Abstract
Entecavir (ETV) is a potent and selective inhibitor of hepatitis B virus replication. In HBeAg-positive and HBeAg-negative lamivudine-naïve patients with chronic hepatitis B (CHB), treatment with ETV at a dose of 0.5 mg daily is associated with a more potent viral suppression, a higher rate of biochemical remission and a greater improvement of liver histology compared to Lamivudine (LAM). After 3 years of ETV treatment, the majority of patients (94%) may achieve serum HBV DNA levels undetectable by sensitive PCR assays. ETV treatment of patients with LAM-resistant HBV mutants requires a higher daily dose of 1 mg yet, potent HBV suppression at 3 years is achieved only in 40% of them while the cumulative rate of genotypic HBV resistance increases from 6% in the first year to >30% in year 3. ETV resistance of HBV is rare in lamivudine-naïve patients with a reported rate of <1% after three years of treatment. In conclusion, ETV is a very potent anti-HBV drug with a high genetic barrier to resistance, highly effective in lamivudine-naïve CHB patients and most promising for their long-term treatment but not very suitable for CHB patients harboring LAM–resistant HBV mutants.
Introduction
Chronic hepatitis B virus (HBV) infection is a major health problem, affecting hundreds of millions of people worldwide. It may progress to cirrhosis and hepatocellular carcinoma (HCC) and is responsible for almost 1 million deaths annually (CitationLee 1997; CitationMaddrey 2000). Eradication of HBV infection with currently available therapies is not really possible. Resolution of the infection, as indicated by HBsAg loss and development of anti-HBs, is also extremely rare. Thus, the most realistic goal of hitherto approved therapies for chronic hepatitis B (CHB) is potent and durable suppression of viral replication aiming at stop of progression and remission/regression of the underlying liver disease (CitationDi Marco et al 2004; CitationLiaw et al 2004). More specifically, in patients with HBeAg-positive CHB the end point and goal of treatment is HBeAg seroconversion, while in patients with HBeAg-negative CHB is durable suppression of HBV to HBV DNA levels undetectable by real time PCR assays (CitationLok et al 2001).
Six drugs have been approved for the treatment of chronic hepatitis B, standard interferon-α (IFN-α), the Pegylated form (PEG)-IFN-α-2a, and the nucleos(t)ide analogues Lamivudine (LAM), Adefovir Dipivoxil (ADV), Entecavir (ETV) and most recently Telbivudine (LdT).
Interferon-alpha
Interferon-alpha is the first drug approved for the treatment of CHB. In HBeAg-positive CHB finite course of treatment with standard IFN-α has achieved HBeAg loss in 33% of treated patients vs 12% of placebo-treated controls, p = 0.0001 (CitationWong et al 1993), with responses being durable after stopping treatment (CitationNiederaou et al 1996). In HBeAg-negative CHB, long-term biochemical remission has been reported in 15%–25% of patients treated for 6 or more months with a significant percentage of them (31.6%) also loosing HBsAg (CitationHadziyannis et al 1990; Manesis and Hadziyanni et al 1990; CitationPapatheodoridis et al 2001). On the other hand PEG-IFN-α-2a either alone or in combination with LAM in HBeAg-positive CHB, achieved similar rates of HBeAg seroconversion in 32% and 27% of patients, respectively and both regimens were superior to LAM monotherapy (32% vs 19%, p < 0.001 and 27% vs 19%, p = 0.02) (CitationLau et al 2005) (see also ). In HBeAg-negative CHB, PEG-IFN-α-2a alone or in combination with LAM achieved similar rates of virologic remission (HBV-DNA < 20.000 cp/mL) in 43% and 44% of patients, respectively and again the two regimens were clearly superior to LAM monotherapy (29%, p = 0.007 and p = 0.003, respectively) (CitationMarcellin et al 2004). Furthermore, follow-up of patients, 2 years after discontinuation of treatment has shown that the response appears to be durable (CitationMarcellin et al 2006). Treatment with PEG-IFN-α-2a is probably superior to standard IFN-α, but there is no comparative study between the two forms of interferon-α applied in courses of the same duration. Although treatment with IFN-αs has many adverse events and the disadvantage of subcutaneous administration, these are the only available drugs that can achieve HBsAg seroconversion in finite courses of treatment of less than one year duration (CitationWong et al 1993; CitationMarcellin et al 2004; CitationLau et al 2005).
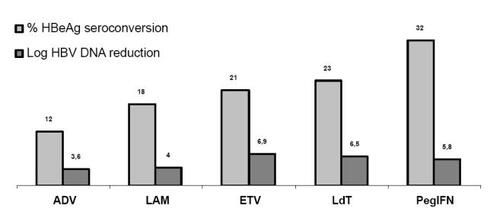
Figure 1 Comparison of responses to treatment (HBeAg seroconversion and log reduction of serum HBV DNA) of HBeAg positive chronic hepatitis B. CitationLau et al. 2005. N Engl J Med, 352:2682–95. Dienstag et al. 1999. N Engl J Med, 341:1256–63. CitationMarcellin et al. 2005. EASL, Abstract 73. Lai et al. 2005. AASLD, Abstract 72404.(LB1) Chang et al. 2004 AASLD, Abstract 70. Telbivudine package insert. CitationMarcellin et al. 2003. N Engl J Med. 348:808–16. Chang et al. 2006. N Engl J Med. 354:1001–10.
