnancy, increase basal prolactin concentration. It is uncertain whether the amount of estrogens in hormonal contraceptives is able to induce hyperprolactinemia:heterogeneous data concerning oral estroprogestinic anti-contraceptive therapy are reported. Studies on women taking low-dose-estrogen compounds (less than 50 micrograms of estradiol) did not show a significant elevation response as compared to pretreatment levels (CitationDe Leo et al 1991) or control patients with intrauterine devices (CitationHwang et al 1986). In the studies documenting hyperprolactinemia, the incidence among women on oral contraceptives is variously reported, from 12% (CitationLuciano et al 1985) to 30% (CitationReyniak et al 1980). In the study by Luciano et al, hyperprolactinemia, assessed by multiple blood sampling, appeared transient and resolved spontaneously in about 50% of cases (CitationLuciano et al 1985). In some of these studies, no correlation was found between the dosage of estrogenic component of the combined oral contraceptives, or duration of their use, and prolactin levels (CitationReyniak et al 1980; CitationDavis et al 1984). Little is known, however, about dose-dependency of estrogen effects on plasma hormone levels. Hormone replacement therapy is usually prescribed as medium- to high-dose formulations. In surgically or naturally menopausal women receiving different kinds of hormone replacement therapies, over 2 years and 6 months, no significant differences in serum prolactin levels were found during subsequent treatment cycles, serum prolactin showing varying levels always within normal range (CitationFoth and Romer 1997).
No increase in basal prolactin levels is reported during therapy with estrogen plus antiandrogen cyproterone acetate alone (CitationAcien et al 1997) or with additional spironolactone (CitationKelestimur and Sahin 1998), nor during hirsutism treatment with flutamide (CitationMuderris and Bayram 1999; Taner et al 2002). A significant decrease in prolactin levels has been reported in patients treated with flutamide alone (Akaza et al 1993) or with flutamide and an oral contraceptive formulation with ethinyl estradiol and cyproterone acetate (Taner et al 2002). However, in 60 postmenopausal women receiving replacement therapy with estrogen plus anti-androgen cyproterone acetate, a significant increase in hyperprolactinemic response to dopaminergic drug sulpiride was observed (CitationPaoletti et al 2001), though normal basal prolactin levels were confirmed.
Other drugs
Fenfluramine is a sympathomimetic amine used as an appetite suppressant. It causes hyperprolactinemia by promoting the release of serotonin and blocking its neuronal uptake, that leads to an increased serotoninergic activity and postsynaptyc stimulation of 5HT2A (). In healthy subjects, prolactin release by fenfluramine is dose-dependent, negatively correlated with age and increased in young females, as compared to males (CitationNewman 1998).
One case report documented hyperprolactinemia during chronic anticolvulsivant therapy with fenitoin and phenobarbital after the addition of oral fluoresone 750 mg daily. The pathogenetic mechanism is unclear (CitationRossi et al 1983).
Hyperprolactinemia in four patients on protease inhibitors presenting with galactorrhea likely resulted from the enhancement of dopamine antagonistic effects of other simultaneously administered drugs (prokinetics and fluoxetine) through inhibitory action on cytochrome P 450 system (CitationHutchinson et al 2000).
Cholinomimetic drugs with central effect, such as physostigmine, have been differently reported either to have no effect, to increase, or to inhibit prolactin release, though physostigmine may produce significant increases in plasma prolactin concentrations in humans after intravenous infusion. In three separate studies, reported by CitationRisch et al 1982, conducted collaboratively by the National Institute of Mental Health and the University of California at San Diego, prolactin rise following physostigmine and arecoline administration correlated with plasma concentrations of beta-endorphin immunoreactivity, cholinergic stimulation of hypothalamic beta-endorphin possibly representing an interesting example of peptidergic modulation of hypothalamic-pituitary hormonal regulation (CitationRisch et al 1982).
