Abstract
Palonosetron (Aloxi®, Onicit®, Paloxi®) is a second-generation 5-HT3 receptor antagonist (RA) with an extended half-life of ~40 hours and high binding affinity for the 5-HT3 receptor that is markedly different from other 5-HT3 RAs. Phase III trials demonstrate that a single dose of palonosetron compared with traditional 5-HT3 RAs is more effective in preventing chemotherapy-induced nausea and vomiting (CINV) during the first 24 hours following chemotherapy (acute CINV), and also exhibits prolonged efficacy to provide significantly better protection from CINV in the delayed and overall phases. This superior and extended protection from CINV conferred by palonosetron following a single intravenous dose before chemotherapy simplifies dosing schedules. Recent research has focused on optimization of palonosetron-based antiemetic regimens, particularly in combination with steroids and neurokinin-1 RAs. The available clinical data indicate high control rates for palonosetron, suggesting a synergistic potential for protection in patients scheduled to receive emetogenic drug regimens.
Introduction
Complete control of chemotherapy-induced nausea and vomiting (CINV) remains a primary goal of chemotherapy treatment (CitationKoeller et al 2002). Some 15 years after the launch of the first-generation 5-HT3 receptor antagonists (5-HT3 RAs), available in Europe or North America such as ondansetron (Zofran®, GlaxoSmithKline), granisetron (Kytril®, Roche), dolasetron (Anzemet®, Aventis), and tropisetron (Navoban®, Novartis), which heralded a major advance in the treatment of acute CINV, some patients are still not treated adequately. Failure to gain effective control over CINV can have important consequences that may include extended or unplanned hospitalization or delay or refusal of chemotherapy as a result of vomiting and nausea, potentially leading to a reduction in antineoplastic efficacy (CitationGrunberg et al 2000). Despite efforts to combat CINV, it remains one of the worst side effects experienced () (CitationCoates et al 1983; CitationGriffin et al 1996; Citationde Boer-Dennet et al 1997; CitationLindley et al 1999; CitationGrunberg et al 2000; CitationKoeller et al 2002), with 59% of patients reporting nausea to such an extent that it severely impacts daily living (CitationDe Moor et al 2003).
Table 1 Patient perceptions of the most severe side effects of cancer chemotherapy
CINV can be categorized in simplified regulatory terms as either acute (up to 24 hours postchemotherapy) or delayed (after 24 hours postchemotherapy), while chemotherapy regimens are subdivided into levels 1–5 according to their emetogenic potential. Among them, level 3–4 regimens result in an emesis frequency of 30%–90%, and are traditionally considered moderately emetogenic (MEC), while level 5 results in >90% patients experiencing emesis with highly emetogenic (HEC) chemotherapy (CitationHesketh et al 1997).
International guideline development is a continuous and dynamic effort towards indication of the better antiemetic treatment in light of the most recent data published in literature. According to the most recent indication for CINV prevention, 5HT3 RAs are unanimously recognised to be the foundation of antiemetic therapy. 5HT3 RAs should then be given in combination with other antiemetic agents, usually corticosteroids such as dexamethasone and NK1 RAs, in accordance to the evaluation of patient-related and treatment-related risk factors, in particular the chemotherapy emetogenicity (HEC or MEC).
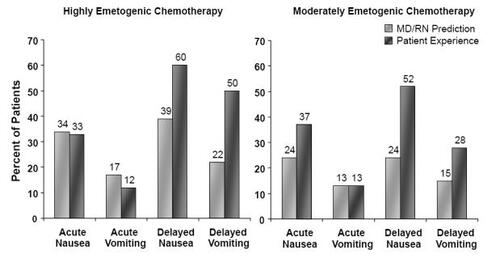
Although current regimens are considered to be quite effective in treating acute CINV, adequate control of delayed symptoms needs improvement. A recent study found acute emesis in 13% and 12% of patients receiving MEC and HEC, respectively, while over 37% and 33% experienced acute nausea with these respective treatments, despite the use of first-generation 5-HT3 RAs plus concomitant corticosteroids as recommended by international guidelines () (CitationGrunberg, Vanden Burgt et al 2004). Furthermore, delayed emesis was evident in 28% and 50% of patients in the MEC and HEC groups, respectively, while delayed nausea was experienced by 52% and 60% of these respective patients (CitationGrunberg, Vanden Burgt et al 2004). Furthermore the extent of CINV is underestimated by both physicians and nurses treating these patients () (CitationEisenberg, Rubenstein et al 2003; CitationGrunberg, Deusson Burgt et al 2004). Clearly, CINV remains a substantial and underrecognized burden.
Figure 1 Perception vs reality: Healthcare providers’ predictions of incidence, and observed incidence, of nausea and vomiting following chemotherapy (Drawn from data in CitationGrunberg, Deusson et al 2004).
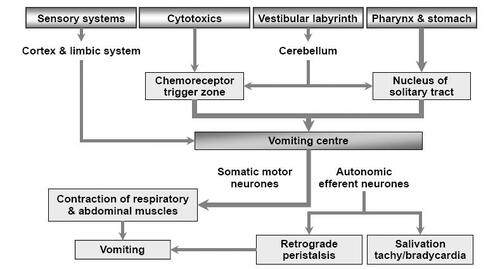
Emesis is believed to be due to complex interactions between a number of neurotransmitters (serotonin (5-HT), neurokinin, dopamine, histamine, and acetylcholine) and receptor subtypes within gastrointestinal (GI) and central pathways. Stimulation of serotonin release from enterochromaffin cells of the GI mucosa is thought to be a major pathway, which triggers emesis through stimulation of the medulla via vagal afferents. There are two central medullary areas implicated, namely the chemoreceptor trigger zone (CTZ) and the vomiting centre. The CTZ integrates bloodborne chemical signals with neuronal inputs from the GI tract, and efferents from the CTZ then signal the collection of brainstem nuclei known as the vomiting centre. The vomiting centre integrates visceral and somatic functions, resulting in the process of vomiting () (CitationHornby 2001).