In view of the above data it is clear that both in HBeAg-positive and HBeAg-negative patients with chronic hepatitis B pegylated IFN-α2a monotherapy in finite courses of 48 weeks duration, achieves higher rates of responses sustained after their discontinuation compared to LAM monotherapy.
Lamivudine
Lamivudine, the first licensed nucleoside analogue (CitationLai et al 1998; CitationDienstang et al 1999), may achieve HBeAg seroconversion, in 18% of HBeAg-positive CHB patients at the first year and in 27% and 40% at the second and third year respectively (CitationLai et al 1998; CitationDienstang et al 1999; CitationLiaw et al 2000; CitationLeung et al 2001). In HBeAg-negative CHB, LAM therapy for one year resulted in undetectable serum HBV DNA in 65% of patients (CitationTassopoulos et al 1999). The high rate of virologic response at one year is not sustainable after discontinuation of therapy, while prolongation of treatment is associated with decreasing rates of virologic response due to the emergence of LAM-resistant HBV mutants in progressively increasing rates reaching levels above 60% in 4 to 5 years time (CitationLai et al 1998; CitationDienstang et al 1999; CitationLiaw et al 2000; CitationLeung et al 2001; CitationPapatheodoridis et al 2002; CitationLok et al 2003). The development of viral resistance may be associated with worsening of liver histology, exacerbation of liver disease and even with liver failure and deaths especially in patients with cirrhosis (CitationDienstag et al 2003; CitationLok et al 2003; CitationDi Marco et al 2004; CitationLiaw et al 2004; CitationPapatheodoridis et al 2005).
Adefovir dipivoxil
Adefovir dipivoxil, a nucleotide analogue, the second licensed oral anti-HBV agent, is effective in the treatment of both wild type and LAM-resistant HBV (CitationHadziyannis et al 2003; CitationMarcellin et al 2003; CitationPeters et al 2004). In HBeAg-positive CHB, HBeAg seroconversion was achieved in 12% of patients at the first year, increasing to 29% and 43% at the second and third year of therapy respectively (CitationMarcellin et al 2003, Citation2005). In HBeAg-negative CHB, long-term ADV therapy is associated with undetectable serum HBV DNA in 71%, 79%, 65% and 67% at year 2, 3, 4 and 5, respectively (CitationHadziyannis et al 2003, Citation2005a, Citation2005b, Citation2006). ADV resistance is delayed and infrequent developing after the first year of treatment. Genotypic HBV resistance may emerge in 3%, 11%, 18% and 29% of patients at years 2, 3, 4 and 5 of treatment, respectively (CitationHadziyannis et al 2003, Citation2005a, Citation2005b, Citation2006). However, virologic and biochemical breakthroughs at year 5 are restricted only to 16% and 11% respectively (CitationHadziyannis et al 2006). In patients with LAM-resistant HBV strains, treatment with ADV alone or in combination with LAM has similar rate of virological (26% vs 35%) and biochemical response (47% vs 53%) at one year (CitationPeters et al 2004). However, longer duration of treatment has shown that adding adefovir on lamivudine rather than switching from lamivudine to adefovir represents the treatment of choice in patients developing LAM-resistant HBV mutants (CitationFung et al 2005, Citation2006; CitationLampertico et al 2006; CitationRapti et al 2007).
Telbivudine
Telbivudine (LdT, β-L-2’-deoxythimidine, Idenix, Cambridge, MA) is an L nucleoside analogue of thymidine, approved by FDA on late 2006 for the treatment of chronic hepatitis B under the trade name of Tyzeka at a daily dose of 600 mg. It is a reverse transcriptase inhibitor and acts by competing with the natural substrate thymidine 5’ triphosphate and its incorporation into viral DNA causes chain termination, resulting in inhibition of HBV replication. It is a potent anti-HBV agent inhibiting the synthesis both of the first and the second DNA strand of the virus and in phase II studies it has been found to be more effective than lamivudine. In HBeAg-positive patients it achieved a greater reduction in HBV DNA levels (6.01 log10 vs 4.57 log10, p < 0.05) as well as higher rates of HBV DNA undetectability (61% vs 32%, p < 0.05) and normalization of ALT levels (86% vs 63%, p < 0.05) (CitationLai et al 2004; CitationLai et al 2005a). The year 1 and 2 results of a very large registration trial in 1367 individuals with HBeAg-positive and -negative CHB, the GLOBE trial, have been reported in the 2005 AASLD (CitationLai et al 2005b) and the 2006 EASL (CitationThongsawat et al 2006) and AASLD (CitationLai et al 2006a) Meetings. The following important observations came out from the analyses of the results at weeks 52 and 104 of this pivotal LdT study:
LdT is equally safe but more effective than lamivudine in terms of absolute HBV DNA reduction from baseline (HBeAg-positive: 6.5 log10 vs 5.5 log10 at week 52, p < 0.05 and 5.7 log10 vs 4.4 log10, p < 0.05, at week 104, respectively, HBeAg-negative: 5.2 log10 vs 4.4 log10, p < 0.05 at week 52 and 5 log10 vs 4.2 log10, p < 0.05 at week 104, respectively) and time of undetectability. However there is no significant difference from LAM in HBeAg loss and seroconversion to anti-HBe (26% vs 23% at week 52, 35% vs 29% at week 104 and 22% vs 21% at week 52, 30% vs 25% at week 104, respectively) (CitationLai et al 2006b).