Controversial data are available on the effect of chemotherapy and immunosuppression on prolactin levels. In a study of 20 women with breast cancer who underwent high-dose chemotherapy and autologous blood stem-cell transplantation, plasma prolactin levels increased significantly during conditioning and within 30 days after transplant, remaining however within the normal age- and sex-adjusted range in all cases (CitationHinterberger-Fischer M et al 2000). In the same study (CitationHinterberger-Fischer MM et al 2000), the use of antiemetic drugs induced further increase in prolactin levels. However, this increase did not affect disease-free survival after transplant. These results are in line with those of previous studies that analyzed the long-term effect of allogeneic bone marrow transplantation on pituitary, gonad, thyroid and adrenal function in adult patients (CitationKauppila M et al 1998). Disturbances in GH, adrenal and prolactin were documented, but appeared to occur less frequently than functional impairment of hypothalamus-pituitary-gonad/thyroid axis after intensive treatment and bone marrow transplantation (CitationKauppila M et al 1998a). Thus, it is very well known that chemotherapy induces frequently hypogonadism in both men and women by a direct toxic action on the gonad (CitationFairley KF et al 1972; CitationShamberger RC et al 1981), but significant prolactin increases are not frequent and are normally mild (CitationKauppila M et al 1998b). Hyperprolactinemia has been documented in the majority of patients treated with irradiation because of CNS malignancies (CitationConstine LS et al 1987). In patients with CNS tumor, the risk to develop iatrogenic hyperprolactinemia is mostly dependent on the radiation dose, with the majority of subjects who receive more than 55 Gy experiencing this endocrine disturbance (CitationConstine LS et al 1987), but is also dependent on the concomitant use of chemotherapy which increases the probability to develop both prolactin and thyroid abnormalities (CitationConstine LS et al 1987).
References
- AcienPMauriMGutierrezMClinical and hormonal effects of the combination gonadotrophin-releasing hormone agonist plus oral contraceptive pills containing ethinyl-oestradiol (EE) and cyproterone acetate (CPA) versus the EE-CPA pill alone on polycystic ovarian disease-related hyperandrogenismHum Rep1997124239
- AmsterdamJDGarcia-EspanaFGoodmanDBreast enlargement during chronic antidepressant therapyJ Affect Disord19974615169479619
- AguilarELopezFPinillaLEffects of pimozide and domperidone administration on prolactin levels in neonatally estrogenized female ratsRev Esp Fisiol19854118364041067
- AndersonIMCowenPJClomipramine enhances prolactin and growth hormone responses to L-tryptophanPsychopharmacology19868913133016787
- AntorRFSexauerJDRandallCLAmoxapine elevates serum prolactin in depressed menJ Affect Disord19835305106229562
- ApiquianRFresarAUlloaREAmoxapine as an atypical antipsychotic: a comparative study versus risperidoneNeuropsychopharmacology20053022364415956984
- AshPRBoumaDExaggerated hyperprolactinemia in response to thiothixeneArch Neurol19813853457247797
- AtmacaMKulogluMTezcanEQuetiapine is not associated with increase in prolactin secretion in contrast to haloperidolArch Med Res200233562512505103
- BarbieriCLarovereMTMariottiGProlactin stimulation by intravenous labetalol is mediated inside CNSClin Endocrinol (Oxf)198216615197105431
- BartGBorgLSchlugerJHSuppressed prolactin response to dynorphin A1-3 in methadone-maintained versus control subjectsJ Pharmacol Exp Ther2003306581712730354
- BasturkMKaraaslanFEselEEffects of short and long term lithium treatment on serum prolactin levels in patients with bipolar affective disorderProg Neuropsychopharmacol Biol Psychiatry2001253152211294478
- BaumgartnerAGrafKJKurtenIProlactin in patients with major depressive disorder and in healthy subjects. II. Longitudinal study of basal prolactin and post-TRH-stimulated prolactin levelsBiol Psychiatry198824268853135848
- BenkerGJaspersCHauslerGControl of prolactin secretionKlin Wochenschr1990681157672126309
- BliesenerNYokusogluHQuednowBBUsefulness of bromocriptine in the treatment of amisulpride-induced hyperprolactinemia: a case reportPharmacopsychiatry2004371899115467977
