Abstract
Objective:
To investigate the possible role and tolerability of high-dose (>160 mg/day) oxycodone controlled release (CR) for the treatment of cancer and non-cancer pain.
Design:
227 patients with cancer or non-cancer pain were enrolled in an open-label, multi-center, Italian study in order to assess the adequacy of their existing pain management (using a numerical rating scale [NRS]) and the possible benefit high-dose oxycodone CR may offer patients experiencing uncontrolled pain.
Results:
Pain was poorly controlled at baseline, with only 18.1% of patients reporting adequate pain relief (NRS <3.5). All other patients reported uncontrolled pain, with an average NRS of 7.81. At baseline assessment, 47.89% of patients had been in pain for up to 3 months, 32.82% for 3–6 months, and 19.19% for more than 6 months. After baseline assessment, patients were switched to oxycodone CR monotherapy. The starting dose was individualized to each patient and titrated up over a 3- to 4-day period until effective pain management was achieved. Treatment was continued for an average of 37.24 days during the study. Pain control (final mean NRS of 2.85) was attained with an average dose of oxycodone CR 221.84 mg/day. Standard adverse events (including constipations, nausea, and vomiting) were recorded in 39.64% of patients receiving high-dose oxycodone CR monotherapy. Side-effects tended to subside after the initial week of treatment and did not result in any participants leaving the study.
Conclusion:
High-dose oxycodone CR can achieve rapid and effective management of moderate to severe cancer and non-cancer pain with minimum side-effects.
Introduction
Living with pain is a reality that affects millions of people worldwide. Cancer pain alone is a significant issue, affecting an estimated 17 million people every year (CitationGoudas et al 2005). Evidence suggests that around 70% of patients living with cancer have to endure chronic pain: 70% of which is caused by the disease, 20% by therapy, and 10% by other factors (CitationItalian Department of Health 2007). Non-cancer-related pain also affects a significant number of people worldwide (CitationFurlan et al 2006) and is thought to affect over a third of the population in developed countries. Data from the International Association for the Study of Pain (IASP) indicate that chronic pain is more common among women, affecting 39.6% of women compared with a prevalence of 31.0% among men (CitationYang et al 2007).
It has been shown on a pan-European level that, in addition to its impact on the quality of life (QoL) of affected individuals and their families, chronic pain also has a significant social and economic burden. Persistent pain can affect a patient’s ability to perform simple tasks which, combined with a lack of availability of treatments and effective therapies, can undermine their ability to work. This is not only valid at a European level, but also holds true on a national scale. In Italy, 83.2% of cancer patients were found to be in pain as a result of their condition, and, although 97% of them were receiving therapy, the treatment was not appropriately tailored to relieve the intensity of pain experienced (CitationCostantini et al 2005).
Pain is often the main presenting symptom when patients visit their primary care physician. The location and nature of the pain can inform doctors on the cause and possible pathology of a patient’s condition. In Italy, however, diagnosis and subsequent treatment choice are often inadequate, with a significant 42% of cancer patients describing their pain as ‘intolerable’ despite treatment (CitationItalian Medical Oncology Association 2007).
The European Pain in Cancer (EPIC) Survey findings provided further insight into the nature and prevalence of cancer pain at presentation (CitationEuropean Pain in Cancer Survey 2007). The survey encompassed 12 European countries and involved a total of 4824 patients, of whom 457 were Italian. Across Europe 73% of patients reported pain associated with their cancer, with approximately one in three (33%) identifying it as the main symptom of their condition. This number was higher for Italy, where almost all Italian participants (95%) reported pain associated with their cancer, and 42% identified pain as their key symptom.
More than half (56%) of patients reported pain at the time of questioning, while 96% of patients in the survey experienced pain at least once a month, and 75% weekly. At the time of the survey, nearly 47% of patients had been suffering pain for at least 3 months, and 6% reported having been in constant pain for more than a year.