Treatment failures and HBV resistance are less frequent with LdT compared to LAM (treatment failure in HBeAg- negative patients 0.9% vs 7.6%, p < 0.001 and in HBeAg-positive 6.8% vs 18.8%, p < 0.001, respectively) (CitationLai et al 2006b).
Both in LdT and LAM treated HBeAg-positive patients, the rates of HBeAg seroconversion are significantly increased if a profound and rapid HBV suppression to undetectability of serum HBV DNA at week 24 is achieved, this effect being also associated with enhanced T-cell reactivity to HBc protein (CitationCooksley et al 2006).
Entecavir
Entecavir, a novel carbocyclic analog of 2’ deoxyguanosine (), has been approved in 2005 in USA and in 2006 in Europe for naïve and lamivudine-resistant chronic hepatitis B treatment. Data on its safety, side effects, short- and long-term efficacy and HBV resistance in the various subsets of HBV patients are critically reviewed in this article.
In the HBeAg response rates achieved with one year of therapy by ETV, the other three approved nucleos(t)ides and pegylated interferon-alfa2a together with their potency in terms of log reduction in HBV DNA levels are shown in a comparative way.
Entecavir in chronic hepatitis B
Entecavir therapy in treatment-naïve patients with chronic hepatitis B
The safety and efficacy of ETV were initially evaluated in a randomized, placebo-controlled, dose-escalating study (CitationDe Man et al 2001). In patients with CHB, ETV was administered at doses of 0.05 mg, 0.1 mg, 0.5 mg and 1 mg and compared to placebo for 28 days (CitationDe Man et al 2001). All patients were followed-up 24 weeks off therapy. All doses of ETV were well tolerated and were associated with a significant decline in serum HBV DNA levels. However, a slower rebound of viremia in the post treatment follow-up period was observed with the 0.5 and 1 mg of ETV compared with the other two lower doses (p < 0.05) (CitationDe Man et al 2001). During the post-treatment period, 9% of ETV treated patients experienced hepatic flares defined as elevation in ALT greater than twice the baseline level and more than 10 times the upper limit of normal. None of these flares were associated with elevated bilirubin levels and were not clinically significant. This study has showed that ETV is a potent inhibitor of HBV in humans, but longer duration dosing trials should be performed before definite conclusions about the safety and the role of ETV in the treatment of chronic hepatitis B infection are made.
In another double-blind randomized study, the safety and efficacy of 3 doses of ETV were evaluated and compared to LAM in patients with CHB (CitationLai et al 2002). Entecavir doses of 0.01 mg, 0.1 mg, 0.5 mg and LAM 100 mg daily were administered for 24 weeks. Complete response was defined as undetectable serum HBV DNA by bDNA (cut-off 0.7 Meq/mL) at week 22, with normal ALT and undetectable HBeAg for HBeAg-positive patient at baseline. Treatment was discontinuated in individuals with complete response and they were followed-up for 12 weeks. Partial response was defined as undetectable serum HBV DNA by bDNA assay at week 22, but without loss of HBeAg for HBeAg-positive patients or with elevated ALT for HBeAg-negative patients at baseline. In these patients, LAM 100 mg were given for 48 weeks. The nonresponders (detectable HBV DNA by bDNA) were managed after week 24 by their physician and followed-up for 12 weeks. One hundred sixty-nine patients (81% HBeAg-positive) completed 24 weeks of treatment period. At the end of therapy a significantly higher proportion (83.7%) of patients receiving 0.5 mg of ETV had undetectable HBV DNA levels (bDNA < 0.7Meq/mL) compared to LAM 100 mg (57.5%, p = 0.008), while the response to the ETV 0.1 mg dose was similar with LAM (61.8% vs 57.5%). Serum HBV DNA undetectable by the b-DNA assay and ALT normalization were observed in 23.1% and 50%, 61.8% and 83.3%, 83.7% and 69%, 57.5% and 59.1% at the 0.01, 0.1, 0.5 mg ETV and the 100 mg LAM, doses respectively (CitationLai et al 2002). Complete or partial response occurred in 23%, 62%, 84% and 57% of patients in the 0.01 mg, 0.1 mg and 0.5 mg ETV and LAM group, respectively. After discontinuation of treatment, ALT elevation > 3xULN was observed in 21% of patients in the 0.01 mg ETV group compared to 10.5% in the LAM group and in 3% and 4.5% in the 0.1 and 0.5 mg ETV groups respectively. On the basis of the results of this study it was considered that the optimal dose of ETV for the treatment of naïve patients should be 0.5 mg daily (CitationLai et al 2002).
