Abstract
The topoisomerase I inhibitor topotecan is the only single-agent therapy approved for the treatment of recurrent small cell lung cancer (SCLC). Many patients with recurrent SCLC may be predisposed to treatment-related adverse events because of the presence of comorbidities such as advanced age, renal impairment, or extensive prior therapy. In this setting, disease stabilization is considered a treatment benefit, and quality-of-life effects and toxicity profiles of treatments should be considered. The approved regimen of topotecan 1.5 mg/m2 on days 1 to 5 of a 21-day cycle is active and has generally mild nonhematologic toxicity and a well-established hematologic toxicity profile characterized by reversible, manageable, and noncumulative neutropenia. Alternative dosing and treatment schedules may lower the incidence of hematologic toxicities while maintaining antitumor efficacy in this patient population. Therefore, topotecan may provide physicians with an effective and versatile therapeutic option for the treatment of patients with relapsed SCLC.
Introduction
Lung cancer is the most common cancer worldwide, with an estimated 174,470 new cases diagnosed and 162,460 deaths in the United States in 2006 alone (CitationJemal et al 2006). Non-small cell lung cancer is the leading cause of cancer-related death in both men and women and frequently presents as advanced disease (CitationHo et al 2006). Consequently, most patients with non-small cell lung cancer are treated with palliative therapy (CitationHo et al 2006). Docetaxel, pemetrexed, and erlotinib are approved for second-line use, and oral topotecan, polyglutamated paclitaxel, and gefitinib have been evaluated in phase III trials (CitationHo et al 2006). Small cell lung cancer (SCLC), which accounts for approximately 18% of all lung cancers in the United States, is typically an aggressive disease with a high incidence of early and distant metastases (CitationArdizzoni 2004). Most patients with SCLC are not diagnosed until their cancer is in an advanced stage, and the majority of cases are unresectable. Consequently, first-line therapies are typically aggressive, especially for limited-stage disease. Although several agents are active against SCLC and initial response rates to standard first-line therapies are as high as 90% in patients with limited-stage disease, most patients with SCLC experience disease relapse (CitationOkuno and Jett 2002). The 5-year disease-free survival rate is 5% to 10% in this patient population, and relapsed SCLC is considered a chronic disease state by some physicians in the field (CitationArdizzoni 2004). As such, symptom palliation is an important endpoint for patients with extensive-stage disease, and stable disease may be considered a treatment benefit. Therefore, effective and well-tolerated agents that differ from those of therapeutic regimens used in the first-line setting are required.
Management of relapsed SCLC can be challenging for many reasons. Approximately 70% of patients diagnosed with lung cancer in the United States are >65 years old and have comorbidities (eg, impaired pulmonary, hepatic, or renal function or poor performance status) that may limit the use of aggressive therapies such as platinum-based combination regimens (CitationHurria and Kris 2003; CitationArdizzoni 2004; CitationGarst et al 2005). Moreover, administration of many first-line therapies results in cumulative toxicities, which may reduce the patient’s ability to tolerate subsequent lines of therapy. Therefore, patients with relapsed SCLC may require dose delays, dose reductions, and hematopoietic growth factor support during therapy. Currently, second-line therapies for SCLC include cyclophosphamide, doxorubicin, and vincristine (CAV); single-agent topotecan; and single-agent etoposide (CitationArdizzoni 2004; CitationCiombor and Rocha Lima 2006). Although combination therapies may improve the overall response rate (ORR) compared with single-agent therapies, the duration of response remains limited, and the utility of combination regimens may also be limited because of the potential for overlapping or cumulative toxicities (CitationArdizzoni 2004).
Single-agent therapies are therefore an attractive option for the treatment of relapsed SCLC. Topotecan (Hycamtin®; GlaxoSmithKline, Research Triangle Park, North Carolina, USA) is the only approved single-agent therapy for relapsed SCLC and has demonstrated significant pain palliation compared with CAV in this setting (Citationvon Pawel et al 1999; CitationHycamtin 2006). In patients with relapsed SCLC, topotecan produced ORRs of 14% to 38% in platinum-sensitive SCLC and 2% to 11% in platinum-resistant SCLC, with an additional 17% to 20% of patients achieving stable disease (CitationPerez-Soler et al 1996; CitationArdizzoni et al 1997; Citationvon Pawel et al 1999). Moreover, topotecan is associated with generally mild nonhematologic toxicities and manageable, noncumulative, reversible neutropenia (CitationHycamtin 2006). Therefore, the efficacy and tolerability of topotecan provide physicians with a viable therapeutic option in the treatment of relapsed SCLC.