Patients were asked to indicate the severity of their pain on a numerical rating scale (NRS) that ran from 1 to 10, with 10 indicating the worst pain possible. More than 96% of patients classified their pain as moderate-to-severe, with an NRS of >5. Although 98% of patients involved in the study had received some form of pain treatment, only 24% of pan-European participants considered their treatment effective. This number was even lower for the Italian participants, among whom only 16% felt their treatment was effective. A significant 70% of patients involved in the study reported a belief that effective pharmacological pain control was not possible (CitationEuropean Pain in Cancer Survey 2007).
Poor pain control, as recorded in the study, contributes to the psychosocial and physical effects associated with chronic pain. In addition to the physical sensation of pain, patients can also experience sleep disturbance, fear, anxiety, depression, weight loss, reduced physical activity, social isolation, and problems with social interaction that often result in problems finding and maintaining employment. Chronic pain’s high prevalence and debilitating nature result in a significant combined economic and social burden.
The impact of chronic pain on the patient, society, and the economy highlights the need to focus on, and address, pain on both a national and international scale. Recognition of the importance of finding an efficacious approach to pain management leads to arguments supporting the extensive use of opioids in chronic pain management (CitationBallantyne 2007). Opioids are considered by some to be integral to effective pain management, and key to minimizing the suffering associated with persistent pain (CitationEuropean Pain in Cancer Survey 2007), its deleterious physical effects (CitationCarr et al 1999; CitationKehlet et al 2003; CitationAmerican Academy of Pain Medicine Council on Ethics 2005), and its erosion of a patient’s autonomy, dignity, and decision-making capacity. However, the potentially addictive nature of opioids is also recognized, and caution is advised to ensure that the ‘principle of balance’ is upheld. The principle of balance maintains that efforts to address abuse of therapy may be necessary, but they should not interfere with legitimate medical practice and patient care (CitationBrennen et al 2005; CitationDubois 2005). Furthermore, long-term studies into the use of opioids have not only found that prolonged treatment can improve patients’ functioning and QoL, but also that it is possible to reach, and maintain, a stable (non-escalating) effective dose. The established side-effects associated with opioid use, such as nausea, sedation, constipation, and itching, also tend to subside with prolonged use, as side-effect tolerance appears to develop without an associated abrogation of analgesic effect.
The opioid oxycodone controlled release (CR) is commonly used for pain relief in cancer patients, and may be considered for first-line oral therapy. In a study on visceral pain, CitationStaahl et al (2007) demonstrated that oxycodone CR has superior analgesia to morphine. In addition, the European Federation of Neurological Societies’ (EFNS) recently recommended use of oxycodone alone for the treatment of painful polyneuropathy (PPN) at a level rating of A (that requiring the existence of a strong evidence base) (CitationAttal et al 2006). A recent study investigating the efficacy and tolerability of high-dose oxycodone CR achieved optimum pain control at a dosage of oxycodone CR 224 mg/day; resulting in a significant reduction in pain intensity (final NRS <3) with a favorable side-effect profile (CitationMameli et al 2006).
Yet, published evidence on the use of high-dose oxyco-done CR in patients with terminal cancer remains limited (CitationBercovitch et al 2006). This study sought to address the gap in the evidence base by assessing the possible role and tolerability of high-dose (>160 mg/day) oxycodone CR in the management of cancer and non-cancer pain.
Methods
The study was designed as an open-label, multi-center, observational trial carried out across 10 locations. It ran for 3 months (from 1 April to 30 June 2007) and involved a total of 227 consecutive patients: 207 patients with cancer pain and 20 patients with non-cancer pain. A baseline pain evaluation was carried out using a NRS, and previous treatments were recorded to establish the most commonly prescribed therapy, and then correlated against the patient’s perception of treatment efficacy.
The goal of the study was to reduce the intensity of pain experienced by participants. The study sought to establish whether prescription of an appropriate therapy, as recommended by the World Health Organization’s (WHO) guiding principles: ‘by the clock, by the mouth, by the ladder’, could improve patients’ pain relief (CitationWHO 1996).