- BonuccelliUMurialdoGMartinoEEffects of carbamazepine on prolactin secretion in normal subjects and in epileptic subjectsClin Neuropharmacol19858165743924400
- BressanRAErlandssonKSpencerEPProlactinemia is uncoupled from central D2/D3 dopamine receptor occupancy in amisulpride treated patientsPsychopharmachology(Berl)200417536773
- BrowersJRAssiesJWiersingaWMPlasma prolactin levels after acute and subchronic oral administration of domperidone and of metoclopramide: a cross-over study in healthy volounteersClin Endocrinol (Oxf)198012435407428183
- Bruins SlotLADe VriesLNewman-TancrediADifferential profile of antipsychotics at serotonin 5-HT1A and dopamine D2S receptors coupled to extracellular signal-regulated kinaseEur J Pharmacol2006534637016497294
- BuckleyPFAripipazole: efficacy and tolerability profile of a novel acting atypical antipsychoticDrugs Today (Barc)2003391455112698209
- BunkerMTMarkenPASchneiderhanMEAttenuation of antipsychotic-induced hyperprolactinemia with clozapineJ Child Adolesc Psychopharmacol199776599192542
- BuschDAFangVSMeltzerHYSerum prolactin levels following intramuscolar chlorpromazine: two and three-hours response as predictors of six-hour responsePsychiatry Res19791153945130
- CanusoMCHanauMJhambKKOlanzapine use in women with antipsychotic-induced hyperprolactinemiaAm J Psychiatry199815514589766782
- CassanoGBFagioliniALattanziLAripiprazole in the Treatment of Schizophrenia: A Consensus Report produced by Schizophrenia Experts in ItalyClin Drug Invest200727113
- CavallaroRCocchiFAngeloneSMCabergoline treatment of risperidone-induced hyperprolactinemia: a pilot studyJ Clin Psychiatry2004651879015003071
- CohenIGBiedermanJTreatment of risperidone-induced hyperprolactinemia with a dopamine agonist in childrenJ Child Adolesc Psychopharmacol2001114354011838826
- CohenMADaviesPHDrug therapy and hyperprolactinemiaAdv Drug Reaction Bull19981907236
- ConstineLSRubinPWoolfPDHyperprolactinemia and hypothyroidism following cytotoxic therapy for central nervous system malignanciesJ Clin Oncol198751841513681371
- CooperDSGelenbergAJWojcikJCThe effect of amoxapine and imipramine on serum prolactin levelsArch Intern Med1981141102357018438
- CosiCCarilla-DurandEAssieMBPartial agonist properties of the antipsychotics SSR181507, aripiprazole and bifeprunox at D2 receptors: G protein activation and prolactin releaseEur J Pharmacol20065351354416554049
- CowenPJSargentPAChanges in plasma prolactin during SSRI treatment: evidence for a delayed increase in 5HT neurotransmissionJ Psychopharmacol19971134589443523
- CrawfordAMBeasleyCMJrTollefsonGDThe acute and long term affect of olanzapine compared with placebo and haloperidol on serum prolactin concentrationsSchizophr Res19972641549376336
- CrowleyTJHydinger-MacdonaldMMotility, parkinsonism, and prolactin with thiothixene and thioridazineArch Gen Psychiatry198138668757247630
- DavidSRTaylorCCKinonBJThe effects of olanzapin, risperidone and haloperidol on plasma prolactin levels in patients with schizophreniaClin Ther20002210859611048906
- DavisJRSelbyCJeffcoateWJOral contraceptive agents do not affect serum prolactin in normal womenClin Endocrinol (Oxf)198420427346609028
- DelvaNJBrooksDLFranklinMEffects of short-term administration of valproate on serotonin-1A and dopamine receptor function in healthy human subjectsJ Psychiatry Neurosci2002274293712491576
- De LeoVLanzettaDVanniALLow estrogen oral contraceptives and the hypothalamo-pituitary axisContraception199144155611832628
- Di RenzoGAmorosoSTagliatelaMPharmacological characterization of serotonin receptors involved in the control of prolactin secretionEur J Pharmacol198916237132524399
- DorevitchAAronzonRStarkMPsychotic exacerbation attributed to low-dose bromocriptine treatment of galactorrhea and hyperprolactinemiaActa Obstet Gynecol Scand19917037561746267
- EmilianoABFFudgeJLFrom galactorrhea to osteopenia, Rethinking serotonin-prolactin interactionsNeuropsychopharmacology2004298334614997175
- FairleyKFBarrieJUJohnsonWSterility and testicular atrophy related to cyclophosphamide therapyLancet1972156894110052
- FoleyKFKastREReview of evidence that posttransplantation psychiatric treatment commonly affects prolactin levels and thereby influences graft fateGen Hosp Psychiatry200628230316675366