5-HT3 receptor antagonists
The first generation of 5-HT3 RAs – ondansetron, granisetron, dolasetron, and tropisetron – are remarkable by virtue of their similarities rather than differences and have similar efficacies in preventing acute CINV when administered at therapeutically equivalent doses, although they have a more limited impact on delayed symptoms (CitationHesketh 2000). They are widely believed to be similar to the point that until recently they have been regarded as therapeutically equivalent and interchangeable in clinical guidelines (CitationKoeller et al 2002). A more detailed observation and the advent of the second-generation 5-HT3 RA palonosetron is, however, changing this ethos.
Palonosetron: a second-generation 5-HT3 receptor antagonist
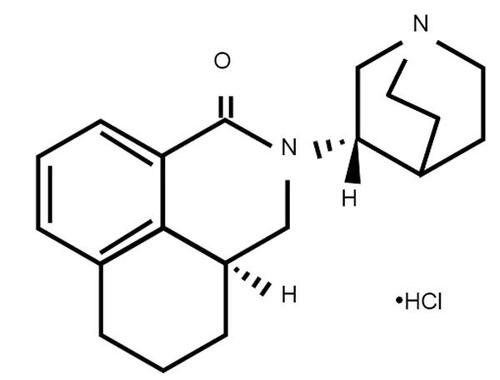
Palonosetron () is the first 5-HT3 RA to show an efficacy that is superior to other 5-HT3 RAs against CINV due to MEC in FDA registration trials (CitationEisenberg et al 2003; CitationGralla et al 2003; CitationRubenstein et al 2003). It was first approved by the FDA in 2003 and is currently licensed in the USA for the prevention of acute nausea and vomiting associated with MEC and HEC, and the prevention of delayed nausea and vomiting associated with MEC. More recently, palonosetron has gained approval in Europe for the prevention of acute nausea and vomiting associated with HEC, and the prevention of nausea and vomiting associated with MEC. It is postulated that the superior clinical efficacy of palonosetron, including the control of delayed CINV following a single dose prior to chemotherapy, can be related to the >30-fold higher binding affinity for the 5-HT3 receptor subtype and a 4–10-fold longer half-life compared to first-generation 5-HT3 RAs (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGrunberg et al 2003). Despite having a longer half-life and higher binding affinity, however, palonosetron has a similar tolerability profile to the first-generation 5-HT3 RAs, demonstrating minimal side effects. Headache and constipation are the most commonly reported adverse events for both generations of 5-HT3 RAs (CitationHesketh et al 1996; CitationEisenberg, Figueroa-Vadillo et al 2003; CitationEisenberg, Rubenstein et al 2003; CitationGralla et al 2003; CitationGrunberg et al 2003; CitationRubenstein et al 2003).
Pharmacokinetics and pharmacodynamics
The pharmacokinetics of palonosetron have been studied both in healthy volunteers (CitationStoltz et al 2004) and cancer patients receiving highly emetogenic cisplatin (CitationEisenberg et al 2004), with generally similar kinetics being shown in both studies. Intravenously administered palonosetron (0.3–90 μg/kg) showed overall elimination half-life values of approximately 40 hours as result of low clearance values (1.11–3.90 mL/min/kg) and a large volume of distribution (3.85–12.6 L/kg). At the currently approved IV dose of 0.25 mg, palonosetron shows to be widely distributed in the body, with a volume of distribution of 8.3 L/kg, and to be bound to plasma proteins at a proportion of 62% (CitationAloxi® prescribing information 2003/2005). Palonosetron is metabolized by cytochrome P450 enzymes, mainly CYP2D6, with minor contributions from CYP1A2 and CYP3A4 (CitationAloxi® prescribing information 2003/2005). It is noteworthy that the clinical pharmacokinetic parameters are not significantly different between poor and extensive metabolizers of CYP2D6 substrates (CitationAloxi® prescribing information 2003/2005) The major metabolites are N-oxide-palonosetron and 6-S-hydroxy-palonosetron, both of which have a low affinity for the 5-HT3 receptor and therefore do not contribute to the activity of the parent compound (CitationAloxi® prescribing information 2003/2005).
Drug interactions
Due to its multiple routes of elimination and lack of effect on cytochrome P450 isoenzyme induction or inhibition at therapeutic concentrations, palonosetron has low potential for clinically significant drug interactions (CitationAloxi® prescribing information 2003/2005). This characteristic is of particular value in elderly patients who are often receiving multiple medications (CitationAapro et al 2005). Additionally, no dose adjustments are required in the elderly or in patients with renal or hepatic impairment (CitationAloxi® prescribing information 2003/2005). Likewise palonosetron does not interact with antineoplastic drugs; its chemical stability and the antitumor activity of these medications are maintained during coadministration (CitationAloxi® prescribing information 2003/2005; CitationCantoreggi et al 2003; CitationTrissel and Zhang 2004a, Citation2005a, Citation2005b; CitationXu et al 2004; CitationTrissel and Xu 2005). Any interaction with dexamethasone would be of particular importance as it is usual clinical practice to coadminister a premixed solution of dexamethasone and a 5-HT3 RA over 15 min; however, the label for palonosetron recommends a much quicker intravenous infusion over 30 sec (CitationAloxi® prescribing information 2003/2005). An admixture of palonosetron and dexamethasone in polyvinylchloride minibags or in polypropylene syringes has been shown to be physically compatible and chemically stable for at least 48 hours when stored as indicated (CitationTrissel and Zhang 2004b). Furthermore, a recent open-label study has evaluated the safety and efficacy of palonosetron and dexamethasone coadministered over 10–15 min, which has confirmed that coadministration is both safe and effective (CitationHajdenberg et al 2006).
