Abstract
Atopic dermatitis (AD) is a multifactorial chronic remittent skin disease which requires long-term treatment. Pimecrolimus cream 1% is a nonsteroid selective inhibitor of inflammatory cytokines and effective in the treatment of AD. Various clinical trials have shown its long-term safety and efficacy in pediatric and adult patients suffering from mild to moderate AD. In this article we discuss data which has assessed the impact of AD on the patient’s quality of life, and the consequent role of topical anti-inflammatory therapy for long-term AD treatment.
Introduction
Atopic dermatitis (AD) is a chronic relapsing cutaneous disease characterized by dry and scaly skin, inflammation, and intense itching. A point prevalence up to 30% in some industrialized countries and a steady increase in disease prevalence has been observed (CitationZuberbier et al 2006). AD is more frequent in children; up to 20% of children are affected (CitationBreuer et al 2005). In adults, we have previously shown a point prevalence of 1.6% if current symptoms are considered (CitationWorm et al 2006). In addition to genetic factors, several trigger factors play a role in the development of AD (CitationLeung and Bieber 2003). These include allergens, microbial infections and, as recently shown, a disrupted skin barrier function (CitationMarenholz et al 2006).
Accordingly, the treatment of AD is complex. One major basic measure is the regular usage of emollients. Furthermore, an anti-inflammatory topical treatment is crucial to ending the itching-scratching cycle in affected patients. Corticosteroids have long been the standard treatment; calcineurin inhibitors now represent an additional treatment option. These offer long-term treatment, with stabilization of the chronic disease and an improvement in quality of life. This is of great importance, as it is known that AD has a greater impact on the quality of life than the other chronic skin diseases (CitationFinlay and Khan 1994). In addition, AD has a negative effect on the mental health of patients (CitationKiebert et al 2002).
The recently published International Study Of Life with ATopic Eczema (ISOLATE) enrolled a total of 2002 patients from eight countries (France, Germany, Mexico, Netherlands, Poland, Spain, UK, and US). It presents for the first time a large amount of quality of life data from affected AD patients. In the following we will review published clinical data on pimecrolimus and discuss its impact on the medical treatment of AD patients.
Structure and mechanism
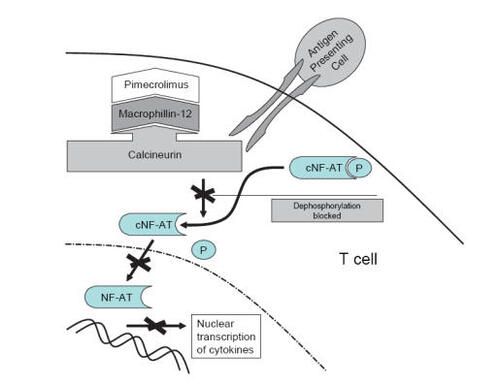
Pimecrolimus is an ascomycin macrolactam derivative and was developed specifically for the treatment of inflammatory skin diseases. By binding to the cytosolic receptor macrophilin-12 with high affinity in T lymphocytes and forming a complex structure, the activity of the calcium dependent phosphatase calcineurin is blocked (). The inhibition of calcineurin prevents from NF-AT dependent transcription of genes encoding the TH2-type cytokines IL-4 and IL-10 but also TH1-type cytokines IL-2 and IFN-γ. Pimecrolimus also decreases cytokine production from mast cells and influences the release of preformed mediators (CitationZuberbier et al 2001; CitationStuetz et al 2006), while not affecting Langerhans cells (CitationMeingassner et al 2003; CitationHoetzenecker et al 2004).
Figure 1 Mechanism of calcineurin inhibitors: By binding to macrophillin-12, calcineurin is blocked which in turn inhibits the translocation of NF-AT into the nucleus and the transcription of pro-inflammatory cytokines of activated T cells. Adapted with permission from CitationEichenfield LE, Beck L. 2003. Elidel (pimecrolimus) cream 1%: A nonsteroidal topical agent for the treatment of atopic dermatitis. J Allergy Clin Immunol, 111:1153–68. Copyright© 2003 Elsevier.
Infants as well as children with extended atopic lesions are at high risk for local side effects and systemic absorption through the skin. Therefore, even minimal systemic absorption is an important safety aspect in infants. It is essential to exclude a possible systemic exposure to pimecrolimus when applied extensively on affected skin. However, previous pharmacokinetic studies in pedriatic patients have shown that systemic absorption of pimecrolimus is low, even in infants with large body surface area involvement (CitationHarper et al 2001; CitationBillich et al 2004; CitationDraelos et al 2005; CitationMeingassner et al 2005). This low level of systemic absorption might be explained by the lipophilicity of the molecule and its high molecular weight (810 Da). Consequently, none of the systemic side effects associated with corticosteroids are expected with pimecrolimus.