Efficacy in HBe-positive patients
In a randomized phase III double-blind trial, 715 treatment-naïve, HBeAg-positive (+) CHB patients were assigned to receive either 0.5 mg of ETV or 100 mg of LAM once daily for a minimum period of 52 weeks (CitationChang et al 2006a). According to the study protocol clinical-management decisions were made at week 52 on the basis of the results of HBV DNA levels (b-DNA) and HBeAg assays on serum samples obtained at week 48. Patients who had a complete response defined as undetectable serum HBV DNA levels (< 0.7 Meq/mL) and HBeAg loss and the nonresponders defined by serum HBV DNA levels ≥ 0.7 Meq/mL discontinued treatment at week 52. Patients who achieved only virologic response [undetectable serum HBV DNA levels (<0.7 Meq/mL), without HBeAg loss] were continued on therapy up to 96 weeks (CitationChang et al 2006a). At week 48, histologic improvement (necroinflammation score) occurred in significantly higher proportion of patients treated with ETV compared to LAM (72% vs 62%, p = 0.009). Serum HBV DNA levels became undetectable by PCR methods in 67% and 36% (p < 0.001) of the patients treated with ETV and LAM, respectively. Moreover, the mean reduction from baseline in the serum HBV DNA levels at week 48 was 6.9 log and 5.4 log in the ETV and LAM group respectively (p < 0.001). Although ETV achieved a significantly greater suppression of HBV DNA levels than LAM, the rate of HBeAg loss and HBeAg seroconversion did not differ significantly between the two treatment groups being 22% and 21% in the ETV vs 20% and 18% in the LAM arm (CitationChang et al 2006a, ). At week 48, 21% of the patient in the ETV group and 19% of those in the LAM group had achieved a complete response, 70% and 46% respectively had only virologic response and 5% and 26% respectively had no response. Among patients with response at week 48, 82% in the ETV and 73% in the LAM group sustained their response 24 weeks after discontinuation of treatment. There was no evidence of emergence of ETV resistant variants among 339 evaluated patients and although 6 (2%) of ETV treated individuals experienced a virologic rebound during the first year of therapy, samples obtained from these patients retained full phenotypic susceptibility to ETV. The adverse events were similar in the two groups. In the ETV group 3% of patients experienced alanine aminotransferase flares (ALT levels more than twice the baseline level and more than 10 times the ULN) during treatment while such flares were observed in 6% of the patients in the LAM group. All flares in the ETV group were associated with a decline of HBV DNA levels by ≥ 2 log10 and all but one were self-limited with continuation of treatment, without any evidence of hepatic decompensation. On the other hand half of the flares in the LAM group were associated with increasing HBV DNA levels and in one patient hepatic decompensation developed. Post-treatment ALT flares were observed in 1% in the ETV group and in 7% of patients in the LAM group (CitationChang et al 2006a).
Table 1 Comparison of entecavir vs lamivudine in HBeAg positive and HBeAg-negative treatment-naïve patients with chronic hepatitis B (CHB)
The efficacy of extension of ETV and LAM treatment to week 96 in those HBeAg-positive patients who had only a virological response at week 48 without HBeAg seroconversion, have also been evaluated (CitationGish et al 2005). During this period return of ALT to normal was achieved in 50% of the ETV and 42% of the LAM-treated patients. The cumulative virologic response rate at week 96 (), defined by HBV DNA <300copies/mL, was 80% in the ETV and 39% in the LAM group (p < 0.001). Despite the significantly greater suppression of HBV DNA in the ETV group, the cumulative HBeAg seroconversion rate did not differ significantly between the two treatment groups () Furthermore, patients achieving only virologic response during the second year of therapy could receive double dose of ETV (1 mg) for at least one additional year. Thus 122 from 151 eligible HBeAg-positive patients were enrolled and evaluated at week 144. Serum HBV DNA <300copies/mL and ALT normalization were observed in 87% and 85% of patients respectively, while HBeAg loss and HBeAg seroconversion were achieved in 31% and 16% of them (CitationChang et al 2006b).
Efficacy in HBeAg-negative patients
In a randomized double-blind trial, treatment with ETV 0.5 mg was compared to LAM 100 mg once daily for at least 52 weeks (CitationLai et al 2006b). According to the study protocol, similarly with the trial in HBeAg-positive patients, clinical-management decisions were made at week 52 on the basis of the results of HBV DNA levels (b-DNA) and ALT levels on serum samples obtained at week 48. Response was defined as undetectable serum HBV DNA (<0.7Meq/mL by the b-DNA assay) and ALT levels below 1.25 times the upper limit of normal (ULN) and non response as a serum HBV DNA levels ≥0.7 Meq/mL. Treatment was discontinued at week 52 both in responders and non-responders. Patients with only virologic response (= undetectable serum HBV DNA levels <0.7 Meq/mL) and ALT levels ≥1.25xULN were offered continued therapy for up to 96 weeks. Histologic improvement (reduction of necroinflammation) occurred at week 48 in significantly more patients in the ETV than in the LAM group (70% vs 61%, p = 0.01). Undetectable serum HBV DNA levels by PCR and ALT normalization were observed in 90% and 78% and in 72% and 71% of patients treated with ETV and LAM, respectively (CitationLai et al 2006b). Eighty-five percent of patients in the ETV group and 78%, (p = 0.04) in the LAM group had responded at week 48 while 10% and 11% of each group had only virologic response. At the end of 24 weeks follow-up the response was sustained in 48% and 35% of the patients in the LAM and ETV group, respectively. There was no evidence of ETV resistance at week 48, in paired samples from 211 randomly selected patients in the ETV group but 5 (2%) of these patients experienced virologic rebound. Genotypic analysis from these patients revealed no emerging substitutions that confer resistance to ETV. Twenty-five patients (8%) in the LAM group had a virologic rebound during the treatment period (CitationLai et al 2006b). The adverse events between the two treatment groups were similar. Three patients in the ETV group experienced ALT flares, associated with a reduction of HBV DNA levels by at least 2 log10, that resolved spontaneously, while such flares occurred in 5 patients in the LAM group. In two of these 5 patients alanine aminotransferase flares were associated with a reduction of HBV DNA levels by at least 2 log10 and in the other 3 with increasing HBV DNA levels. One of the last experienced the development of ascites. Post-treatment ALT flares occurred in 8% of the patients in the ETV and in 11% in the LAM group (CitationLai et al 2006b).