Affinity and potency
Palonosetron has at least a 30-fold higher affinity for the 5-HT3 receptor compared to the other first-generation 5-HT3 RAs. At clinically relevant doses, it does not display any appreciable binding to a range of other receptors, including dopaminergic, muscarinic, adrenergic, and opioid receptors (CitationAloxi® prescribing information 2003/2005; CitationWong et al 1995). In addition to a higher affinity, intravenously administered palonosetron has a higher potency at the 5-HT3 receptor, being three-fold more potent than granisetron and up to 55 times more potent than ondansetron in animal models (CitationEglen et al 1995). The distinct pharmacokinetics and dynamics displayed by palonosetron compared to first-generation 5-HT3 RAs appear to translate into a distinct clinical profile, providing extended relief from CINV (CitationEisenberg, Rubenstein et al 2003; CitationGralla et al 2003; CitationEisenberg et al 2004).
Optimizing dosage of palonosetron
The optimal dose of palonosetron was evaluated in a randomized, double-blind, multicenter, dose-ranging phase II trial (CitationEisenberg et al 2004). A total of 161 chemotherapy-naïve patients (80.0% male) were randomized to one of five groups to receive between 0.3 and 90 μg/kg palonosetron as a single intravenous bolus dose 30 min prior to receiving HEC (largely high dose cisplatin: ≥70 mg/m2). The pooled 0.3–1 μg/kg dose was chosen as a suboptimal dose. Dexamethasone was not given prophylactically and was available only as a rescue medication. The primary endpoint was 24-hour complete response (CR) (no emesis and no need for rescue medication). Efficacy was evaluated up to day 7. Complete control was included as a secondary endpoint, defined as no emesis, mild or no nausea, and no need for rescue medication.
In the 148 evaluable patients, acute CR rates of 40%–50% were achieved across the effective dose range (3–90 μg/kg). The dose–response curve demonstrated a threshold relationship that is typical for 5-HT3 RAs (CitationRubenstein et al 2003; CitationEisenberg et al 2004). Complete control in the acute phase was achieved in 39%–48% of patients across this dose range and again showed a threshold dose–response relationship. Prolonged protection following a single dose on day 1 was observed up to day 7. Compared with the low efficacy of the pooled suboptimal cohort of 0.3–1 μg/kg (CR 24%), the lowest effective doses were the 3 and 10 μg/kg doses, demonstrating 46% and 40% CR rates in the acute period, respectively. Based on these data, doses of 0.25 mg and 0.75 mg (equivalent to 3 μg/kg and 10 μg/kg, respectively) were selected for phase III trials.
Efficacy
The efficacy of palonosetron in the prevention of nausea and vomiting induced by chemotherapy has been demonstrated in three randomized, stratified, double-blind, parallel-arm, active comparator-controlled phase III trials (CitationEisenberg, Rubenstein et al 2003; CitationGralla et al 2003; CitationAapro et al 2006). Two of these studies examined efficacy of the two selected doses of palonosetron, 0.25 mg and 0.75 mg in MEC patients compared to ondansetron 32 mg (CitationGralla et al 2003) or dolasetron 100 mg (CitationEisenberg et al 2003), while the third compared the two doses of palonosetron to ondansetron 32 mg in patients receiving HEC (CitationAapro et al 2006). All pivotal studies were designed to show that at least one of the two palonosetron doses (0.25 mg and 0.75 mg) was at least as effective as the comparator.
To reflect the actual clinical situation, heterogeneous mixed-sex study populations were recruited (51%–82% female) that were chemotherapy experienced or chemotherapy naïve (40%–66% naïve) (CitationAloxi® prescribing information 2003/2005; CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003). A number of moderately emetogenic agents were included in the studied regimens: methotrexate 250 mg/m2, cyclophosphamide <1,500 mg/m2, doxorubicin >25 mg/m2, cisplatin <50 mg/m2, or any dose of carboplatin (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003). Chemotherapy regimens for the HEC study included cisplatin ≥60 mg/m2, cyclophosphamide >1,500 mg/m2, and dacarbazine (CitationAapro et al 2006). Either no (CitationGralla et al 2003) or minimal (~5% of patients) (CitationEisenberg et al 2003) corticosteroids were administered in the MEC studies, although 67% of the patients in the HEC study received concomitant dexamethasone on day 1 (CitationAapro et al 2006). The primary endpoint of all three studies was the CR rate, defined as no emetic episode and no use of rescue medication during the first 24 hours. A series of secondary endpoints, such as rates of delayed CR (24–120 hours), overall CR (0–120 hours), and complete control (no emetic episode, no rescue medication, and no more than mild nausea), percentage of patients with no emetic episodes, and percentage of patients with no nausea on a daily basis, were also included. The results reported hereon are for the 0.25 mg dose of palonosetron, since this is the dose that has been approved for use.