Safety profile of topotecan
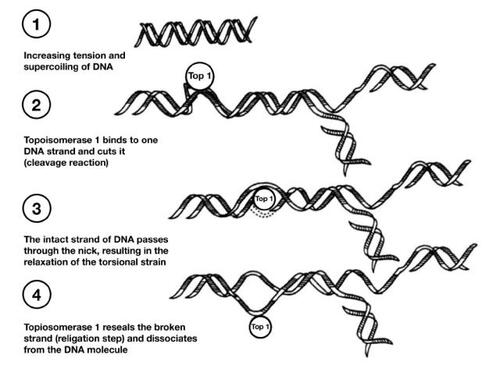
Topotecan, a topoisomerase I inhibitor with broad antitumor activity, is currently approved in more than 70 countries for the second-line treatment of metastatic ovarian cancer and in more than 30 countries for the treatment of platinum-sensitive relapsed SCLC (CitationArmstrong et al 2005). This agent was also recently approved in the United States for the treatment of stage IVB recurrent or persistent cervical cancer not amenable to curative procedures (CitationHycamtin 2006). Topotecan binds to topoisomerase I and forms a complex on the DNA, thereby leading to double-stranded DNA breaks during DNA replication and, ultimately, cell death () (CitationRothenberg 1997). This unique mechanism of action makes topotecan a valuable tool in many treatment regimens. Of note, topotecan is the only approved single-agent regimen that has demonstrated symptom palliation in patients with SCLC, improving dyspnea, anorexia, hoarseness, fatigue, and interference with daily activities (Citationvon Pawel et al 1999).
Figure 1 Mechanism of action of topotecan. Copyright © 1997. Reprinted with permission from CitationRothenberg ML. 1997. Topoisomerase I inhibitors: review and update. Ann Oncol, 8:837–55
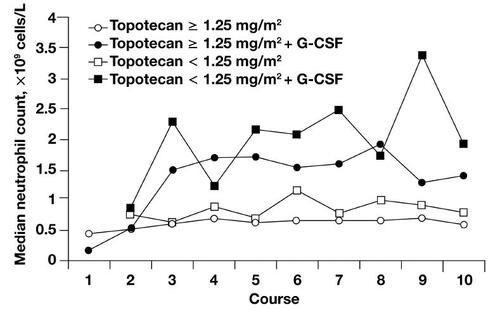
Topotecan is generally well tolerated, with nonhematologic toxicities that include nausea, vomiting, and dyspnea and are generally mild to moderate in severity (CitationTreat et al 2004). The hematologic profile includes dose-limiting neutropenia that is noncumulative, reversible, and generally manageable with dose reductions, treatment delays, and growth factor support () (CitationArmstrong et al 2005). Other grade 3/4 hematologic toxicities include thrombocytopenia and anemia (CitationArmstrong et al 2005).
Figure 2 Median neutrophil nadirs by treatment course, dose, and granulocyte-colony stimulating factor (G-CSF). After the first course, patients may have received G-CSF or reduced doses of topotecan; some patients may have discontinued topotecan because of myelosuppression. Copyright © 2005. Reprinted with permission from CitationArmstrong DK, Spriggs D, Levin J, et al. 2005. Hematologic safety and tolerability of topotecan in recurrent ovarian cancer and small cell lung cancer: an integrated analysis. Oncologist, 10:686–94
Topotecan is approved for use in patients with SCLC who have adequate bone marrow reserves (baseline neutrophil count of >1.5 × 109/L and platelet count of >100 × 109/L) (CitationHycamtin 2006). Recovery of cell counts after treatment-induced suppression may be achieved with growth factor support, such as granulocyte colony-stimulating factor (G-CSF), or transfusion (CitationArmstrong and O’Reilly 1998; CitationHycamtin 2006). If treatment is delayed because of prolonged myelosuppression, dose reductions in 0.25 mg/m2 increments can be considered (CitationArmstrong and O’Reilly 1998).