Low systemic drug exposure in pimecrolimus treated infants was observed in an open-label, non-controlled study by CitationStaab et al (2005). The drug concentrations remained below 2 ng/mL in most cases (96%).
Clinical efficacy and safety
The efficacy and safety of pimecrolimus was analysed in various clinical studies related to short- and long-term management of AD and early intervention. Especially infants from 3 to 12 months and children up to 17 years of age were treated with pimecrolimus in the context of a novel nonsteroidal therapy. A significant reduction in AD flares and pruritus, and a reduced application rate of topical corticosteroids were observed. In addition, systemic adverse events were rare, probably unrelated to pimecrolimus and not clinically relevant. In the majority of cases only local reactions occurred such as skin burning, flush symptomatic, and skin irritation, which normally disappeared within 1 week of treatment. On the other hand, a slightly increased incidence of viral skin infections during the application of pimecrolimus has been shown; in this case, it is recommended to interrupt the application until the infection subsides (CitationEichenfield et al 2002; CitationKapp et al 2002; CitationWahn et al 2002; CitationHo et al 2003; CitationPapp et al 2005).
In adult patients, the treatment with pimecrolimus resulted also in a significant reduction of topical corticosteroids usage. Furthermore, a pronounced reduction of pruritus and number of AD flares throughout the treatment period were observed. During therapy, quality of life also improved by 35% in patients using pimecrolimus, compared with 11% in the control group after 6 months (CitationMeurer et al 2004).
Currently, pimecrolimus has been approved as second-line therapy for the treatment of mild to moderate AD in non-immunocompromized children ≥2 years old and in adult patients. In 2005 the US Food and Drug Administration (FDA) modified the labeling of pimecrolimus (and tacrolimus) and included a black box warning which is the highest level of five possible warning categories found in the package insert (CitationAaronson 2006). This warning states that although a direct causal relationship has not been established, cases of malignancy (eg, skin and systemic) have been reported in patients treated with pimecrolimus, implying that this treatment bears a potential risk to patients if used in a long-term manner.
In contrast, data from randomized controlled studies suggest that pimecrolimus poses a lower risk of cancer than its vehicles or topical corticosteroids (CitationHultsch et al 2005). In the case of pimecrolimus, out of 19,000 treated subjects 2 cases of cancers were reported (CitationHultsch 2005), while out of 4000 subjects treated with corticosteroids or the comparator vehicle, 5 cases of cancers were described.
Impact of pimecrolimus on the quality of life
For many patients and caregivers of children with AD, the skin disease was not restricted only to the visible symptoms, but also had a severe impact on their overall quality of life. The data from ISOLATE demonstrate that 75% of patients and caregivers regarded an improvement in their quality of life via effective AD control as most important (CitationZuberbier et al 2006).
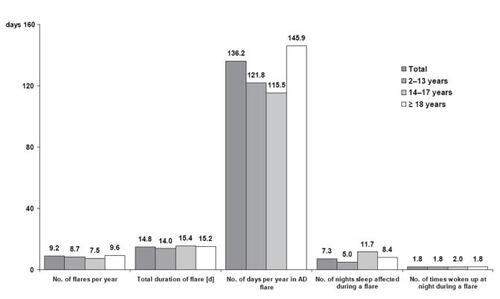
The characteristics of the study population are summarized in . About 50% of AD patients had been diagnosed in the first 3 years of life, 24% after the age of 18 years. Over 50% suffered from moderate AD and 32% had a severe skin status. On average, every patient suffered from 9 flares per year with an average duration of 15 days, resulting in 136 flare days per year. During a flare, sleep disturbance occurred in an average of 7 nights and patients woke up almost twice a night.
Figure 2 Disease characteristics in patients (children, adolescents, adults) with AD (n = 2002). Adapted with permission from CitationZuberbier T, Orlow SJ, Paller AS, et al. 2006. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol, 118:226–32. Copyright© 2006 Elsevier.