Palonosetron and acute CINV
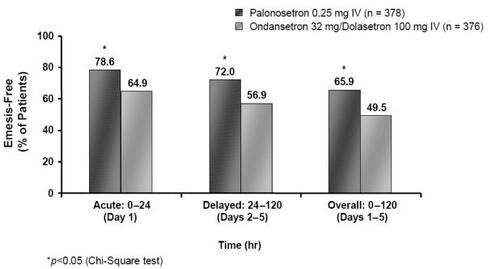
Pooled analysis (CitationRubenstein et al 2003) of the identical MEC studies (CitationEisenberg, Rubenstein et al 2003; CitationGralla et al 2003) demonstrated a statistically significant improvement in CR rates for the acute phase with palonosetron 0.25 mg (n = 378) compared to results pooled for the ondansetron 32 mg/dolasetron 100 mg (n = 376) arms (72.0% vs 60.6%, p = 0.0012) (). Furthermore, the number of patients experiencing no emetic episodes in the acute period was again statistically superior in the palonosetron group (78.6% vs 64.9%, p = 0.0001) () (CitationGrunberg et al 2003; CitationData on file Helsinn Healthcare SA). The improvements in CR and emesis-free rates reported in the pooled analysis in favor of palonosetron over ondansetron and dolasetron are particularly interesting since this is the first time that a 5-HT3 RA has been clinically differentiated from others in the class.
Figure 4 Percentage of MEC patients achieving complete response rates in the acute, delayed, and overall phases following treatment with palonosetron 0.25 mg or ondansetron 32 mg/dolasetron 100 mg (Drawn from data in CitationRubenstein et al 2003; CitationGrunberg, Vanden Burgt et al 2004).
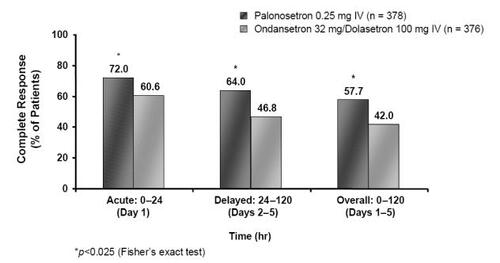
Figure 5 Percentage of emesis-free MEC patients in the acute, delayed and overall phases following treatment with palonosetron 0.25 mg or ondansetron 32 mg/dolasetron 100 mg (Drawn from data in CitationRubenstein et al 2003).
In patients receiving HEC, a group in whom CINV is generally more difficult to control, palonosetron 0.25 mg (n = 223) proved to be at least as effective as ondansetron 32 mg (n = 221) in the acute period, with 59.2% and 57% of patients, respectively, achieving CR rates (CitationAapro et al 2006). Secondary endpoints further confirmed the improved efficacy of palonosetron in the acute phase, with an increased proportion of patients experiencing no emetic episodes (68.2% 60.2% with ondansetron), and a significantly longer time to first emetic episode (120 hours 42.7 hours with ondansetron, p = 0.023) (CitationAapro et al 2006). As is usual in clinical practice, 67% of the patients in this study also received prophylactic dexamethasone prior to chemotherapy. In this subgroup of patients, the trend towards improved acute CR rates in favor of the palonosetron arm (n = 150) over the ondansetron group (n = 147) was almost 10% higher, a difference which, although not statistically significant, indicates a clinically relevant superiority (64.7% vs 55.8%) (CitationAapro et al 2006).
Palonosetron and delayed CINV
In addition to being more efficacious against acute CINV, palonosetron is further distinguished from other 5-HT3 RAs as it shows improved efficacy in preventing delayed CINV in patients receiving MEC. In the pooled analysis (CitationEisenberg et al 2004) from the two MEC studies (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003), a CR for the delayed period was seen in 64.0% of patients given palonosetron 0.25 mg compared to 46.8% receiving ondansetron 32 mg/dolasetron 100 mg (p = 0.001) (). The improved efficacy was more pronounced from days 2–3, the point at which patients are most at risk of experiencing delayed CINV (day 2: 72.0% vs 55.1%, p <0.001; day 3: 76.5% vs 61.4%, p < 0.001) (CitationData on file Helsinn Healthcare SA). Response rates were also better with palonosetron compared with ondansetron/dolasetron for the overall phase (57.7% vs 42.0%, p <0.001) (). Moreover, the percentage of patients with no emetic episodes was significantly higher in the palonosetron group than in the ondansetron/dolasetron group during the delayed and overall time periods () (72.0% vs 56.9%, p < 0.001, and 65.9% vs 49.5%, p <0.001, respectively; CitationData on file Helsinn Healthcare SA).
In patients receiving HEC, palonosetron 0.25 mg demonstrated a trend towards superior CR rates over ondansetron 32 mg in the delayed and overall categories (45.3% vs 38.9%, and 40.8% vs 33.0%, respectively), which became statistically significant in the subgroup of patients receiving concomitant dexamethasone administered on day 1 (delayed phase: 42% vs 28.6%, p = 0.021; overall phase: 40.7% vs 25.2%, p = 0.005) (CitationAapro et al 2006).
It is well established that the strongest predictive factor for delayed CINV is the presence of acute CINV, and therefore it has been speculated that the impact of palonosetron on delayed CR may be due to the superior acute efficacy or a ‘carryover effect’. Recent analysis of the two phase III MEC studies (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003) has attempted to unravel this effect by separating out patients with and without acute CRs and evaluating the proportions of these patients who then had delayed CRs (CitationGrunberg, Vanden Burgt et al 2004). If improved rates of delayed CR are to be attributed to a carryover effect, one would expect no difference between the two groups of patients who received no relief from acute vomiting; however, this was not the case. In the palonosetron group, 23% of patients compared to 12% (p = 0.027) of patients in the comparator (ondansetron/dolasetron) group who had acute CINV went on to experience a CR for delayed CINV. The implication of this study is that palonosetron displays an inherently different pharmacology, which alone amongst the 5-HT3 RAs confers valuable efficacy against delayed CINV.