A recent integrated analysis of topotecan for the treatment of SCLC and ovarian cancer identified several risk factors for hematologic toxicity during the first course of treatment, including extensive prior chemotherapy, advanced age, and impaired creatinine clearance (CitationArmstrong et al 2005). Previous therapies and advanced age may affect bone marrow reserves, and impaired renal function may result in lower clearance rates of topotecan, thereby prolonging exposure. Furthermore, many patients with SCLC have prior exposure to agents that have cumulative myelotoxicity and nephrotoxicity (eg, platinum-based regimens) as first-line treatments, and the initiation of topotecan treatment at a reduced starting dose may be warranted in these patients.
Standard dosing regimen
Topotecan 1.5 mg/m2 on days 1 to 5 of a 21-day cycle is an approved treatment regimen for recurrent SCLC (CitationHycamtin 2006). At this dose, topotecan has demonstrated antitumor activity in both chemosensitive (defined in phase III studies as disease responding to chemotherapy but subsequently progressing = 60-days after chemotherapy and in phase II studies as disease responding to chemotherapy but subsequently progressing = 90-days after chemotherapy) and chemoresistant SCLC (CitationHycamtin 2006).
In a pivotal, multicenter, phase III trial in patients with recurrent SCLC, patients were randomized to receive topotecan or CAV combination therapy (Citationvon Pawel et al 1999). Although CAV is not a standard second-line therapy for SCLC, its toxicity and efficacy profile is well established, and the therapy has been widely used. In this trial, a total of 446 courses of topotecan and 359 courses of CAV were administered to 107 and 104 patients, respectively (Citationvon Pawel et al 1999). The ORR (topotecan, 24%; CAV, 18%) and median overall survival (mOS; topotecan, 25.0-weeks; CAV, 24.7-weeks) were similar in patients treated with topotecan compared with patients treated with CAV (Citationvon Pawel et al 1999). Moreover, the median time to response (topotecan, 6.0-weeks; CAV, 6.1-weeks), time to progression (topotecan, 13.3-weeks; CAV, 12.3-weeks), and response duration (topotecan, 14.4-weeks; CAV, 15.3-weeks) were similar between groups (Citationvon Pawel et al 1999). Furthermore, the toxicity profile was similar for both arms. Hematologic toxicity was the most common toxicity in both arms, and reports of high-grade neutropenia were comparable between groups (topotecan, 70%; CAV, 72%). Grade 4 thrombocytopenia (topotecan, 29%; CAV, 5%) and grade 3/4 anemia (topotecan, 41%; CAV, 19%) were more frequently reported in patients treated with topotecan than in patients treated with CAV (Citationvon Pawel et al 1999). Notably, greater symptom palliation was observed in patients treated with topotecan than in those treated with CAV for dyspnea (p = 0.002), anorexia (p = 0.042), hoarseness (p = 0.043), fatigue (p = 0.032), and interference with daily activities (p = 0.023; Citationvon Pawel et al 1999). Therefore, topotecan and CAV had similar efficacy and tolerability, but topotecan was superior to CAV in providing symptom palliation, an important consideration for maintaining quality of life (QOL) throughout the continuum of care.
Recently, the efficacy and safety of topotecan (1.5 mg/m2 on days 1 to 5 of a 21-day cycle) were retrospectively analyzed using patient data from 5 large phase II and III trials of relapsed SCLC () (CitationEckardt et al 1996; CitationArdizzoni et al 1997; CitationDepierre et al 1997; Citationvon Pawel et al 1999; Citationvon Pawel et al 2001; CitationTreat et al 2004). The ORR was 15%, although patients with chemosensitive disease had a higher ORR than patients with chemoresistant/chemorefractory disease (19% versus 4%, respectively) () (CitationTreat et al 2004). Moreover, stable disease, which is also considered a benefit of treatment in this patient population, was experienced by 20% of patients (22% of patients with chemosensitive disease and 16% of patients with chemoresistant disease) (CitationTreat et al 2004). Neutropenia and leukopenia, though transient, were the most commonly reported grade 3/4 hematologic toxicities, experienced by 90% and 82% of patients, respectively (CitationTreat et al 2004). Transient grade 3/4 thrombocytopenia and anemia were experienced by 55% and 33% of patients, respectively. Nonhematologic toxicities were generally mild to moderate and included dyspnea (12%) and asthenia (8%). Similar to the pivotal phase III trial (Citationvon Pawel et al 1999), this study found that, in the 3 trials that evaluated symptom palliation, topotecan treatment was associated with palliation of a variety of symptoms including dyspnea, cough, chest pain, anorexia, insomnia, and interference with daily activities () (CitationTreat et al 2004).