- FothDRomerTProlactin serum levels in postmenopausal women receiving long-term hormone replacement therapyGynecol Obstet Invest4412469286727
- FowlieSBurtonJHyperprolactinaemia and nonpuerperal lactation associated with clomipramineScott Med J198732523602989
- FrantzAGProlactinN Engl J Med1978298201339087
- FreemanMEKanyicskaBLerantAProlactin : structure, function and regulation of secretionPhysiol Rev200080152363111015620
- FricMLauxGPlasma prolactin level and incidence of adverse endocrinologic effects during therapy with atypical neurolepticsPsychiatric Prax200330Suppl 2S97101
- FryePEPariserSFKimMHBromocriptine associated with symptom exacerbation during neuroleptic treatment of schizoaffective schizophreniaJ Clin Psychiatr1982432523
- FujinoTKatoHYamashitaSEffects of domperidone on serum prolactin levels in human beingsS Endocrinol Jpn1980275215
- GibneyJSmithTPMc KennaTJThe impact on clinical practice of routine screening for macroprolactinemiaJ Clin Endocrinol Metab20059039273215811931
- GoffDCPoseverTHerzLAn exploratory haloperidol-controlled dose finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disordersJ Clin Phychopharmacol199818296304
- GoldMSRedmondDEJrDonabedianRKIncrease in serum prolactin by endogenous and esogenous opiates. Evidence for antidopamine and antipsychotic effectAm J Psychiatry197813514151630292
- GoldsteinJMQuetiapine fumarate: a new atypical antipsychoticDrugs Today (Barc)19993519321012973385
- GoldsteinSRSelective estrogen receptor modulators: a new category of compounds to extend postmenopausal women’s healthInt J Fertil Womens Med199944221610569450
- GoodnickPJRodriguezLSantanaOAntispychotics: impact on prolactin levelsExpert Opin Pharmacother2002313819112387684
- GoyotGDebrayQHugRMeasurement of basal plasma levels of three anterior pituitary hormones during acute or chronic treatment with tricyclic antidepressantsEncephale19851145514017937
- GreenAIBrownWAProlactin and neuroleptic drugsEndocrinol Metab Clin North Am198817213232897910
- GruenPGSacharEJAltmanNRelation of plasma prolactin to clinical response in schizophrenic patientsArch Gen Psychiatry1978351222729592
- HaddadPMWieckAAntidepressant-induced hyperprolactinaemiaJ Psychopharmacol200014Suppl 3A28
- HaddadPMWieckAAntipsychotic induced hyperprolactinemia: mechanisms, clinical features and managementDrugs200464229131415456328
- HalbreichUKahnLSHyperprolactinemia and schizophrenia: mechanisms and clinical aspectsJ Psychiatr Pract200393445315985953
- HalbreichUKinonBJGilmoreJAElevated prolactin level in patients with schizophreniaMechanisms and related adverse effects Psychoneuroendocrinology200328Suppl 15367
- HamnerMBArvanitisLAMillerBGPlasma prolactin in schizophrenia subjects treated with Seroquel (ICI 204, 636)Psychopharmacol Bull199632107108927658
- Hinterberger-FischerMOgrisEKierPElevation of plasma prolactin in patients undergoing autologous blood stem-cell transplantation for breast cancerAm J Clin Oncol200023325910955855
- HutchinsonJMurphyMHarriesRGalactorrhoea and hyperprolactinemia associated with Protease InhibitorsLancet20003561003411041407
- HwangPLNgCSCheongSTEffect of oral contraceptives on serum prolactin: a longitudinal study in 126 normal premenopausal womenEndocrinol (Oxf)19862412733
- JarvinenARagoLMannistoPTEffects of central and peripheral type benzodiazepine ligands on thyrotropin and prolactin secretionNeuropeptides199221183911321364
- KaneJMLeuchtSCarpenterDExpert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summaryJ Clin Psychiatry200364Suppl 1251914640142
- KapurSZipurskyRJonesCA positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancyArch Gen Psychiatry200057553910839333
- KapurSZipurskyRBRemingtonG5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigationAm J Psychiatry199815592189659858
- KauppilaMKoskinenPIrjalaKLong-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adultsBone Marr Transplant1998a223317
- KauppilaMViikariJIrjalaKThe hypothalamus-pituitary-gonad axis and testicular function in male patients after treatment for haematological malignanciesJ Intern Med1998b244411169845857
- KearnsAEGoffDCHaydenDLRisperidone-associated hyperprolactinemiaEndocr Pract20006425911155212