Patients included in the study were over 18 years of age, had a baseline NRS score of >4 and were able to take oral medication. Patients were excluded from the study if they were undergoing current radiotherapy treatment, required modification of adjuvant treatments, or were known to be intolerant to oxycodone.
Baseline assessments included cause of pain, duration of pain experienced prior to study, history of pain relief prescribed, treatment setting, and intensity of pain felt (indicative of treatment efficacy) using a NRS. After baseline assessment, patients were switched to oxycodone CR monotherapy and monitored for at least 21 days. The change in therapy followed recommendations outlined in CitationDoyle et al (1997). Existing therapy was halted completely as study patients reported uncontrolled pain on current treatment (NRS >4) and were then switched to oxycodone CR. After the change from previous treatment to oxycodone CR, patients’ pain score was evaluated and the high-dose oxycodone CR dosage was titrated up until adequate control (NRS ≤2.9) was achieved.
Duration of therapy varied depending on date of enrolment in the study, but high-dose oxycodone monotherapy was taken by study participants for an average of 37.24 days. Oxycodone CR was chosen as the reference drug because of its pharmacokinetics, pharmacodynamics, and demonstrable efficacy in both cancer and non-cancer pain. The setting for treatment administration was not altered for the study. Initial dose was individualized to each patient, using standard conversion tables (CitationDoyle et al 1997) and consideration of pharmacological history (previous treatment and dosage used), and titrated up (over a 3- to 4-day period) until effective pain control was achieved.
Results
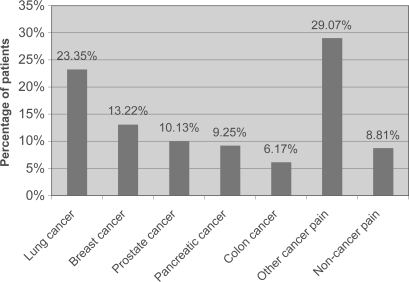
During the 3-month study period, 227 patients from 10 sites were evaluated. The patient population comprised 40% women (n = 90) and 60% male (n = 137). Patients were aged 27–84 years (average 63.76 years). Within the 91.18% (n = 207) of patients with cancer, the following categories were defined: 23.34% (n = 53) lung; 13.21% (n = 30) breast; 10.13% (n = 23) prostate; 9.25% (n = 21) pancreas; and 6.16% (n = 14) colon (; ).
Table 1 Summary of patient demographics at baseline (n = 227)
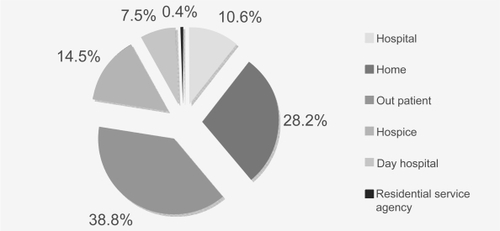
Study participants were administered treatment in a number of different settings: outpatient (n = 88); in the home (n = 64); hospice (n = 33); secondary care hospital (n = 24); day hospital (n = 17); and residential service agency (n = 1) (see ).
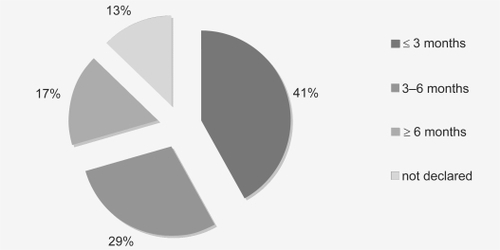
Of the 198 participants who responded to questioning at baseline, 47.98% (n = 95) had been in pain for ≤3 months, 32.83% (n = 65) for 3–6 months, and 19.19% (n = 38) for ≥6 months ().