Palonosetron and nausea
Underestimated by physicians and research nurses, nausea remains a significant problem for most chemotherapy patients despite the use of modern antiemetics () (CitationCoates et al 1983; CitationGriffin et al 1996; Citationde Boer-Dennert et al 1997; CitationLindley et al 1999; CitationGrunberg et al 2000; CitationGrunberg, Deusson et al 2004). There are two main reasons for this: difficulties in researching this highly subjective issue preclinically, and a lack of clinical research due to an underestimation of the extent of the problem. It may be that nausea is in fact regulated also by as yet unknown neurotransmitter pathways separate from those that control vomiting, thus accounting for the difficulties in effective treatment with the currently available range of drugs. However nausea is regulated physiologically, palonosetron appears superior to the first-generation 5-HT3 RAs in providing relief.
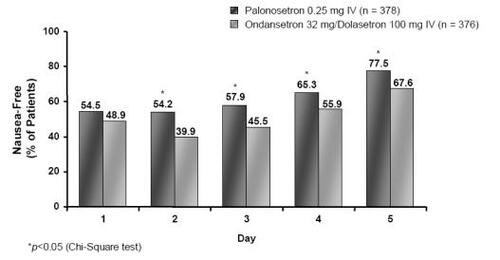
Severity of nausea was evaluated daily in the palonosetron trials over 0–120 hours using a four-point Likert scale (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003). Pooled analysis (CitationDecker et al 2006) of both MEC studies (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003) demonstrated a statistically significant improvement in the proportion of nausea-free patients in the palonosetron 0.25-mg arm in days from 2 to 5 compared with the ondansetron 32 mg/dolasetron 100 mg group (). Of particular note is the increased efficacy of palonosetron on days 2–3, which is known as the most difficult period for CINV control (day 2: 54.2% vs 39.9%, p < 0.0001; day 3: 57.9% vs 45.5%, p = 0.0006) (CitationDecker et al 2006).
Figure 6 Percentage of nausea-free MEC patients on a daily basis following treatment with palonosetron 0.25 mg or ondansetron 32 mg/dolasetron 100 mg (Drawn from data in CitationRubenstein et al 2003).
The functional impact of delayed nausea on daily life activities was demonstrated using the validated FLIE instrument to be significantly less with palonosetron (65% of patients reported no impact on daily life – NIDL) than with ondansetron/dolasetron (54% reported NIDL) in the pooled analysis of the MEC trials (p < 0.01; CitationDecker et al 2006; Citationde Moor and Cunningham 2005). In the study of patients receiving HEC that utilized the same FLIE measurement, functional impact from delayed nausea was also less in the patients given palonosetron with concomitant dexamethasone (55% reported NIDL) than in those receiving ondansetron with dexamethasone (46% reported NIDL). Although the differences were not statistically superior in this subset, they were however clinically relevant and indicative of a meaningful difference to patients (CitationAapro et al 2006).
Palonosetron and steroids
Palonosetron is clearly efficacious for delayed CINV when given alone. This raises the possibility of being able to reduce steroid treatment, which is unpopular with patients because of its associated side effects (CitationVardy et al 2006) and consequently reduces adherence and quality of life. The HEC trial by CitationAapro et al (2006) administered a single dose of dexamethasone to 67% of the patients on day 1 of chemotherapy, thus allowing a subgroup analysis of patients receiving palonosetron with concomitant dexamethasone. In the delayed period, 42% of patients receiving palonosetron 0.25 mg plus dexamethasone experienced a CR compared to 28.6% in the ondansetron 32 mg plus dexamethasone group (p = 0.021).
Synergy between palonosetron and dexamethasone has been further evaluated in a recent open-label, phase II study by CitationHajdenberg et al (2006) in patients receiving MEC. Fifteen min prior to commencing chemotherapy, an infusion of palonosetron 0.25 mg plus dexamethasone 8 mg over a 10–15-min period was administered to 33 chemotherapy-naïve and non-naïve patients. In this albeit small sample (32 completers), 84.4% of patients had a CR during the acute period, and 59.4% demonstrated a CR both in the delayed and overall time periods. The proportion of patients with no emetic episodes was 90.6%, 81.3%, and 71.9% for the acute, delayed, and overall phases, respectively. Perhaps more importantly from a patient’s perspective, palonosetron used together with dexamethasone given on day 1 only was also associated with an increased number of nausea-free patients throughout days 1–3 (CitationHajdenberg et al 2006) when compared to palonosetron given alone (using pooled data) (CitationDecker et al 2006) from the two MEC trials () (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003). Some 78.1% of patients were nausea-free on day 1, increasing to 81.3% on day 2. Overall, rates of nausea control ranged between around 66% and 81% over days 1–5.
Table 2 Percentage of nausea-free patients in a pooled analysis of patients receiving single infusion of palonosetron 0.25 mg prior to MEC (CitationDecker et al 2006) compared with patients receiving concomitant infusion of palonosetron 0.25 mg + dexamethasone 8 mg (CitationHajdenberg et al 2006) on Day 1 only.
These studies are encouraging in that they suggest a single dose of steroid plus palonosetron can provide efficacy throughout acute and delayed CINV, thus simplifying the dosing regimens that currently involve daily administration of 5-HT3 RAs and dexamethasone (CitationRoila et al 2006). This convenient and simple method of CINV control could improve patient compliance and, indeed, physician adherence to international guidelines.