Table 1 Topotecan SCLC trials included in an integrated analysis
Table 2 Antitumor response in the intent-to-treat population in a pooled analysis of patients with SCLC
Table 3 Proportion of patients with SCLC treated with topotecan who had symptom improvement
Although the standard 5-day topotecan regimen is generally active and well tolerated, heavily pretreated patients and patients with comorbidities may be predisposed to adverse events (CitationArmstrong et al 2005). Patients with advanced SCLC generally have a poor prognosis and may benefit from regimens that are more convenient and have improved tolerability compared with the standard regimen. Patients may have QOL benefits even in the absence of objective responses if disease stabilization and symptom palliation occur. Accordingly, investigations have been undertaken to assess the value of alternative regimens and dosing strategies of topotecan.
Alternative dosing regimens
Multiple treatment regimens and dosing strategies of topotecan have been investigated to optimize tolerability and convenience while maintaining antitumor efficacy. A summary of topotecan treatment regimens and associated responses is presented in and (CitationPerez-Soler et al 1996; Citationvon Pawel et al 1999; CitationKoschel et al 2000; CitationOgawara et al 2000; CitationArdizzoni et al 2003; CitationTakeda et al 2003; CitationTreat et al 2004; CitationChristodoulou et al 2006; CitationMurphy et al 2006; CitationShipley et al 2006).
Figure 3 Overall response rates (ORRs) and stable disease (SD) responses to various topotecan treatment regimens.
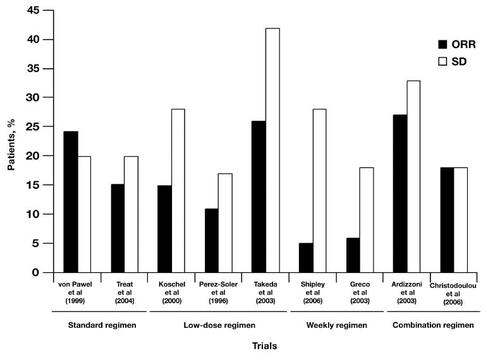
Table 4 Efficacy and safety of various topotecan treatment regimens
Low-dose topotecan
Reductions in the standard topotecan dose may improve hematologic toxicities and the need for treatment delays in patients with risk factors for myelosuppression (eg, advanced age, extensive pretreatment, prior platinum therapy or radiation therapy, and renal impairment) (CitationArmstrong et al 2005). For example, in a phase II, single-arm, multicenter trial of low-dose topotecan (1.25 mg/m2 on days 1 to 5 of a 21-day cycle) in patients with SCLC (N = 171), the reported ORR (15%) and median overall survival ([mOS] 22.4-weeks) were similar to results reported in studies using the standard regimen (CitationKoschel et al 2000). Importantly, the rate of myelosuppression was substantially reduced compared with myelosuppression rates reported in studies using the standard regimen, with grade 3/4 neutropenia, thrombocytopenia, and anemia reported in 10%, 5%, and 1% of cycles, respectively (CitationKoschel et al 2000). Furthermore, no high-grade nonhematologic toxicities were reported. Similar ORRs (11%) were reported with this regimen in a trial of topotecan in patients with etoposide- and cisplatin-refractory SCLC (N = 28) (CitationPerez-Soler et al 1996). Moreover, 17% of patients achieved stable disease, and the mOS was 20-weeks (CitationPerez-Soler et al 1996). A higher incidence of grade 3/4 myelosuppression was reported in this trial than that reported in the Koschel et al study (2005), with grade 3/4 granulocytopenia and thrombocytopenia occurring after 70% and 31% of cycles, respectively (CitationPerez-Soler et al 1996). This increased incidence of high-grade hematologic toxicity compared with the toxicity associated with other low-dose regimens may be related to previous cisplatin therapy, a known risk factor for myelosuppression. Even with the high incidence of myelosuppression in this trial, there were no withdrawals because of drug-related toxicity, and toxicity was reversible, with recovery from thrombocytopenia occurring within 21-days in most cases (CitationPerez-Soler et al 1996). No grade 3/4 nonhematologic toxicities were reported (CitationPerez-Soler et al 1996).