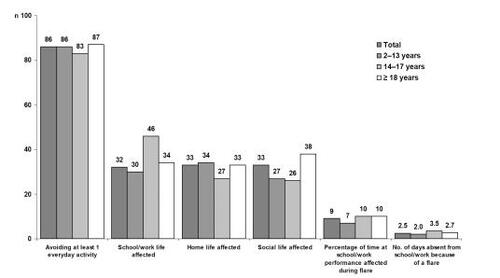
Regarding the consequences of AD flares on patient’s lifestyle, 86% of AD patients avoided at least one type of everyday activity during a flare period, eg, swimming or wearing revealing clothes. In the same way parameters like school/work life, home life, and social life were altered during a flare in almost 33% of the patients. In 9% of patients, school/work performance and productivity was influenced negatively. Patients with AD were absent about 2.5 days per year due to AD from school/work ().
Figure 3 Lifestyle consequences of AD flares on patients and caregivers (n = 2002). Adapted with permission from CitationZuberbier T, Orlow SJ, Paller AS, et al. 2006. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol, 118:226–32. Copyright© 2006 Elsevier.
The ISOLATE data also demonstrate the emotional aspects of AD. At least 43% of the patients suffering from AD were fairly or very concerned about being seen in public while experiencing a flare. More than 1 in 3 patients (36%) was impaired by a flare in terms of self-confidence. Every second patient (51%) was always or sometimes unhappy or depressed respectively, while 27% of the patients had experienced bullying because of their AD ().
Table 1 Emotional aspects of AD (n = 2002)
Further aspects of patients’ social lives were affected by AD flares, including relationships. About 30% of the patients or caregivers believed that either their or their child’s AD had an effect on other household members. Twenty-one percent of adult patients indicated problems in forming relationships with a partner, and 12% had experienced difficulties in established relationships due to an AD flare. Furthermore, almost half of the patients (42%) felt embarrassed to be seen by their partner during a flare ().
Table 2 Effects of AD on relationships (n = 2002)
The ISOLATE data also demonstrated data on the “quality of life index for atopic dermatitis” (QoLIAD) for AD patients older than 13 years and the “parent’s index of quality of life – atopic dermatitis” (PIQoL-AD) for caregivers of children aged 2–13 years. While adolescent and adult patients were worried (73%) and embarrassed (44%) about their appearance and did not want people to see their skin during a flare (63%), the caregivers were concerned regarding the choice of clothes (71%) and had side effects of AD treatments in mind (64%) (). The topical corticosteroid treatment especially worried about 89% of the caregivers and 83% of adolescent and adult patients.
Table 3 QoLIAD and PIQoL-AD responses
The perceptions of patients and caregivers regarding topical AD treatment are shown in . Skin atrophy after the application of topical corticosteroids still remains the most important anticipated side effect.
Table 4 Patient perception of topical AD treatment side effects
The treatment with pimecrolimus was associated with a statistically significant improvement in quality of life after 6 months of treatment compared to baseline in adult patients and caregivers. Interestingly, those caregivers who had previously reported concerns about local treatment with corticosteroids reported a significantly greater improvement in quality of life than did those without such concerns. The application of corticosteroids on the other hand was less frequent and for shorter periods than recommended by the doctor. This is in line with previous studies showing the steroid sparing effect during long-term pimecrolimus treatment (CitationMeurer et al 2004).
Other recent studies have also reported the positive impact of pimecrolimus therapy on patients’ quality of life (CitationMcKenna et al 2006; CitationSunderkötter et al 2006). The data were assessed with the “Dermatology Life Quality Index” (DLQI) and the “Children’s Dermatology Life Quality Index” (CDLQI) respectively and the PIQoL-AD. In the course of treatment a significant improvement in the quality of life could be demonstrated (DLQI p < 0.0001, PIQoL-AD p < 0.052) together with an effective and well tolerated therapy.
Conclusion
AD is one of the most frequent chronic inflammatory skin diseases, and it has a great impact on the quality of life on affected patients. Data from the ISOLATE study have allowed for the first time a detailed analysis of social and emotional consequences in AD patients. The severity of these consequences indicates the necessity of effective treatment of AD. These consequences can be alleviated via long-term topical treatment with topical calcineurin inhibitors.