Twenty-six patients in the ETV and 28 in the LAM group continued therapy up to week 96 (CitationShouval et al 2006). The cumulative virologic response through week 96 was 94% and 77%, (p < 0.0001) among ETV and LAM treated patients respectively, while biochemical response occurred in 89% and 84% of them (CitationShouval et al 2006).
In the response rates at year 1 and 2 in HBeAg-positive and HBeAg-negative patients treated by ETV or LAM are depicted. It is obvious that ETV is superior to LAM in terms of liver histology improvement, HBV DNA suppression, ALT normalization and the viral resistant strains development rate. Moreover despite the higher rate of HBV DNA suppression in ETV group the rate of HBeAg seroconversion was similar between the two groups for the treatment period.
Data from the above studies also indicated that response rate to ETV did not differ between patients with and without cirrhosis on liver biopsy (CitationSchiff et al 2005). Although the number of cirrhotic patients was small in the two studies, histologic improvement occurred in a similar rate between patients with or without cirrhosis on liver biopsy at baseline, 76% vs 72% in HBeAg-positive and 74% vs 70% in HBeAg-negative patients, respectively (CitationSchiff et al 2005).
Efficacy of entecavir in patients with lamivudine- and adefovir-resistant HBV mutants
The efficacy and safety of ETV in LAM-resistance patients were initially evaluated in a double-blind randomized dose-ranging trial. In this study, 182 patients with HBeAg- positive and -negative CHB and LAM-resistant HBV were enrolled and treated with 3 different daily doses of ETV (0.1 mg, 0.5 mg and 1 mg) in comparison to LAM 100 mg daily (CitationChang et al 2005). A significant higher proportion of patients in the ETV 1 mg (79%) and 0.5 mg (51%) groups achieved serum HBV DNA levels < 0.7 Meq/mL (by bDNA assay) compared to the LAM group (13%, p < 0.0001), after 24 weeks of treatment (CitationChang et al 2005). Moreover the proportion of patients with undetectable serum HBV DNA by PCR assay was higher in ETV 1 mg (26%) and 0.5 mg (26%) groups compared to LAM (4%, p < 0.01) at 48 weeks of treatment. HBeAg seroconversion was achieved in a minority of HBeAg-positive patients in all treatment groups (CitationChang et al 2005). The rate of biochemical remission at week 48 was superior in ETV 1 mg (68%) and 0.5 mg (59%) than in LAM (6%, p < 0.001). In this study superiority of ETV at dose of 1 mg for the treatment of LAM-resistant patients was revealed. At baseline, 87% of the patients had lamivudine resistance-associated substitutions and at week 48, 80% of them retained this substitution, regardless of treatment group. Entecavir-associated resistance substitutions (rtT184, rtS202 and rtM250) were detected in 6 patients at baseline and emerged in 2 entecavir-treated patients (1 receiving 0.5 mg and 1 receiving 0.1 mg) during treatment period, but only one patient experienced viral rebound at week 48 (CitationChang et al 2005). Viral rebound was also observed in 5 ETV treated patients, but genotypic analysis could not reveal any entecavir-resistance mutations (CitationChang et al 2005).
In a randomized phase III trial, in HBeAg-positive CHB patients with LAM-resistant HBV, ETV 1 mg daily was compared with LAM 100 mg daily for at least 52 weeks (CitationSherman et al 2006). In this study clinical management decisions were again made at week 52, based on week 48 results. Responders defined by serum HBV DNA levels < 0.7 Meq/mL by bDNA assay and HBeAg loss at week 48 and nonresponders (HBV DNA levels > 0.7 Meq/mL) discontinued treatment and followed-up for 24 weeks period. Patients with only virologic response at week 48 as defined by serum HBV DNA levels < 0.7 Meq/mL by bDNA assay, without HBeAg loss, continued study medication until week 96 (CitationSherman et al 2006). At week 48, histologic improvement observed in a higher proportion of patient in the ETV than in LAM group (55% vs 28%, p < 0.001) (). Moreover the rates of virological response (by PCR assay) and return of ALT to normal were higher in the ETV than in the LAM treated patients, 19% vs 1%, (p < 0.0001) and 61% vs 15%, (p < 0.0001), respectively. The rate of HBeAg seroconversion did not differ between the two groups (ETV group 8% and LAM group 3%, p = 0.06). At baseline 18 (6%) of lamivudine-refractory patients had substitutions that confer resistance to lamivudine and entecavir. Genotypic analysis of paired baseline and week 48 samples were performed for 134 of the 141 ETV-treated patients and reveled 7 patients with mutations associated with ETV resistance at baseline. None of these patients exhibited virologic rebound during the first year of therapy. Two other patients (1.4%) experienced a virologic rebound during ETV-treatment and genotypic analysis revealed mutations that confer resistance to ETV (CitationSherman et al 2006).
Table 2 Comparison of entecavir vs lamivudine in HbeAg positive lamivudine-resistance patients with chronic hepatitis B (CHB)
A number of patients in the ETV and the LAM groups continued treatment for a second year (CitationYurdaydin et al 2006). The cumulative confirmed virologic, serologic (HBeAg seroconversion) and biochemical response at week 96 in the ETV and LAM groups were 30% and 1%, (p < 0.0001), 16% and 4%, (p = 0.0011) and 85% and 29%, (p < 0.0001), respectively. Nine percent of the ETV treated patients exhibited viral rebound due to ETV resistant mutations (CitationYurdaydin et al 2006).