- KelestimurFSahinYComparison of Diane 35 and Diane 35 plus spironolactone in the treatment of hirsutismFertil Steril1998696699457935
- KellerRMonginiFSwitch to quetiapine in antipsychotic agent-related hyperprolactinemiaNeurol Sci200223233512522680
- KelleySRKamalTJMolitchMEMechanism of verapamil calcium channel blockade-induced hyperprolactinemiaAm J Physiol19962701 Pt1E961008772480
- KimKSPaeCUChaeJHEffects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidoneJ Clin Psychiatry2002634081312019665
- KinonBJAhlJLiu-SeifertHImprovement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotic or risperidone to olanzapinePsychoneuroendocrinology2006315778816488084
- KinonBJBassonBRGilmoreJAStrategies for switching from conventional antipsychotic drugs or risperidone to olanzapineJ Clin Psychiatry2000618334011105736
- KinonBJGilmoreJALiuHPrevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidonePsychoneuroendocrinology2003a28Suppl 2556812650681
- KinonBJGilmoreJALiuHHyperprolactinemia in response to antipsychotic drugs; characterization across comparative clinical trialsPsychoneuroendocrinology2003b28Suppl 2S6982
- KinonBJStaufferVLMcGuireHCThe effects of antipsychotic drug treatment on prolactin concentrations in elderly patientsJ Am Med Dir Assoc2003c41899412837139
- KleinbergDLDavisJMde CosterRProlactin levels and adverse events in patients treated with risperidoneJ Clin Psychopharmacol19991957619934944
- KniggeUPHistaminergic regulation of prolactin secretionDan Med Bull199037109242188799
- KniggeUMatzenSWarbergJEffects of H2-receptor antagonists on prolactin secretion: specificity and mediation of the responseActa Endocrinol (Copenh)198711546182888250
- KolakowskaTWilesDHGelderMGClinical significance of plasma chlorpromazine levels. II. Plasma levels of the drug, some of its metabolites and prolactin in patients receiving long-term phenothiazines treatmentPsychopharmacology (Berl)19764910178814
- KopecekMBaresMSvarcJHyperprolactinemia after low dose of amisulprideNeuro Endocrinol Lett2004254192215665803
- KuhnKUMeyerKMaierWFlupenthixole—a partial atypical neurolepticFortschr Neurol Psychiatr200068Suppl 1S384110907612
- KunwarARMegnaJLResolution of risperidone-induced hyperprolactinemia with substitution of quetiapineAnn Pharmacother200337206812549948
- LaakmannGSchuleCBaghaiTEffects of mirtazapine on growth hormone, prolactin, and cortisol secretion in healthy male subjectsPsychoneuroendocrinology1999247698410451911
- LafuenteAMarcoJEsquifinoAIOpioids and the pulsatile prolactin secretory pattern: effects of hyperprolactinemiaVet Hum Toxicol19943652487900271
- LaineKAnttilaMHeinonenELack of adverse interactions between concomitantly administered selegiline and citalopramClin Neuropharmacol199720419339331518
- LambertsSWvan KoetsvelPMHoflandLJThe effect of clozapine on prolactin secretion at the level of the lactotrophLife Sci1990461013192325501
- LeeBHKimYKParkSHUsing aripiprazole to resolve antipsychotic induced symptomatic hyperprolactinemia: a pilot studyProg Neuropsychopharmacol Biol Psychiatry2006307141716571367
- LeePAKellyMRWallinJDIncreased prolactin levels during reserpine treatmemt of hypertensive patientsJAMA1976235231617131202
- LeuchtSPitschel-WalzGEngelRRAmisulpride, an Unusual “Atypical” Antipsychotic: A Meta- Analysis of Randomized Controlled TrialsAm J Psychiatry20021591809011823257
- Levi-MinziSBermanzohnPCSirisSGBromocriptine for “negative” schizophreniaCompr Psychiatry199132210161679383
- LiebermanJADopamine partial agonists: a new class of antipsychoticsCNS Drugs2004182516715015905
- LucianoAAShermanBMChaplerFKHyperprolactinemia and contraception: a prospective studyObstet Gynecol198565506103982724
- Mc CallumRWSowersJRHershmanJMMetoclopramide stimulates prolactin secretion in manJ endocrinol Metab197642114852
- McKeageKPloskerGLAmisulpride: a review of its use in the management of schizophreniaCNS drugs2004189335615521794
- ManciniAMGuitelmanAVargasCAEffect of sulpiride on serum prolactin levels in humansJ Clin Endocrinol Metab19764218141249187
- MannistoPTKorttilaKSeppalaTSerum prolactin after a single and subchronic oral administration of chlorpromazine and sulpiride. A cross over study in healthy volunteersArzneimittelforschung1978287681580204
- MarkenPAHaykalRFFisherJNManagement of psychotropic-induced hyperprolactinemiaClin Pharm19921185161341991