Palonosetron + neurokinin-1 antagonists
Different mechanisms may lie behind acute and delayed CINV (CitationHesketh 2000; CitationHesketh 2003), therefore it is to be expected that combinations of antiemetic drug classes will optimize CINV therapy. Aprepitant (Emend®, Merck and Co), a neurokinin-1 (NK-1) antagonist, is a valuable addition to antiemesis regimes and it is recommended in patients scheduled to receive cisplatin-based and anthracycline-cyclophosphamide (AC)-based chemotherapy (CitationRoila et al 2006).
A recent open-label, phase II study (n = 58) evaluating the efficacy and safety of triple therapy with palonosetron, dexamethasone, and aprepitant for prophylaxis of CINV in HEC and MEC (including AC-based chemotherapy) patients with various types of cancers uncovered synergies between aprepitant and palonosetron (CitationGrote et al 2006). Administration of palonosetron 0.25 mg in combination with dexamethasone (12 mg on day 1, 8 mg on days 2–3) and standard doses of aprepitant (125 mg on day 1, 80 mg on days 2–3) achieved 87.9% CR rates in the acute period, while 77.6% of patients achieved CR in both the delayed and overall phases. Some 93.1% of these patients were emesis-free during both the acute and delayed phases, and 91.4% were emesis-free in the overall phase. Furthermore, 70.7% of patients were nausea-free in the acute period, and nausea control was generally maintained during all 24-hour intervals observed. Whether this highly efficacious treatment regimen can be simplified is the subject of further studies. It may prove possible to maintain this high level of efficacy while reducing the dose of dexamethasone in line with the study by CitationHajdenberg et al (2006).
Tolerability and safety
Adverse reactions
Despite having a longer half-life and duration of action, clinical trial data for MEC and HEC patients demonstrate a comparable safety profile for palonosetron in terms of frequency and severity to that of the first-generation 5-HT3 RAs (CitationAloxi® prescribing information 2003/2005; CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003; CitationAapro et al 2006). Headache (9%) and constipation (5%), generally of mild intensity, are the most frequently reported treatment-related adverse reactions (CitationAloxi prescribing information 2003/2005). Postmarketing data from the USA, where 1.3 million doses have been distributed (CitationBissoli et al 2005) in 15 months, have confirmed the good tolerability and safety profile evident during the phase III clinical trials, with no suggestion of an unexpected pattern or incidence of adverse reactions.
Combination therapy
In the phase II study of MEC patients by Hajdenberg and colleagues, a combination regimen of palonosetron 0.25 mg plus dexamethasone 8 mg was shown to be safe and well tolerated, with the majority of adverse events that were reported being of mild–moderate severity (CitationHajdenberg et al 2006). Moreover, the safety profile of palonosetron 0.25 mg used as a triple therapy with dexamethasone and aprepitant in MEC and HEC patients was consistent with expectations of these treatment regimens, with adverse events again reported to be of mild or moderate intensity and resolved quickly (Grote et al 2005).
Cardiovascular safety
A theoretical cardiovascular risk has been noted for several first-generation 5-HT3 RAs (CitationNavoban® prescribing information 2001; CitationAloxi® prescribing information 2003/2005; CitationAnzemet® prescribing information 2003), notably dolasetron (CitationHesketh et al 2000) and tropisetron due to increased cardiovascular conduction times, particularly mean prolongation of the QTc interval. Administration of palonosetron 0.25 mg resulted in a 1- to 3-ms increase in QTc interval during phase III studies, which compared well to ondansetron and dolasetron (both 5-ms) (CitationEisenberg, Figueroa-Vadillo et al 2003; CitationGralla et al 2003; CitationAapro et al 2006). Prolongation of the QTc interval was not clinically significant in any of these study groups, confirming that although cardiovascular problems are a theoretical risk, the risk does not translate into a clinical problem.
The effect of antiemetic therapy on cardiovascular parameters in the elderly subpopulation is a very important evaluation since age-related cardiac comorbidities are common in these patients (CitationGridelli et al 2004). It has been shown that the incidence of cardiovascular adverse events in elderly patients treated with palonosetron 0.25 mg is low (<2% of patients) and similar to that of ondansetron 32 mg/dolasetron 100 mg, despite 30% of these patients having some cardiovascular disease or impairment at study enrolment (CitationAapro et al 2005). Very small changes in the QTc interval were observed with palonosetron (-1-ms) and with ondansetron/dolasetron (4-ms) in this group of patients; however, consistent with previous reports (CitationAloxi prescribing information 2003/2005), no severe or serious treatment-related cardiac events were observed in patients receiving any of the antiemetic agents in these studies.
Conclusion and expert opinion
Clinicians have waited since the introduction of the first-generation 5-HT3 RAs for the next step forward in the treatment of CINV. Over this time there has been a rise in the emetogenic potential of increasingly complex chemotherapy regimens without a corresponding evolution in antiemetic therapeutics. During the past three years, significant progress has been made with the advent of the second-generation 5-HT3 RA palonosetron and the NK-1 RA aprepitant.
Our hopes from the phase III trials of palonosetron have indeed been realized. Palonosetron has proven to be an excellent addition to the armamentarium against CINV, specifically against delayed and overall CINV, which until now has proved difficult to treat with conventional 5-HT3 RAs. Not only is palonosetron effective against acute and delayed CINV, it is effective after a single dose, thus providing a simpler and convenient treatment option. An outstanding feature of palonosetron is improved nausea control, which does not seem to be adequately controlled with older 5HT3 RAs or with the new class of NK1 RAs. Improving the convenience of treatment regimens is important both for physicians and patients. Patient adherence, particular from those patients most at risk such as the elderly or young women, will improve with easier treatment regimens (CitationDe Moor et al 2003). A higher degree of attention may be paid to patients’ symptoms having an impact on their functioning during daily life by evaluating patient’s quality of life with specific tools (FLIE, Osoba).