Topotecan doses as low as 1.0 mg/m2 have demonstrated efficacy in the treatment of recurrent SCLC. For example, treatment of patients with advanced SCLC with low-dose topotecan (1.0 mg/m2 on days 1 to 5 of a 21-day cycle) was reported to produce response rates comparable with those that have been reported for standard therapy (26%) (CitationOgawara et al 2000). Moreover, the mOS (35.1-weeks) and the median 1-year survival rate (32%) were consistent with those seen in trials of other approved regimens in this setting (CitationOgawara et al 2000). Notably, no grade 4 nonhematologic toxicities were observed (CitationOgawara et al 2000). In a similar phase II trial of topotecan (1.0 mg/m2 on days 1 to 5 of a 21-day cycle) in patients with relapsed SCLC (N = 50), the ORR was 26% and the mOS was 262-days (37.4-weeks) (CitationTakeda et al 2003). Although myelosuppression (leukopenia, neutropenia, anemia, and thrombocytopenia) was the most commonly reported toxicity, it was generally manageable and there were few associated complications (CitationTakeda et al 2003).
The administration of topotecan at the low doses of 1.0 mg/m2 and 1.25 mg/m2 results in efficacy comparable with that of the standard dosing regimen and a lower incidence of treatment-related adverse events. Therefore, low-dose topotecan therapy may be appropriate for patients who exhibit risk factors for treatment-related myelosuppression such as advanced age, extensive pretreatment, or renal impairment.
Weekly topotecan
Weekly schedules of topotecan have also been investigated with the intention of minimizing myelosuppression, dose reductions, and treatment delays associated with the standard 5-day regimen. Moreover, a weekly dosing regimen may provide a greater convenience to patients receiving topotecan in combination with other agents administered on a weekly basis, such as gemcitabine. This regimen is generally active and well tolerated in patients with recurrent SCLC, and only moderate myelosuppression is typically observed in response to the weekly administration of topotecan.
Weekly topotecan (4 mg/m2 for 12 consecutive weeks) was associated with comparable activity and a lower incidence of myelosuppression compared with published results of the standard 5-day regimen (CitationShipley et al 2006). In patients with either chemosensitive or chemoresistant SCLC (N = 81), the ORR was 5%, and stable disease was experienced by 28% of patients (CitationShipley et al 2006). Patients with chemosensitive SCLC were approximately twice as likely to respond to this weekly topotecan regimen versus patients with chemoresistant disease (6% versus 3%, respectively); however, the mOS (4.5-months [19.4-weeks]) was comparable between treatment groups (chemosensitive, 5.6-months [24.1-weeks]; chemoresistant, 3.2-months [13.8-weeks]; p = 0.05) (CitationShipley et al 2006). Grade 3/4 neutropenia or thrombocytopenia was observed in 17 (21%) and 22 (27%) patients, respectively (CitationShipley et al 2006).
Results of a phase II study of weekly topotecan (4 mg/m2 for 12-weeks) also suggested that this regimen is active and well tolerated in patients with untreated extensive SCLC who were elderly with poor performance status or severe coexistent medical illness (N = 39) (CitationMurphy et al 2006). Of 31 evaluable patients, 4 (13%) patients experienced a partial response and 20 (65%) patients experienced stable disease. Grade 3/4 thrombocytopenia occurred in 3 (10%) patients, and grade 3/4 neutropenia occurred in 11 (35%) patients (CitationMurphy et al 2006).