- MarkianosMHatzimanolisJLycourasLProlactin responses to acute clomipramine and haloperidol of male schizophrenic patients in a drug-free state and after treatment with clozapine or with olanzapineSchizophr Res200256111712084414
- MaskallDDZisAPLamRWProlactin response to buspirone challenge in the presence of dopaminergic blockadeBiol Psychiatry19953823598547445
- MatsuokaINakaiTMiyakeMEffects of bromocriptine on neuroleptic-induced amenorrhea, galactorrhea and impotenceJpn J Psychiatry Neurol198640639462885437
- Mc CanceSLCohenPRCowenPJLithium increases 5-HT-mediated prolactin releasePsychopharmacology (Berl)198999276812508166
- MelkerssonKDifferences in prolactin elevation and related symptoms of atypical antipsychotic in schizophrenic patientsJ Clin Psychiatry200566761715960571
- MeltzerHBastaniBJayathilateKFluoxetine, but not tricyclics antidepressants, potentiates the 5hydroxytryptophan-mediated increase in plasma cotisol and prolactin secretion in subjects with major depression or with obsessive compulsive disorderNeuropsychopharmacology1997171119194044
- MeltzerHYBuschDAFangVSSerum neuroleptic and prolactin levels in schizophrenic patients and clinical responsePsychiatry Res19839271836580660
- MeltzerHYFangVSThe effect of neuroleptic on serum prolactin in schizophrenic patientsArch Gen Psychiatry197633279861259521
- MeltzerHJFangVSTricouBJEffect of antidepressants on neuroendocrine axis in humansAdv Biochem Psychopharmacol198232303166124090
- MeltzerHYGudelskyGADA and SE effects of clozapine. Implication for a unique clinical profileArzeimittelforschung19924226872
- MeltzerHYMaesMEffects of buspirone on plasma prolactin and cortisol levels in major depressed and normal subjectsBiol Psychiatry199435316238011800
- MillerKKManagement of hyperprolactinemia in patients receiving antipsychoticsCNS Spectr20049Suppl 7283215303078
- MolitchMEPathologic hyperprolactinemiaEndocrinol Metab Clin North Am1992218779011486880
- MolitchMEMedication-induced hyperprolactinemiaMayo Clin Proc2005801050716092584
- MonteleonePZontiniGSteardoLFailure of the GABAergic drug, sodium valproate, to reduce basal plasma prolactin secretion in chronic schizophreniaPsychoneuroendocrinology198510475803003777
- MontgomeryJWinterbottomEJessaniMPrevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatmentJ Clin Psychiatry2004651491815554761
- MorosiniPPCampanellaNGhirelliSCimetidine and hyperprolactinemiaBoll Soc Ital Biol Sper1979551417551779
- MuderrisIIBayramFClinical efficacy of lower dose flutamide 125 mg/day in the treatment of hirsutismJ Endocrinol Invest199922165810219882
- MullerEELocatelliVCellaSProlactin lowering and-releasing drugs. Mechanisms of action and therapeutic applicationsDrugs1983253994326133737
- NewmanMEShapiraBLererBEvaluation of central serotonergic function in affective and related disorders by the fenfluramine challenge test: a critical reviewInt J Neuropsychopharmacol19981496911281946
- NielsenJLPlasma prolactin during treatment with nortriptylineNeuropsychobiology198065257366815
- NuttDMiddletonHFranklinMThe neuroendocrine effects of oral imipraminePsychoneuroendocrinology198712367753432499
- OtaniKYasuiNKanekoSTrazodone treatment increases plasma prolactin concentrations in depressed patientsInt Clin Psychopharmacol199510115177673654
- PandurangiAKNarasimhachariNBlackardWGRelation of serum molindone levels to serum prolactin levels and antipsychotic responseJ Clin Psychiatry198950379812676994
- PaneraiAEPetragliaFSacerdotePMainly μ-opiate receptors are involved in LH and PRL secretionEndocrinology1985117109692990867
- PaolettiAMFlorisSManniasMEvidence that cyproterone acetate improves psychological symptoms and enhances the activity of the dopaminergic system in postmenopauseJ Clin Endocrinol Metab2001866081211158017
- PaparrigopoulosTLiappasJTzavellasEAmisulpiride-induced hyperprolactinemia is reversible following discontinuationProg Neuropsychopharmacol Biol Psychiatry20073092616938372
- PerretGHuguesJNLouchahiMEffect of a short-term oral administration of cimetidine and ranitidine on the basal and thyrotropin-releasing hormone-stimulated serum concentrations of prolactin, thyrotropin and thyroid hormones in healthy volunteers. A double-blind cross-over studyPharmacology19863210183081918