Treatment efficacy, convenience, compliance, and cost-effectiveness can also be improved in clinical practice using combination therapy strategies. Studies to date have clearly demonstrated that palonosetron coadministered with dexamethasone offers enhanced anti-CINV protection with a single-dose infusion (CitationHajdenberg et al 2006), and substantial benefits in preventing CINV are also evident when used in a triple-therapy combination alongside dexamethasone and aprepitant (CitationGrote et al 2006).
Further optimization of antiemetic therapy will require evaluation of drug combinations in a variety of patient groups in order to elucidate any additional synergistic efficacy. Other groups and areas that require specific attention include patients receiving multiday emetogenic therapy, an area where also guidelines for antiemetic prevention may need further improvement, those undergoing radiotherapy with or without concomitant chemotherapy, and those receiving high-dose chemotherapy with hematopoietic stem-cell support.
In addition an effort directed to improve both patients’ and medical professionals’ compliance with an optimal antiemetic schedule will benefit of a better knowledge of longer acting agents that can be given once or antiemetic schedules given on Day 1 only, that are able to ensure protection against nausea and vomiting both in the acute and the delayed setting.
Five-year review
The full potential of the second-generation 5-HT3 RA palonosetron has yet to be explored; combinations with other drug classes will provide a more tailored, individual approach to CINV prophylaxis, and drug regimens will be optimized according to patient risk factors. It is not unreasonable to speculate that this approach will lead to a majority of patients being CINV-free within this timeframe.
Although therapy optimization will require combinations of various drugs, the treatment regimens will become simpler since more will be known regarding the duration of action of drugs. A standard regimen will probably involve a single steroid dose plus a long-acting 5-HT3 RA prior to chemotherapy of moderate to high emetic risk, with administration of an NK-1 antagonist throughout each higher risk chemotherapy cycle.
We will have a number of neurokinin RAs, perhaps with differing pharmacology, thus providing improved efficacy compared to aprepitant, much like the improvement over first-generation 5-HT3 RAs that the pharmacologically distinct palonosetron has provided. Studies of these new drugs and various combinations will also have lead to a better understanding of the complexities of the vomiting and nausea response.
We are now entering an era of change in the conduction of clinical studies. The fact that we can provide many patients with prophylaxis from vomiting is a tribute to the progress made over the past two decades, but we are now realizing that this is not enough. Nausea remains a challenge that is underaddressed and one that physicians are starting to regard more seriously. As a result, future clinical trials should evaluate endpoints that are most relevant to patients.
Key issues
Key issues are as follows:
CINV remains a clinical challenge, particularly the treatment of delayed CINV.
The second-generation 5-HT3 RA palonosetron is pharmacologically differentiated from the first-generation 5-HT3 RAs with a 4–10-fold longer half-life and a >30-fold greater affinity for the 5-HT3 receptor.
A single dose of palonosetron prior to chemotherapy provides protection over the entire overall phase (days 1–5) and particularly over the first three-day period when CINV risk is greatest, hence providing a simple, yet effective, regimen.
In comparison to the first-generation 5-HT3 RAs, the second-generation 5-HT3 RA palonosetron provides improved protection against both acute and delayed CINV.
Palonosetron demonstrates improved efficacy against nausea and maintenance of daily activities, in addition to reducing the number of emetic episodes.
Combining administration of palonosetron with aprepitant and dexamethasone appears to confer a synergistic benefit in preventing acute and delayed CINV.
References
- AaproMSGrunbergSMManikhasGMA phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapyAnn Oncol2006171441916766588
- AaproMSMacciocchiAGridelliCPalonosetron improves prevention of chemotherapy-induced nausea and vomiting in elderly patients: Results from two randomized, controlled clinical trialsJ Support Oncol2005336974 Erratum in: J Support Oncol, 4:107 (2006)16218261
- Aloxi® (palonosetron HCl) prescribing information. 2003/2005. Bloomington, MN, USA: MGI PHARMA, INC.:(2003); Damastown, Dublin, Republic of Ireland: Helsinn Birex Pharmaceuticals Ltd.(2005).