Evaluation of pain intensity was also assessed at study outset. The overall NRS calculated for participants at the outset of the study was 7.73, indicating that pain was uncontrolled among most patients. Baseline analysis reported controlled pain (defined as NRS 2.9, p < 0.00001) in 8.1% (n = 41) of patients, all of whom were receiving high-dose oxycodone CR (an average dose of 241 mg/day). All other patients reported uncontrolled pain, with an average NRS of 7.81. Of note, 3.96% (n = 9) recorded an NRS of 5, while a significant 24.67% (n = 56) indicated a NRS of 8, and 10.13% (n = 23) recorded a maximum 10 on the NRS (; ).
Figure 4 Pain severity as classified by study participants at baseline using numerical rating scale (NRS).
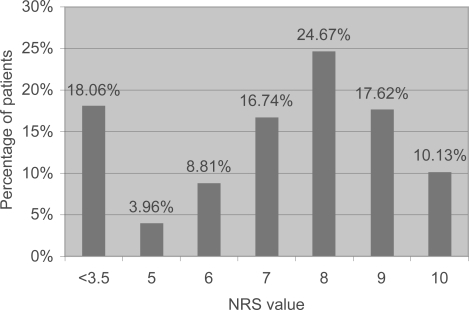
Table 2 Summary of pain control at baseline (n = 198)
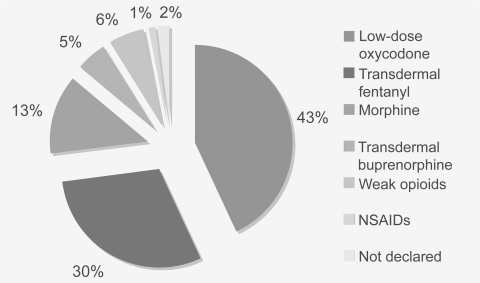
A range of pharmacological agents were in use among patients who reported uncontrolled pain (): transdermal fentanyl (30.0%); morphine (12.8%); transdermal buprenorphine (5.3%); weak opioids (6.2%); and non-steroidal anti-inflammatory drugs (NSAIDs) (1.3%). Of patients who reported poor pain control, 42.2% were treated with low-dose (<80 mg/day) oxycodone (either oxycodone/acetaminophen or oxycodone CR). Analysis found that breakthrough pain had been treated with immediate-release morphine (33.02%), transmucosal fentanyl (24.18%), NSAIDs (12.09%), immediate-release oxycodone (in the form of oxycodone/acetaminophen) (4.65%), and weak opioids (0.9%).
During the study, all patients experiencing uncontrolled pain were switched from their original treatment to oxycodone CR monotherapy. The initial dose administered was titrated up until effective pain relief was achieved. Pain control (NRS ≤ 2.9) was attained using an average dose of oxycodone CR 221.84 mg/day, resulting in an average NRS of 2.85 at study end (p < 0.00001). All patients on oxycodone CR in the study were treated for an average treatment duration of 37.24 days. Side-effects (including constipation, nausea, and vomiting) were recorded among 39.64% of patients on high-dose oxycodone CR monotherapy, but tended to subside after the first week of treatment and did not result in any patients leaving the study ().
Table 3 Summary of study findings after patients’ switch from previous pain mediction to high-dose oxycodone CR
Discussion
This study corroborates the poor management of both cancer and non-cancer pain in Italian patients. In a significant number of cases the wrong therapeutic approach was taken: transdermal treatments were used over oral agents for first-line therapy in more than a third of patients (35.3%), despite no prior stabilization of their pain. In addition, the dose prescribed was not optimized in a large number of cases, as highlighted by the use of low-dose oxycodone in 42.2% of patients who reported persistent pain despite treatment. The study demonstrates that oxycodone CR can be used in such patients to achieve rapid control of moderate to severe pain, with minimal side-effects.
Although the mean duration of therapy in this trial was only 37.24 days, a separate study carried out over a 3-year period demonstrated that oxycodone CR can achieve adequate, longer-term pain management of non-cancer pain, without requiring a further increase in dose (CitationPortenoy et al 2007).