- PetitAPiednoirDGermainMLDrug-induced hyperprolactinemia: a case-non-case study from the national pharmacovigilance databaseTherapie2003581596312942857
- PetrilloMPradaAPorroGBPlasmaprolactin and cimetidineLancet1977276171562
- PettyRGProlactin and antipsychotic medications: mechanism of actionSchizophr Res199935SupplS677310190227
- PolleriAMasturzoBMurialdoGDose and sex related effects of aromatic aminoacids decarboxylase inhibitors on serum prolactin in humansActa Endocrinol (Copenh)1980937127355667
- PollockAMc LarenEHSerum prolactin concentration in patients taking neuroleptic drugsClin Endocrinol (Oxf)199849513169876350
- PorterRJMcAllister-WilliamsRHYoungAHAcute effects of venlafaxine and paroxetine on serotonergic transmission in human volunteersPsychopharmacology (Berl)1999146194810525755
- PradalierAVincentDBaerzegarCHyperprolactinemia induced by indoraminTherapie19985350029921044
- PriceLHCharneyDSDelgadoPLEffects of desipramine and fluvoxamine treatment on prolactin response to tryptophan. Serotoninergic function and mechanism of antidepressant actionArch Gen Psychiatry198946625312500111
- PriceLHCharneyDSHeningerGREffects of tranylcypromine treatment on neuroendocrine, behavioral, and autonomic responses to tryptophan in depressed patientsLife Sci198537809184033356
- PurdonSEJonesBDStipENeuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone or haloperidol. The Canadian Collaborative group for research in schizophreniaArch Gen Psychiatry2000572495810711911
- RampelloRRaffaeleRNicolettiGNeurobehavioural syndrome induced by H2 receptor blocker withdrawal: possible role of prolactinClin Neuropharmacol19972049549037573
- ReyniakJVWenofMAubertJMIncidence of hyperprolactinemia during oral contraceptive therapyObstet Gynecol1980558117352067
- RischSCJanowskyDSSiverLJCorrelated cholinomimetic-stimulated beta-endorphin and prolactin release in humansPeptides19823319226289276
- RiveraJLLalSEttigiPEffect of acute and chronic neuroleptic therapy on serum prolactin levels in men and women of different age groupsClin Endocrinol (Oxf)1976527382954220
- RobertsonAGBerryRMeltzerHYProlactin stimulating effects of amoxapine and loxapine in psychiatric patientsPsychopharmacology (Berl)198278287926818584
- RolandiEMagnaniGMilesiGMEffect of a psychoactive drug, trazodone, on prolactin secretion in manNeuropsychobiology1981717197465018
- RomeoJHDombrowskiRKwackYSHyperprolactinemia and verapamil: prevalence and potential association with hypogonadism in menClin Endocrinol (Oxf)19964557158977754
- RossiLBonuccelliUMarcacciGynecomastia in epileptics treated with Phenobarbital, phenytoin and fluoresone: two case reportsItal J Neurol Sci19834207106618859
- SacharEJGruenPHKarasuTBThioridazine stimulates prolactin secretion in manArch Gen Psychiatry1975328856239662
- SantalaMSuvanto-LuukkonenEKyllonenAHyperprolactinemia complicating juvenile granulosa cell tumor of the ovaryGynecol Oncol2001823899111531301
- SchuleCBaghaiTBidlingmaierMEndocrinological effects of mirtazapine in healthy volunteersProg Neuropsychopharmacol Biol Psychiatry20022612536112502011
- SchliengerJLKapferMTSingerLDifferent effects of tricyclic (clomipramine and amitriptyline) and tetracyclic (maprotiline) antidepressors on the release of thyroid stimulating hormone, prolactin and growth hormone to thyrostimulating releasing hormone in patients with psychoaffective disordersActa Psychiatr Belg1980805899
- SchlosserRGrunderGAnghelescuILong term effects of the substituted benzamide derivative amisulpiride on baseline and stimulated prolactin levelsNeuropsychobiology200246334012207145
- SeemanPAtypical antipsychotics: mechanism of actionCan J Psychiatry200247273811873706
- SeppalaTRantaTShrotriyaRCSerum prolactin levels after buspirone in manMed Biol1987656133613693
- ShambergerRCRosenbergSASeippCAEffects of high-dose methotrexate and vincristine on ovarian and testicular functions in patients undergoing postoperative adjuvant treatment of osteosarcomaCancer Treat Rep198165739466791818
- ShinSHObonsawinMCVan VugtDAMorphine can stimulate prolactin release independent of a dopaminergic mechanismCan J Physiol Pharmacol198866138152907416
- SibiliaVNettiCGuidobonoFCimetidine-induced prolactin release: possible involvement of the GABA-ergic systemNeuroendocrinology198540189922986023