- Anzemet (dolasetron mesylate injection) prescribing information2003Kansas City, MOAventis Pharmaceuticals Inc
- BissoliFMcGuigganMBertazzoliMPost-marketing experience of palonosetron confirms a favourable benefit/risk profileSupport Care Cancer200513413 A04–17
- CantoreggiSParisiSVan LaarEPalonosetron, a new 5-HT3 antagonist, does not alter the antitumor efficacy of various chemotherapeutics in tumor bearing rodentsClin Cancer Res200396254s AC207
- CoatesAAbrahamSKayeSBOn the receiving end: Patient perception of the side-effects of cancer chemotherapyEur J Cancer Clin Oncol19831920386681766
- Data on file. Helsinn Healthcare SA
- de Boer-DennertMde WitRSchmitzPIPatient perceptions of the side effects of chemotherapy: The influence of 5-HT3 antagonistsBr J Cancer1997761055619376266
- De MoorCCohenPEisenbergPDOncologists compliance with anti-emetic guidelines and outcomes of patients receiving emetogenic chemotherapyProc Am Soc Clin Oncol200322727
- de MoorCCunninghamRSImproving the functional status of patients with cancer by more effectively preventing chemotherapy-induced nausea and vomiting (CINV): a comparison of palonosetron (PALO) vs ondansetron (OND) or dolasetron (DOL)J Support Oncol200535 Suppl 3256PA12
- DeckerGMDeMeyerESKiskoDLMeasuring the maintenance of daily life activities using the Functional Living Index-Emesis (FLIE) in patients receiving moderately emetogenic chemotherapyJ Support Oncol20064354116444851
- EglenRMLeeC-HSmithWLPharmacological characterization of RS 25259-197, a novel and selective 5-HT3 receptor antagonist, in vivoBr J Pharmacol199511486067773547
- EisenbergPFigueroa-VadilloJZamoraRImproved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: Results of a phase III, single-dose trial versus dolasetronCancer20039824738214635083
- EisenbergPMacKintoshFRRitchPEfficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical studyAnn Oncol200415330714760130
- EisenbergPDRubensteinEBCohenLPerceptions and satisfaction (S) of oncologists and oncology (RNs) nurses of antiemetic therapy (AET) for chemotherapy-induced nausea and vomiting (CINV) vs patient experiences after emetogenic chemotherapy (CT) in the USProc Am Soc Clin Oncol200322740 A2974
- GrallaRLichinitserMVan der VegtSPalonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetronAnn Oncol2003141570714504060
- GridelliCAaproMFactors influencing the choice of 5-HT3-receptor antagonist antiemetics: focus on elderly cancer patientsSupport Care Cancer2004124879614999555
- GriffinAMButowPNCoatesASOn the receiving end V: Patient perceptions of the side effects of cancer chemotherapy in 1993Ann Oncol19967189958777177
- GroteTHajdenbergJCartmellACombination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitantJ Support Oncol20064403817004515
- GrunbergSVanden BurgtJBerrySPrevention of delayed nausea and vomiting (D-CINV): Carryover effect analysis of pooled data from 2 phase III studies of palonosetron (PALO)J Clin Oncol200422A8051
- GrunbergSMDeusonRRMavrosPIncidence of chemotherapy-induced nausea and emesis after modern antiemeticsCancer20041002261815139073
- GrunbergSMKoellerJMPalonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesisExpert Opin Pharmacother20034229730314640928
- GrunbergSMZhangMZhangQHospital service use associated with chemotherapy-induced emesis and nausea for patients with principal or secondary diagnosis of cancerEur J Cancer200036Suppl 3S28 Abstract #7111056305
- HajdenbergJGroteTYeeLInfusion of Palonosetron plus dexamethasone for the prevention of chemotherapy-induced nausea and vomitingJ Support Oncol200644677117080735
- HeskethPNavariRGroteTDouble-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron methylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy- Induced Emesis Prevention GroupJ Clin Oncol199614224298708713
- HeskethPJKrisMGGrunbergSMProposal for classifying the acute emetogenicity of cancer chemotherapyJ Clin Oncol19971510398996130
- HeskethPJVan BelleSAaproMDifferential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonistsEur J Cancer20033910748012736106
- HeskethPJComparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomitingCancer Invest2000181637310705879
- HornbyPJCentral neurocircuitry associated with emesisAm J Med2001111Suppl 8A106S112S11749934
- KoellerJMAaproMSGrallaRJAntiemetic guidelines: creating a more practical treatment approachSupport Care Cancer2002105192212324805
- LindleyCMcCuneJSThomasonTEPerception of chemotherapy side effects: cancer versus non-cancer patientsCancer Pract19997596510352062
- Navoban (tropisetron hydrochloride) prescribing information2001Avondale, Auckland, New ZealandNovartis New Zealand Limited
- RoilaFHeskethPJHerrstedtJAntiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer. Prevention of chemotherapy- and radiotherapy-induced emesis: Results of the 2004 Perugia International Antiemetic Consensus ConferenceAnn Oncol20061720816314401
- RubensteinEBGrallaRJEisenbergPPalonosetron (PALO) compared with ondansetron (OND) or dolasetron (DOL) for prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV): Combined results of two phase III trialsProc Am Soc Clin Oncol200322729 A2932
- StoltzRCyongJ-CShahAPharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjectsJ Clin Pharmacol2004445203115102873
- TrisselLAXuQAPhysical and chemical stability of palonosetron hydrochloride with Topotecan Hydrochloride and Irinotecan Hydrochloride during simulated Y-site administrationInt J Pharm Comp20059238
- TrisselLAZhangYCompatibility and stability of Aloxi (palonosetron hydrochloride) admixed with dexamethasone sodium phosphateInt J Pharmaceut Compound2004a8398403
- TrisselLAZhangYPhysical and chemical stability of palonosetron HCl with cisplatin, carboplatin, and oxaliplatin during simulated Y-site administrationJ Onc Pharm Pract2004b101915
- TrisselLAZhangYPalonosetron HCl compatibility and stability with doxorubicin HCl and epirubicin HCl during simulated Y-site administrationAnn Pharmacother2005a39280315613463
- TrisselLAZhangYPhysical and chemical stability of palonosetron hydrochloride with fluorouracil and with gemcitabine hydrochloride during simulated Y-site administrationInt J Pharm Comp2005b93202
- VardyJChiewKSGalicaJSide effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapyBr J Cancer20069410111516552437
- WongEHFClarkRLeungEThe interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitroBr J Pharmacol199511485197773546
- XuQATrisselLAStability of palonosetron hydrochloride with paclitaxel and docetaxel during simulated Y-site administrationAm J Health Syst Pharm2004611596815372835