Pain is one of the most common symptoms associated with cancer. It becomes more frequent as the cancer progresses, but may also be present during the early stages of the disease. Pain associated with cancer is largely due to the condition itself, but can (in 20% of cases) be caused by treatment complications.
An overlap in pain caused by antineoplastic treatments and that caused by cancer was recorded in around 10% of patients in a recent study (n = 1095) by CitationCaraceni et al (1999). Sources of cancer pain in the study were classified as bone lesion (due to metastases or to tumor invasion of bone and joint structures) in 41% of patients, neoplastic visceral damage in 28%, and lesions of the nervous system in 28% of cases. These were further classified into the following pain pathophysiologies: somatic, visceral, and neuropathic. The most common pathophysiology was somatic-nociceptive pain (32.0%), followed by somatic-neuropathic (23.0%), pure visceral pain (15.0%), pure neuropathic pain (7. 7%), and other syndromes of mixed pathophysiologies.
The aim of treatment in patients with advanced-stage cancer is improved QoL, which requires both efficacious treatment and adequate pain relief. Clinical experience confirms that optimal palliative care in cancer is associated with maximal pain relief. Yet, despite international guidelines on cancer-pain management (CitationWHO 1996) and availability of treatments that are effective in approximately 70%–90% of cases, up to 40% of cancer patients in Italy remain under-treated.
The European Society for Medical Oncology (ESMO) advocates the use of opioids as first-line therapy in moderate to severe cancer pain (CitationESMO Guidelines Task Force 2005CitationESMO Guidelines Task Force 2007). Guidance suggests that strong opioids can be safely administered, in increasing amounts, until pain relief is achieved. No ceiling dose is outlined, and no standard initial dose is suggested; the correct dose is one that achieves effective pain relief, and may vary among patients. The WHO recommends that pharmacological pain management should be guided by the core principles of: ‘by the mouth, by the clock, by the ladder’ (CitationWHO 1996). ‘By mouth’ embodies the recommendation of oral therapy use (where possible) as it can be rapidly modified to changing intensities of pain. Transdermal formulations are recommended only in patients in whom pain management is stable, not as first-line therapy.
Oxycodone is derived from thebaine, an opium alkaloid. It is a semi-synthetic, pure opioid receptor agonist analgesic drug that appears to work by stimulating the μ-opioid receptors found in the central nervous system (CitationGlare et al 1993; CitationReder et al 1993; CitationCitron et al 1998; CitationNapp Pharmaceuticals 2007). Oxycodone is metabolized by the liver (by the cytochrome P450 2D6) to form oxymorphone and noroxycodone, which has only weak affinity for μ-opioid receptors. Despite it being a potent analgesic, oxymorphone accounts for only 10% of oxycodone metabolites. Oxycodone, rather than oxymorphone, appears to be responsible for both analgesia and side-effects. Recent studies have demonstrated a role for oxycodone as first-line pain therapy in place of morphine (CitationRiley et al 2006), and evidence for its use, in both cancer and non-cancer pain, is growing.
Conclusion
Oxycodone CR is an oral therapeutic option approved for the treatment of moderate to severe pain. It has a similar safety and efficacy profile to morphine, but offers several therapeutic advantages. The results of its use at high dosages for pain relief in cancer and non-cancer patients demonstrate that an adequate dose can bring previously uncontrolled moderate to severe pain under control rapidly, with minimal side-effects. However, further randomized, controlled studies involving oxycodone CR and a control arm are required to increase evidence for the efficacy of oxycodone CR use in cancer and non-cancer pain.
Acknowledgements
The authors would like to thank Dr Berenato, Dr Cianfanelli, Dr Iodice, Dr Liotta and Dr Mazzocchi for their invaluable co-operation in this study and the editorial assistance of Alison Chisholm of Horizon Medical Publishing.