After several years of use of adefovir dipivoxil, HBV resistance to this compound has been recognized among patients under long-term ADV monotherapy particularly after the second year of treatment with genotypic ADV resistance reaching 29% at year five. In vitro studies have shown that ETV inhibits effectively the replication of ADV resistant HBV mutants (CitationBrunelle et al 2005). However, clinical studies on the efficacy of ETV in patients with ADV-resistant HBV mutants have not been performed yet, but in one recent report ETV treatment in two patients with ADV resistance showed a ≥3 log10 decline of HBV DNA levels after 6 months of therapy (CitationFung et al 2005).
Entecavir resistance
Entecavir resistance was first been identified in two patients with LAM-resistant strains, who experienced virologic breakthrough after more than 1 year of ETV therapy (CitationTenney et al 2004). Genotypic analysis of the polymerase region before the initiation of ETV treatment, had shown substitutions conferring HBV resistance to LAM therapy: rtL180M, rtM204V, rtV173L in both patients. Genotypic analysis of the polymerase region after virologic breakthrough under ETV treatment showed that in addition to the LAM-resistance substitutions, the unique substitutions rtI169T (domain B) and rtM250V (domain E) in the first patient and the rtS184G (domain B) and rtS202I (domain C) in the second one emerged (CitationTenney et al 2004). Phenotypic analysis of recombinant HBV genomes, containing patients’ RT domains or specific mutations, was performed. Substitution rtI169T alone or in combination with LAM-resistance substitutions did not decrease the susceptibility to ETV. Substitution rtM250V alone conferred a low level of ETV resistance, while in combination with LAM-resistance substitutions, a >1,000-fold reduction in ETV susceptibility was observed. Substitutions rtS184G and rtS202I alone did not confer resistance to ETV, while either substitution in combination with LAM-resistance substitutions slightly reduced the susceptibility to ETV. HBV containing all 4 substitutions rtL180M, rtM204V, rtS184G and rtS202I exhibited the highest level of resistance (>1,000-fold reduction in ETV susceptibility) (CitationTenney et al 2004). It is obvious that the pattern of ETV-resistance is more complicated than LAM or ADV resistance because 3 or 4 substitutions in the polymerase region are required. Moreover, LAM-resistance substitutions are necessary for the development of ETV resistant mutant. Until now three patterns of ETV resistance have been detected in LAM resistant strains: (1) substitutions rtI169T and rtM250V, (2) substitutions rtS184G and rtS202I and (3) substitutions rtS202G (CitationVillet et al 2005).
In vitro studies have shown that ETV-resistant strains have no effect on the susceptibility to ADV. In 2 patients with ETV-resistance, ADV was administered in a dose of 10 mg daily with marked reduction in the viral load (CitationTenney et al 2004). Thus, on clinical grounds ADV seems quite effective in patients with HBV mutants resistant to ETV and is probably the treatment of choice.
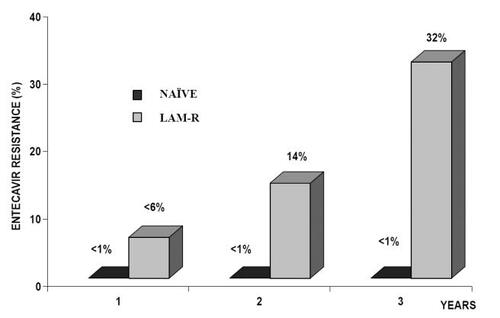
No evidence of resistance up to week 96 has been reported among ETV-treated nucleoside naïve patients (CitationGish et al 2005; CitationChang et al 2006a; CitationColonno et al 2006a; CitationLai et al 2006b; CitationShouval et al 2006). However, data from a recent study of 3 years of ETV treatment have shown that the previous concept of no-resistance in naive patients on ETV monotherapy is under question (CitationColonno et al 2006b). In this study, 3 HBeAg-positive patients experienced a virologic breakthrough with genotypically confirmed resistance in the third year of treatment. Although this data, obtained under a complex study design, are compatible with a rate of resistance lower than 1% at the third year of treatment, this has to be confirmed following a wide use of the drug in clinical practice (). Moreover, while ETV genotypic resistance in LAM resistant mutants has been reported to be very low (6%) during the first year of therapy increasing to approximately 14% at year two, new calculations on genotypic resistance have disclosed a cumulative rate of more than 30% at year 3 () (CitationColonno et al 2006b). Though a number of these patients may remain in low serum HBV-DNA levels, viral rebound increases from 1% at the first to 10% at the second and 25% at the third year of treatment. For the time being the rate of biochemical resistance remains unknown because the definition of biochemical breakthrough (ALT > 2x baseline or >10xULN) applied in this study (CitationColonno et al 2006b) actually refers to biochemical flares and not to biochemical breakthroughs which represent increase of normal ALT values to >1.25xULN.
Figure 3 The rate of Entecavir resistance in Naïve and Lamivudine-resistance chronic hepatitis B patients during 3 years of treatment. Colonno RJ. AASLD 2006. Abstract 110.