- SlaterSLLipperSShillingDJElevation of plasmaprolactin by monoamine-oxidase inhibitorsLancet19772275669882
- SmithSNeuroleptic-associated hyperprolactinemia. Can it be treated with bromocriptine?J Reprod Med199237737401359137
- SmithSWheelerMJMurrayRThe effect of antipsychotic–induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axisJ Clin Psychopharmacol2002221091411910254
- SowersJRSharpBMc CallumRWEffect of domperidone, an extracerebral inhibitor of dopamine receptors, on thyrotropin, prolactin, rennin, aldosterone and 18- hydroxycorticosterone secretion in manJ Clin Endocrinol Metab198254869717037817
- SpigsetOMjorndalTThe effect of fluvoxamine on serum prolactin and serum sodium concentration: relation to platelet 5HT2A receptor statusJ Clin Psychopharmacol19971729279241009
- SpitzerMSajjadRBenjaminFPattern of development of hyperprolactinemia after initiation of haloperidol therapyObstet Gynecol19989169359572212
- ŠsagudMPivacNMuck-SelerDEffects of sertraline treatment on plasma cortisol, prolactin and thyroid hormones in female depressed patientsNeuropsychobiology2002451394311979064
- StannilandCTaylorDTolerability of atypical antipsychoticsDrug Saf20002219521410738844
- StevensJRKymissisPIBakerAJLElevated prolactin levels in male youths treated with risperidone and quetiapineJ Child Adolesc Psychopharmacol20051589390016379509
- SteinerJCassarJMashiterKEffect of methyldopa on prolactin and growth hormoneBr Med J19761118681268617
- StreetJKinonBStaufferVTranPBysmesterFTyeNHerreraJBreierATollefsonGOlanzapine in dementiaOlanzapine (Zyprexa®): a novel antipsychotic2000PhiladelphiaLippincott Williams and Wilkins Healthcare41627
- SulimanAMSmithTPGibneyJFrequent misdiagnosis and mismanagement of hyperprolactinemic patients before the introduction of macroprolactin screening: application of a new strict laboratory definition of macroprolactinemiaClinical Chemistry2003491504912928232
- TamagnaEILaneWHerschmanJMEffect of chronic metoclopramide therapy in serum pituitary hormone concentrationHorm Res1979111619574121
- TaylorDMAripiprazole: a review of its pharmacology and clinical useInt J Clin Pract200357495412587943
- TogoTIsekiEShojiMProlactin levels in schizophrenic patients receiving perospirone in comparison to risperidoneJ Pharmacol Sci2003912596212686750
- TollefsonGDBeasleyCMJrTranPVOlanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trialAm J Psychiatry1997154457659090331
- TollinSRUse of dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disordersJ Endocrinol Invest2000237657011194712
- TranPVHamiltonSHKuntzAJDouble blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disordersJ Clin Pharmacol19971740718
- TurronePKapurSSeemanMVElevation of prolactin levels by atypical antipsychoticsAm J Psychiatry2002159133511772702
- UrbanRJVeldhuisJDA selective serotonin reuptake inhibitor, fluoxetine hydrochloride, modulates the pulsatile release of prolactin in postmenopausal womenAm J Obstet Gynecol19911641 Pt 1147521986602
- Van VugtDAMeitesJInfluence of endogenous opiates on anterior pituitary functionFed Proc198039253386247213
- VeldhuisJDBorgesJLDrakeCRDivergent influences of the structurally dissimilar calcium entry blockers, diltiazem and verapamil on TRH and GnRH stimulated anterior pituitary hormoneJ Clin Endocrinol Metab19856014493880562
- VercelliniPSacerdotePTrepidiLVeralipride for hot flushes induced by a gonadotropin-releasing hormone agonist: a controlled studyFertil Steril199462938427926138
- Von BardelebenUBenkertOHolsboerFClinical and neuroendocrine effects of zotepine-a new neuroleptic drugPharmacopsychiatry19872028342883678
- WahlROstroffRReversal of symptomatic hyperprolactinemia by aripiprazoleAm J Psychiatry20051621542316055781
- WetzelHWiesnerJHiemkeCAcute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteersJ Psychiatr Res199428461737897617
- WieckAHaddadPMHyperprolactinemia caused by antipsychotic drugsBMJ2002324250211823343
- WieckAHaddadPMAntipsychotic-induced hyperprolactinemia in women: pathophysiology, severity and consequencesBr J Psychiatry200318219920412611781
- ZemishlanyZMcQueeneyRGabrielSMNeuroendocrine and monoaminergic responses to acute administration of alprazolam in normal subjectsNeuropsychobiology19902312482098668