Combination regimens
The goal of combination therapy in the treatment of SCLC is to promote synergistic antitumor activity in an attempt to yield a higher ORR and an improved duration of response compared with the results obtained with monotherapy. However, combination therapies may be associated with increased incidence of overlapping toxicities, and it is unclear whether these regimens are more effective than single-agent therapies in the treatment of recurrent SCLC. Multiple clinical trials with a number of investigational drug combinations and schedules have been undertaken to determine the benefit of topotecan combination therapy in the treatment of recurrent SCLC.
A phase II trial of second-line topotecan (0.75 mg/m2 on days 1 to 5 of a 21-day cycle) in combination with cisplatin (60 mg/m2 on day 1) was conducted in patients with either refractory or sensitive SCLC (N = 110) (CitationArdizzoni et al 2003). The ORR was 27% (chemosensitive SCLC, 29%; chemorefractory SCLC, 24%) (CitationArdizzoni et al 2003). Despite differences in response rates, mOS was similar between patients with chemosensitive SCLC and patients with chemorefractory SCLC (chemosensitive, 6.4-months [27.5 -weeks]; chemorefractory SCLC, 6.1-months [26.2-weeks]) (CitationArdizzoni et al 2003). The most prevalent and severe toxicity was myelosuppression, with grade 4 neutropenia occurring in 62% of patients with chemosensitive SCLC and 49% of patients with chemorefractory SCLC (CitationArdizzoni et al 2003). Nonhematologic toxicities were generally mild, with grade 3/4 nausea, vomiting, and diarrhea occurring in ≤10% of all patients (CitationArdizzoni et al 2003). The most commonly reported nonhematologic adverse event was fatigue/malaise, which occurred in 52% of chemosensitive patients and 68% of chemorefractory patients (CitationArdizzoni et al 2003). Therefore, based on this phase II trial, topotecan and cisplatin combination therapy is active and tolerable in the second-line treatment of SCLC (CitationArdizzoni et al 2003).
Combination therapies with alternative topotecan dosing schedules have also been investigated for the treatment of SCLC. In a phase II trial of topotecan (0.9 mg/m2) and cisplatin (20 mg/m2) administration on days 1 to 3 every 3-weeks in patients with recurrent SCLC (N = 34), the ORR was 18%, with chemosensitive patients experiencing a greater overall response (24%) compared with chemorefractory patients (8%; CitationChristodoulou et al 2006). The mOS and time to progression for the entire patient population were 6.5-months (28.0-weeks) and 4.4-months (18.9-weeks), respectively, although patients with chemosensitive disease had a longer mOS (chemosensitive SCLC, 7.8-months [33.5-weeks]; chemorefractory SCLC, 6.2-months [26.7-weeks]) and time to progression (chemosensitive SCLC, 5.9-months [25.4-weeks]; chemorefractory SCLC, 3.2-months [13.8-weeks]) than patients with chemorefractory disease (CitationChristodoulou et al 2006). This regimen was well tolerated, and grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 42%, 15%, and 15% of patients, respectively (CitationChristodoulou et al 2006). Alopecia was the most common nonhematologic toxicity, occurring in 6% of patients (CitationChristodoulou et al 2006). This 3-day schedule of topotecan and cisplatin provides patients with SCLC that is either chemosensitive or chemorefractory with a tolerable, efficacious, and potentially more convenient treatment regimen.
The administration of weekly topotecan has also been combined with a number of chemotherapeutic agents including taxanes, temozolomide, and platinum agents. For example, a phase II trial of weekly topotecan (1.75 mg/m2) plus paclitaxel (70 mg/m2) was conducted in patients with recurrent SCLC (N = 41) (CitationStathopoulos et al 2006). The ORR of 27% was similar to that reported for single-agent topotecan therapy (CitationStathopoulos et al 2006). The median duration of response and mOS were 4.0-months (17.2-weeks) and 7.0-months (30.1-weeks), respectively (CitationStathopoulos et al 2006). Myelosuppression was the most commonly reported adverse event, with grade 3/4 neutropenia occurring in 27% of patients (CitationStathopoulos et al 2006).
Although topotecan combination therapies appear to be active in recurrent SCLC, response rates are similar to those reported in response to topotecan monotherapy. The role of topotecan combination therapies for the treatment of patients with SCLC remains to be determined.