As in the case of ETV therapy, patients with LAM-resistant HBV mutants treated with Adefovir monotherapy are also at significantly higher risk to develop ADV resistant HBV mutants than LAM-naïve patients (CitationFung et al 2005, Citation2006; CitationVillet et al 2005; CitationYim et al 2006). On the other hand no patient with LAM-resistant strains treated by the combination of ADV with LAM for up to 4 years, has hitherto developed ADV resistant HBV mutants (CitationLampertico et al 2005; CitationRapti et al 2007). It is therefore reasonable to suggest that in patients with LAM-resistance HBV mutants sequential nucleos(t)ide monotherapy should be avoided and combination therapy be applied by 2 potent antiviral agents with different resistant profiles as is the case of combination of ETV with adefovir and preferable with tenofovir.
Conclusions
The high anti-HBV potency of entecavir, its impressive efficacy in terms of rapid HBV suppression to undetectability of HBV DNA by most sensitive PCR assays, combined with its high genetic barrier to HBV resistance, make ETV monotherapy a very attractive option as first line treatment in lamivudine-naïve CHB patients both HBeAg-positive and HBeAg-negative. However, the hitherto duration of ETV phase III trials is short, their extension design complex and appropriate long-term studies are needed before reaching definite conclusions on its very long-term safety and resistance.
In the setting of cirrhosis and liver transplantation, existing data are promising but limited.
In CHB patients with LAM-resistant HBV mutants, particularly those with advanced chronic liver disease, long term ETV monotherapy should be avoided, however this being true not only for ETV but for any sequential nucleos(t)ide analog monotherapy because of the increased risk for emergence of additional HBV resistance and multi-drug resistant HBV strains. In such setting the addition of adefovir or preferably tenofovir while continuing lamivudine, currently represents the treatment of choice but in the near future combination of ETV with ADV or tenofovir may turn out as the most preferable long term treatment strategy.
References
- BrunelleMNJacquardACPichoudCSusceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovirHepatology2005411391815915463
- ChangTTGishRGHadziyannisSJA dose-ranging study of the efficacy and tolerability of entecavir in lamivudine-refractory chronic hepatitis B patientsGastroenterology2005129119820916230074
- ChangTTGishRGDe ManRA comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis BN Engl J Med2006a35410011016525137
- ChangTTChaoYCKaymakoglouSEntecavir maintained virologic suppression through 3 years of treatment in antiviral-naïve HBeAg(+) patients (ETV 022/901)Hepatology2006b44229A
- ColonnoRJRoseREBaldickCJHigh barrier of resistance results in no emergence of entecavir resistance in nucleoside-naïve subjects during the first two years of therapyJ Hepatol2006a44S182
- ColonnoRJRoseREPokornowskiKAssessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naive patients while resistance emergence increases over time in lamivudine refractory patientsHepatology2006b44229A
- CooksleyHHouJLVitekLImpact of nucleoside treatment on antiviral T-cell reactivity in chronic hepatitis B: major differences depending on early viral suppression, HBeAg status and HBV genotypeHepatology200644547A
- De ManRALeoniekeMMWNevensFSafety and efficacy of oral entecavir given for 28 days in patients with chronic hepatitis B virus infectionHepatology2001345788211526545
- Di MarcoVMarzanoALamperticoPClinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to LamivudineHepatology2004408839115382125
- DienstangJLSchiffERWrightTLLamivudine as initial treatment for chronic hepatitis B in the United statesN Engl J Med199934112566310528035
- DienstagJLGoldiRDHeathcoteEJHistological outcome during long-term lamivudine therapyGastroenterology20031241051712512035
- FungSKAndreonePHanSHAdefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensationJ Hepatol2005439374316168522
- FungSKChaeHBFontanaRJVirological response and resistance to adefovir in patients with chronic hepatitis BJ Hepatol2006442839016338024
- GishRGChangTTDe ManREntecavir results in substantial virologic and biochemical improvement and HBeAg seroconvertion through 96 weeks of treatment in HBeAg(+) chronic hepatitis B patients (Study ETV-022)Hepatology200542267A268A
- HadziyannisSJBramouTMakrisAInterferon a-2b treatment of HBeAg negative/serum HBV-DNA positive chronic active hepatitis type BJ Hepatol199011Suppl 1S113362079567
- HadziyannisSJTassopoulosNCHeathcoteEJAdefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitisBN Engl J Med2003348800712606734
- HadziyannisSJTassopoulosNCHeathcoteEJLong-term therapy with Adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis BN Engl J Med2005a35226738115987916
- HadziyannisSJTassopoulosNCChangTTLong-term Adefovir dipivoxil treatment induces regression of liver fibrosis in patients with HBeAg-negative chronic hepatitis B: results after 5 years of therapyHepatology2005b42754A
- HadziyannisSJTassopoulosNCHeathcoteEJLong-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 yearsGastroenterology200613117435117087951
- LaiCLChienRNLeungNWYA one year trial of lamivudine for chronic hepatitis BN Engl J Med19983396189654535
- LaiCLRosmawatiMLaoJEntecavir is superior than lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infectionGastroenterology20021231831812454840