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- American Academy of Pain Medicine (AAPM) Council on Ethics.2005Ethics charter from American Academy of Pain MedicinePain Med62031215972083
- AttalNCruccuGHaanpaaMEFNS Task Force2006EFNS guidelines on pharmacological treatment of neuropathic painEur J Neurol1311536917038030
- BallantyneJC2007Opioid Analgesia: Perspectives on Right Use and UtilityPain Physician104799117525783
- BercovitchMAdunskyA2006High dose controlled-release oxycodone in hospice careJ Pain Palliat Care Pharmacother2033917182504
- BrennanTJKehletH2005Preventive analgesia to reduce wound hyper-algesia and persistent postsurgical pain. Not an easy pathAnesthesiol1036813
- CaraceniAPortenoyRK1999An international survey of cancer pain characteristics and syndromes. International Association for the Study of Pain (IASP) Task Force on Cancer PainPain822637410488677
- CarrDBGoudasLC1999Acute painLancet3532051810376632
- CitronMLKaplanRParrisWC1998Long-term administration of controlled-release oxycodone tablets for the treatment of cancer painCancer Invest16562719844616
- CostantiniMBeccaroMMerloFISDOC Study Group2005The last three months of life of Italian cancer patients. Methods, sample characteristics and response rate of the Italian Survey of the Dying of Cancer (ISDOC)Palliat Med196283816450880
- DoyleDHanksGWCMacDonaldN1997Modified Oxford Textbook of Palliative Medicine2nd edOxfordOxford Medical Publications
- DuboisMY2005The birth of an ethics charter for pain medicinePain Med6201215972082
- European Pain in Cancer SurveyEuropean Association of Palliative Care2007Half of European cancer patients have moderate to severe pain: one in five patients does not receive treatmentJ Pain Palliat Care Pharmacother2151318032318
- European Society for Medical Oncology (ESMO) Guidelines Task Force2005ESMO Minimum Clinical Recommendations for the management of cancer painAnn Oncol6Suppl 1
- ESMO Guidelines Task Force2007Management of cancer pain: ESMO Clinical RecommendationsAnn Oncol18Suppl 2
- FurlanADSandovalJAMailis-GagnonA2006Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effectsCMAJ17415899416717269
- GlarePAWalshTD1993Dose-ranging study of oxycodone for chronic pain in advanced cancerJ Clin Oncol1197388487060
- GoudasLCBlochRGialeli-GoudasM2005The epidemiology of cancer painCancer Invest231829015813511
- Italian Department of Health2007[online]. Accessed 6 August 2007. URL: www.Redazione-Ministerosalute.it
- Italian Medical Oncology Association (AIOM)2007[online]. Accessed 6 August 2007. URL: www.fondazioneaiom.it
- KehletHDahlJB2003Anaesthesia, surgery, and challenges in postoperative recoveryLancet3621921814667752
- MameliMMauriziPMorinoP2006Adeguata titolazione per un utilizzo di ossicodone CR ad alte dosi: chiave di successo nella risoluzione del dolore? Risultati del progetto DADO (Definizione Alti Dosaggi Ossicodone)Clinical Intervent Aging, Companion SeriesI1
- Napp Pharmaceuticals2007Summary of Product Characteristics: OxyContin tablets562007
- PortenoyRKFarrarJTBackonjaMM2007Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry studyClin J Pain232879917449988
- RederRShiM1993Safety and efficacy of controlled release oral oxycodone: doses greater than 80 mg per day [poster]. 7th World Congress on Pain, Paris, France; August 22–27 1993
- RileyJRossJRRutterD2006No pain relief from morphine? Individual variation in sensitivity to morphine and the need to switch to an alternative opioid in cancer patientsSupport Care Cancer14566415952009
- StaahlCDimcevskiGAndersenSD2007Differential effect of opioids in patients with chronic pancreatitis: an experimental pain studyScand J Gastroenterol423839017354119
- World Health Organization (WHO)1996Cancer pain relief with a guide to opioid availabilityGenevaWorld Health Organization
- YangYChengyuanW2007Guidelines on the basic outcome data from International Association for the Study of PainClin J Pain2354917575497