Discussion
Small cell lung cancer is an aggressive malignancy that has a high response rate to standard first-line therapies but typically relapses. Recurrent SCLC has a poor prognosis and is generally poorly responsive to therapies. Moreover, patients with recurrent SCLC often have comorbidities. In this setting, disease stabilization is considered a treatment benefit, and symptom palliation is an important goal. Antitumor activity must be balanced with tolerability to maximize QOL. Patients with renal impairment and depletion of bone marrow reserves from prior chemotherapy exposure may be predisposed to adverse events. Topotecan provides an attractive therapeutic option for the treatment of SCLC in this setting because it is active and well tolerated, is not associated with cumulative toxicity, and offers flexible dosing and treatment schedules to accommodate patients with comorbidities or concurrent therapy. The reversible and noncumulative hematologic toxicities and mild to moderate nonhematologic toxicities may help to preserve a patient’s QOL during long-term treatment.
Alternative dosing regimens and treatment schedules have demonstrated promising antitumor activity and may provide improved tolerability and patient convenience benefits compared with the standard 5-day regimen. These alternative regimens may be especially beneficial for patients at high risk for myelosuppression, including patients who have impaired renal function, advanced age, or a history of multiple rounds of chemotherapy. Although topotecan-containing combination therapies have not yet demonstrated greater efficacy compared with monotherapy in the treatment of relapsed SCLC, trials are ongoing to optimize combinations and treatment schedules.
In conclusion, topotecan provides physicians with an effective and flexible therapeutic option for the treatment of recurrent SCLC. Further studies to investigate optimal topotecan treatment strategies are warranted and should focus on improving survival and preserving QOL while maintaining efficacy. These issues are important to patients undergoing long-term treatment for SCLC, especially those who may not be able to tolerate aggressive therapeutic regimens.
Future directions
Preliminary studies are underway to investigate alternative treatment strategies that may further enhance a patient’s response to topotecan therapy. One such approach involves administering an induction dose of topotecan to sensitize tumors to subsequent standard therapies. The activity of topotecan in SCLC makes it well suited for use as induction therapy. Moreover, its unique mechanism of action decreases the likelihood of cross-resistance to other therapies. This induction-based approach has previously displayed favorable activity when cyclophosphamide, epiadriamycin, and vincristine were used for induction therapy before platinum-based chemotherapy and concurrent radiotherapy in patients with limited SCLC in a phase II trial (CitationMaranzano et al 2002). Moreover, in a phase I trial of chemotherapy-naive patients with SCLC, induction therapy with topotecan was well tolerated and produced partial responses both in patients with limited-stage disease and in patients with extensive-stage disease (CitationGarst et al 2006). Consistent with the tolerability profile of single-agent topotecan, the dose-limiting toxicity in this regimen was myelosuppression (CitationGarst et al 2006). The maximum tolerated dose of topotecan was 2.25 mg/m2 on days 1 to 3 of a 2-week cycle with G-CSF support (CitationGarst et al 2006). Nonhematologic toxicities were generally not severe and included anorexia, nausea, and 1 renal adverse event (CitationGarst et al 2006).
Individual dose adjustments are also under investigation to reduce toxicities for each patient receiving treatment (CitationHuber et al 2006). This type of personalized approach to patient therapy should allow physicians to determine the maximum tolerated dose for each patient while minimizing toxicity and maintaining efficacy. Preliminary results using this individualized approach have been positive in patients with SCLC (CitationHuber et al 2006).