- LaiCLLimSGBrownNAA dose-finding study of once-daily oral Telbivudine in HBeAg-positive patients with chronic hepatitis B virus infectionHepatology2004407192615349912
- LaiCLLeungNTeoEKA 1-year trial of Telbivudine, Lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis BGastroenterology2005a1295283616083710
- LaiCLGaneELiawYFTelbivudine (LDT) vs. Lamivudine for chronic hepatitis B: first-year results from the international phase III globe trialHepatology2005b42748A
- LaiCLGaneEHsuCWTwo-years results from the globe trial in patients with hepatitis B: greated clinical and antiviral efficacy for telbivudine (LDT) vs lamivudineHepatology2006a44222A
- LaiCLShouvalDLokASEntecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis BN Engl J Med2006b35410112016525138
- LamperticoPViganoMManentiEFive years of sequential LAM to LAM +ADV therapy suppresses HBV replication in most HBeAg-negative cirrhotics, preventing decompensation but not hepatocellular carcinomaHepatology200542582A
- LamperticoPViganoMIavaroneMLow rates of genotypic resistance to adefovir in lamivudine resistant patients treated with adefovir-lamivudine combination therapy for 3 yearsHepatology200644556A
- LauGKKPiratvisuthTLuoKXPeginterferon alfa-2a, lamivudine and the combination for HBeAg-positive chronic hepatitis BN Engl J Med200535226829515987917
- LeeWMHepatitis B virus infectionN Engl J Med19973371733459392700
- LeungNWYLaiCLChangTTExtended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: Results after 3 years of therapyHepatology20013315273211391543
- LiawYFLeungNWYChangTTEffects of extended lamivudine therapy in Asian patients with chronic hepatitis BGastroenterology20001191728010889166
- LiawYFSungJJYChowWCLamivudine for patients with chronic hepatitis B and advanced liver diseaseN Engl J Med200435115213115470215
- LokASHeathcoteEJHoofnagleJHManagement of hepatitis B: 2000 summary of a workshopGastroenterology200112018285311375963
- LokASFLaiCLLeungNLong-term safety of Lamivudine treatment in patients with chronic hepatitis BGastroenterology200312517142214724824
- MaddreyWCHepatitis B: an important public health issueJ Med Virol200061362610861647
- ManesisEHadziyannisSInteferon α treatment and retreatment of hepatitis B e negative chronic hepatitis BGastroenterology2001321101911438498
- MarcellinPChangTTLimSGAdefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitisBN Engl J Med20033488081612606735
- MarcellinPLauGKKBoninoFPeginterferon alfa-2a alone, lamivudine alone and the two combination in patients with HBeAg-negative chronic hepatitis BN Engl J Med200435112061715371578
- MarcellinPChangTTLimSGIncreasing serologic, virologic and biochemical response over time to Adefovir dipivoxil (ADV) 10 mg in HBeAg+ chronic hepatitis B (CHB) patientsJ Hepatol2005422S312
- MarcellinPLauGKKFarciPThe majority of patients with HBeAg-negative chronic hepatitis B treated with Peginterferon alpha-2a (40KD) [PEGASYS] sustain responses 2 years post-treatmentJ Hepatol2006442S275
- NiederaouCHeintgesTLangeSLong term follow up of HBeAg positive patients treated with interferon alpha for chronic hepatitis BN Engl J Med1996334142278618580
- PapatheodoridisGVManesisEHadziyannisSJThe long-term outcome of interferon-a treated and untreated patients with HBeAg-negative chronic hepatitis BJ Hepatol2001343061311281561
- PapatheodoridisGVDimouELarasACourse of virological breakthroughs under long-term lamivudine in HBeAg negative precore mutant HBV liver diseaseHepatology2002362192612085368
- PapatheodoridisGVDimouEDimakopoulosKOutcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudineHepatology200542121915962291
- PetersMGHannHWMartinPAdefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitisBGastroenterology20041269110114699491
- RaptiINDimouEMitsoulaPHadziyannisSJCombination of adefovir dipivoxil with lamivudine vs adefovir alone in lamivudine-resistant HBeAg-negative chronic hepatitis B patientsHepatology2007453071317256746
- SchiffELeeWMChaoYCEfficacy and safety of entecavir (ETV) and lamivudine (LVD) in compensated, cirrhotic patients with chronic hepatitis BHepatology200542583A
- ShermanMYurdaydinCSollanoJEntecavir for the treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis BGastroenterology200613020394916762627
- ShouvalDHatzisGKitisGContinued virologic and biochemical improvement through 96 weeks of entecavir treatment in HBeAg(-) hepatitis B patients (Study ETV-027)J Hepatol200644S182
- TassopoulosNCVolpesRPastoreGEfficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis BHepatology1999298899610051494
- TenneyDJLevineSMRoseREClinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudineAntimicrob Agents Chemother200448349850715328117
- ThongsawatSLaiCLGaneGTelbivudine displays consistent antiviral efficacy across patient subgroups for the treatment of chronic hepatitis B: results from the globe studyJ Hepatol200644S4916360232
- VilletSPichoudCOllivetASequential antiviral therapy leads to the emergence of multiple drug resistant hepatitis B virusHepatology200542581A
- WongDKHCheungAMO’RourkeKEffect of Alpha-Interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B: a meta-analysisAnn Intern Med1993119312238328741
- YimHJHussainMLiuYEvolution of multi-drug resistant hepatitis B virus during sequential therapyHepatology2006447031216941700
- YurdaydinCSollanoJHadziyannisSJEntecavir results in continued virologic and biochemical improvement and HBeAg seroconversion through 96 weeks of treatment in lamivudine-refractory HBeAg (+) chronic hepatitis B patients (ETV-026)J Hepatol200644S36