References
- AaronsonDWThe “black box” warning and allergy drugsJ Allergy Clin Immunol200611740416387582
- BillichAAschauerHAszódiAPercutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimusInt J Pharm2004269293514698574
- BreuerKWerfelTKappASafety and efficacy of topical calcineurin inhibitors in the treatment of childhood atopic dermatitisAm J Clin Dermatol20056657715799678
- DraelosZNayakAPariserDPharmacokinetics of topical calcineurin inhibitors in adult atopic dermatitis: a randomized, investigator-blind comparisonJ Am Acad Dermatol200553602916198779
- FinlayAYKhanGKDermatology Life Quality Index (DLQI) - a simple practical measure for routine clinical useClin Exp Dermatol199419210168033378
- EichenfieldLEBeckLElidel (pimecrolimus) cream 1%: A nonsteroidal topical agent for the treatment of atopic dermatitisJ Allergy Clin Immunol200311111536812743593
- EichenfieldLFLuckyAWBoguniewiczMSafety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescentsJ Am Acad Dermatol20024649550411907497
- HarperJGreenAScottGFirst experience of topical SDZ ASM 981 in children with atopic dermatitisBr J Dermatol2001144781711298537
- HoetzeneckerWMeingassnerJGEckerRCorticosteroids but not pimecrolimus affect viability, maturation and immune function of murine epidermal Langerhans cellsJ Invest Dermatol20041226738415086553
- HoVCGuptaAKaufmannRSafety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infantsJ Pediatr2003421556212584537
- HultschTElidel (pimecrolimus) cream 1% safety update Feb 2005. [online] CS-5: URL: http://www.fda.gov/ohrms/dockets/ac/05/slides/2005-4089s2_02_02_Novartis%20Core%20Safety%20(CS).pdf
- HultschTKappASpergelJImmunomodulation and safety of topical calcineurin inhibitors for the treatment of atopic dermatitisDermatology20052111748716088174
- KappAPappKBinghamAFlare Reduction in Eczema with Elidel (infants) multicenter investigator study group Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drugJ Allergy Clin Immunol20021102778412170269
- KiebertGSorensenSVRevickiDAtopic dermatitis is associated with a decrement in health-related quality of lifeInt J Dermatol200241151812010340
- LeungDYBieberTAtopic dermatitisLancet20033611516012531593
- MarenholzINickelRRuschendorfFFilaggrin loss-of-function mutations predispose to phenotypes involved in the atopic marchJ Allergy Clin Immunol20061188667117030239
- McKennaSPWhalleyDde ProstYTreatment of paediatric atopic dermatitis with pimecrolimus (Elidel, SDZ ASM 981): impact on quality of life and health-related quality of lifeJ Eur Acad Dermatol Venereol2006202485416503881
- MeingassnerJGAschauerHStuetzAPimecrolimus permeates less than tacrolimus through normal, inflamed, or corticosteroid-pretreated skinExp Dermatol200514752716176283
- MeingassnerJGKowalskyESchwendingerHPimecrolimus does not affect Langerhans cells in murine epidermisBr J Dermatol2003149853714616380
- MeurerMFartaschMAlbrechtGCASM-DE-01 Study GroupLong-term efficacy and safety of pimecrolimus cream 1% in adults with moderate atopic dermatitisDermatology20042083657215178928
- PappKAWerfelTFolster-HolstRLong-term control of atopic dermatitis with pimecrolimus cream 1% in infants and young children: a two-year studyJ Am Acad Dermatol200552240615692468
- StaabDPariserDGottliebABLow systemic absorption and good tolerability of pimecrolimus, administered as 1% cream (Elidel) in infants with atopic dermatitis - a multicenter, 3-week, open-label studyPediatr Dermatol2005224657116191004
- StuetzABaumannKGrassbergerMDiscovery of topical calcineurin inhibitors and pharmacological profile of pimecrolimusInt Arch Allergy Immunol200614119921216926539
- SunderkötterCWeissJMBextermöllerRPost-marketing surveillance on treatment of 5, 665 patients with atopic dermatitis using the calcineurin inhibitor pimecrolimus: positive effects on major symptoms of atopic dermatitis and on quality of lifeJ Dtsch Dermatol Ges20064301616638059
- WahnUBosJDGoodfieldMFlare Reduction in Eczema with Elidel (Children) Multicenter Investigator Study GroupEfficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in childrenPediatrics20021101 Pt 1e212093983
- WormMForschnerKLeeHHFrequency of atopic dermatitis and relevance of food allergy in adults in GermanyActa Derm Venereol20068611912216648913
- ZuberbierTChongSUGrunowKThe ascomycin macrolactam pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophilsJ Allergy Clin Immunol20011082758011496246
- ZuberbierTOrlowSJPallerASPatient perspectives on the management of atopic dermatitisJ Allergy Clin Immunol20061182263216815160