References
- ArdizzoniATopotecan in the treatment of recurrent small cell lung cancer: an updateOncologist20049Suppl 641315616145
- ArdizzoniAHansenHDombernowskyPTopotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative GroupJ Clin Oncol199715209069164222
- ArdizzoniAManegoldCDebruyneCEuropean organization for research and treatment of cancer (EORTC) 08957 phase II study of topotecan in combination with cisplatin as second-line treatment of refractory and sensitive small cell lung cancerClin Cancer Res200391435012538462
- ArmstrongDO’ReillySClinical Guidelines for Managing Topotecan-Related Hematologic ToxicityOncologist1998341010388079
- ArmstrongDKSpriggsDLevinJHematologic safety and tolerability of topotecan in recurrent ovarian cancer and small cell lung cancer: an integrated analysisOncologist2005106869416249347
- ChristodoulouCKalofonosHPBriasoulisECombination of topotecan and cisplatin in relapsed patients with small cell lung cancer: a phase II study of the hellenic cooperative oncology group (HeCOG)Cancer Chemother Pharmacol2006572071216028103
- CiomborKKRocha LimaCMManagement of small cell lung cancerCurr Treat Options Oncol20067596816343369
- DepierreAvon PawelJHansKEvaluation of topotecan (HycamtinTM) in relapsed small cell lung cancer (SCLC). A multicentre phase II studyLung Cancer199718Suppl 1359268946
- EckardtJGrallaRPalmerMCTopotecan (T) as second-line therapy in patients (Pts) with small cell lung cancer (SCLC): a phase II studyAnn Oncol19967Suppl 5107
- GarstJBullerRLaneSTopotecan in the treatment of elderly patients with relapsed small-cell lung cancerClin Lung Cancer20057190616354314
- GarstJHerndonJEShafmanTA phase I study of dose-dense topotecan given upfront to standard therapy in patients with small cell lung cancerClin Druv Invest20062625766
- HoCDaviewAMLaraPNSecond-line treatment for advanced-stage non-small cell lung cancer: current and future optionsClin Lung Cancer20067118s125s
- HuberRMReckMGosseHEfficacy of a toxicity-adjusted topotecan therapy in recurrent small-cell lung cancerEur Respir J2006271183916481389
- HurriaAKrisMGManagement of lung cancer in older adultsCA Cancer J Clin2003533254115224973
- Hycamtin® (topotecan hydrochloride) for injection [prescribing information]2006Research Triangle Park, NCGlaxoSmithKline
- JemalASiegelRWardECancer statisticsCA Cancer J Clin2006561063016514137
- KoschelRHuberRMGatzemeierUTopotecan in second-line treatment of small cell lung cancer reduced toxicity with individualized therapyLung Cancer20002042
- MaranzanoECrinoLPiroFLong-term results of induction chemotherapy followed by concurrent chemotherapy and thoracic irradiation in limited small cell lung cancerLung Cancer200237798512057871
- MurhpyPBHainsworthJDSpigelDRTopotecan–single agent activity in a weekly intravenous (IV) schedule for first-line therapy in poor prognosis extensive stage small cell lung cancer (SCLC): a Minnie Pearl Cancer Research Network phase II trial [abstract]Proc Am Soc Clin Oncol20062418s Abstract 17000
- OgawaraMNegoroSNishiwakiYPhase II study of topotecan (TOP) for relapsed small cell lung cancer (SCLC)Lung Cancer200029Suppl 140
- OkunoSHJettJRSmall cell lung cancer: current therapy and promising new regimensOncologist20027234812065796
- Perez-SolerRGlissonBSLeeJSTreatment of patients with small-cell lung cancer refractory to etoposide and cisplatin with the topoisomerase I poison topotecanJ Clin Oncol1996142785908874340
- RothenbergMLTopoisomerase I inhibitors: review and updateAnn Oncol19978837559358934
- ShipleyDLHainsworthJDSpigelDRTopotecan: weekly intravenous (IV) schedule similar to standard 5-day IV schedule as second-line therapy for relapsed small cell lung cancer (SCLC) – a Minnie Pearl Cancer Research Network phase II trial [abstract]J Clin Oncol200624384s Abstract 7083
- StathopoulosGPChristodoulouCStathopoulosJSecond-line chemotherapy in small cell lung cancer in a modified administration of topotecan combined with paclitaxel: a phase II studyCancer Chemother Pharmacol20065779680016142488
- TakedaKNegoroSSawaTA phase II study of topotecan in patients with relapsed small-cell lung cancerClin Lung Cancer20034224814624710
- TreatJHuangCHLaneSRTopotecan in the treatment of relapsed small cell lung cancer patients with poor performance statusOncologist200491738115047921
- von PawelJGatzemeierUPujolJLPhase II comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancerJ Clin Oncol2001191743911251005
- von PawelJSchillerJHShepherdFATopotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancerJ Clin Oncol1999176586710080612